2. 北京市农林科学院畜牧兽医研究所, 北京 100097;
3. 北京市农林科学院-美国俄克拉荷马州立大学动物科学联合实验室, 北京 100097;
4. 甘肃农业大学动物科学技术学院, 兰州 730070;
5. 河北北方学院动物科技学院, 张家口 075000
2. Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, China;
3. Joint Laboratory of Animal Science between Beijing Academy of Agriculture and Oklahoma State University, Beijing 100097, China;
4. College of Animal Science and Technology, Gansu Agricultural University, Lanzhou 730070, China;
5. College of Animal Science Technology, Hebei North University, Zhangjiakou 075000, China
妊娠率是繁殖效率的主要影响因素之一,原因不明的空怀会导致妊娠率下降,显著影响养羊效益。肠道微生物在宿主代谢、免疫、内分泌等方面起着至关重要的作用,并且随着环境、饮食、激素等因素变化而变化[1],妊娠期间的微生物感染是不良妊娠结局的重要风险因素[2]。血清代谢物分析能揭示妊娠相关的潜在代谢变化[3-4],通过检测母体血清代谢物的相对丰度,可以精准地识别健康孕妇的胎龄[5]。妊娠是一个复杂的过程,与激素变化、新陈代谢和免疫系统的调节有关,这些生理变化协同进行,以维持胎儿的生长和发育[6]。微生物与妊娠结局关系的报道多集中在阴道微生物组,未妊娠状态下阴道微生物的物种数量显著低于妊娠状态[7],不同妊娠状态下门和属细菌的相对丰度存在显著差异[8]。相比正常女性的阴道微生物组,异常的阴道微生物组可能导致女性怀孕失败[9]。例如缺乏乳酸杆菌可能增加细菌性阴道病的患病率[10],增加早产和妊娠失败的风险[11]。与阴道微生物群一样,肠道微生物群也参与调控宿主的免疫和生理变化,这些变化对妊娠成功至关重要[12-14]。与未妊娠相比,妊娠会引起母体的代谢发生变化[15]。妊娠期糖代谢和脂代谢变化已经被广泛研究,有研究报道,妊娠会造成血液葡萄糖含量降低及低密度脂蛋白胆固醇、高密度脂蛋白胆固醇和甘油三酯含量增加[16]。氨基酸是调节精子发生、卵子受精和胚胎着床等多种生理过程的信号分子[17]。Phillips等[18]研究发现,与可育小母牛相比,不育小母牛血浆代谢物天冬酰胺、赖氨酸、谷氨酰胺、组氨酸、色氨酸、半胱氨酸和鸟氨酸含量降低,这些代谢物可能作为预测生育能力的生物标志物。微生物相对丰度和代谢物含量可能与妊娠生理状态存在密切关系,绵羊上血清代谢物与妊娠结局关系的研究报道较少,肠道微生物影响绵羊妊娠的机制暂不清楚。因此,本研究采集自由交配后妊娠与空怀绵羊的粪便和血液样本,通过分析不同妊娠状态绵羊的肠道微生物和血清代谢物,以期初步揭示绵羊肠道微生物多样性及血清代谢物与不同妊娠结局(妊娠与空怀)之间的关系。
1 材料与方法 1.1 试验动物试验在河北省张家口市阳原县北京市农林科学院试验基地进行,按照生产计划,320只无病史、断奶后2~4岁杂种母羊(中国美利奴公羊×小尾寒母羊)饲养在半开放式羊舍,饲喂相同饲粮,自由饮水和添食舔砖。饲喂根据《肉羊饲养标准》(NY/T 816—2004)配制的全混合日粮,试验期间平均每只母羊饲喂量为2 kg/d,母羊集中断奶后放公羊入群45 d(2个发情周期以上),公母比例为1 ∶ 8,采用本交方式进行配种,在撤出公羊后30 d使用B超仪(HS-1600V,Honda Electronics公司,日本)经直肠进行妊娠诊断。
1.2 超声波直肠检查法在母羊妊娠早期,用7.5 MHz直肠探头,根据B超图像诊断卵巢、胎囊等器官,将母羊分为妊娠组(P组)和空怀组(C组)。从妊娠组和空怀组各随机选取6只绵羊作为研究对象,所选取的母羊平均体重为(65.70±1.90) kg,胎次为2~3胎。
1.3 粪便标本采集及微生物分析 1.3.1 粪便样本采集佩戴一次性PE手套,从6只妊娠母羊和6只空怀母羊直肠采集粪便,采集后更换手套,将新鲜粪便放入无菌离心管中液氮速冻后-80 ℃保存,用于后续测序分析。
1.3.2 DNA提取和PCR扩增根据E.Z.N.A.® soil DNA Kit试剂盒说明书提取12个样本的微生物群落基因组DNA。提取的DNA用1%的琼脂糖凝胶检测,使用分光光度计(NanoDrop-2000,Thermo Scientific公司,美国)测定DNA浓度和纯度。使用引物338F(5′-ACTCCTACGGGAGGCAGCAG-3′)和806R(5′-GGACTACHVGGGTWTCTAAT-3′)对细菌16S rRNA基因V3~V4可变区进行PCR扩增,回收PCR产物、纯化、检测,并使用微型荧光计(QuantiFluor-ST,Promega公司,美国)对其进行定量。
1.3.3 Illumiana MiSeq测序和数据分析将纯化后的PCR产物等摩尔混合,采用NEXTFLEX Rapid DNA-Seq Kit进行文库构建,使用Illumina MiSeq PE 300平台进行测序(上海美吉生物医药科技有限公司)。使用Fastq软件对原始序列质检,FLASH软件拼接后,根据97%相似水平进行操作分类单元(operational taxonomic units,OTU)聚类,参照16S rRNA数据库(Silva v138/16S_bacteria)对OTU序列分类注释(分类置信度0.7)。数据分析在上海美吉生物医药科技有限公司云平台上进行,由Mother软件计算α多样性指数(Shannon、Simpson、Chao、Coverage和Ace指数)。采用主坐标分析(PCoA)方法分析粪便微生物群落组成的差异性或相似性,并用相似性分析(analysis of similarities,ANOSIM)进行OTU数量的组间差异检验。采用Student t检验法分析2组间微生物门水平上的差异显著性。使用线性判别分析的影响大小(linear discriminant analysis effect size,LEfSe)对2组样本进行线性判别分析(linear discriminant analysis,LDA),在属水平上鉴定差异微生物。所有分析结果均以平均值±标准差表示。
1.4 血清样本采集及代谢组学分析 1.4.1 血清样本采集对采集粪样的母羊进行颈静脉采血,血样在真空促凝管中室温静置4 h后,4 ℃离心(3 000 r/min,10 min)分离血清,-80 ℃保存,用于后续检测分析。
1.4.2 液相色谱串联质谱(LC-MS/MS)检测取100 μL血清加入提取液(甲醇∶水=4 ∶ 1),-10 ℃沉淀后,使用冷冻研磨仪(Wonbio-96c,上海万柏生物科技有限公司)将其粉碎,超声处理30 min(5 ℃,40 kHz),样品经放置沉淀蛋白后离心,将上清液转移到样品瓶中进行LC-MS/MS分析,样品经超高效液相色谱系统(ACQUITY UPLC HSS T3,Waters公司,美国)分离后进行质谱检测,流动相A为水/乙腈(体积比19 ∶ 1,含0.1%甲酸),流动相B为乙腈/异丙醇(体积比1 ∶ 1,含0.1%甲酸)。采用离子喷雾电压和正负离子扫描模式采集样品的质谱信号。为保证分析结果可靠性,将质控样本与所测样本混合分析。
1.4.3 数据处理与分析使用Progenesis QI 2.3软件对原始数据进行峰值校准和检测,生成的数据矩阵中,只保留任意一组80%以上非零值的变量,对数据预处理后,进行log10转换生成最终数据矩阵,将质谱信息与代谢物数据库HMDB(http://www.hmdb.ca/)和Metlin(https://metlin.scripps.edu/)匹配。数据分析在上海美吉生物医药科技有限公司云平台进行。使用R软件包对数据进行主成分分析(PCA)和正交最小偏二乘判别分析(OPLS-DA)。在OPLS-DA模型中计算投影变量重要性(VIP)值,使用R2Y和Q2评价OPLS-DA模型的有效性,结合Student t检验法筛选出组间差异代谢物(P < 0.05,VIP值>1),并绘制差异火山图。对组间差异代谢物映射到KEGG通路上,并进行通路富集分析,使用Scipy v 1.0.0软件精确检验显著富集的通路(P < 0.05)。
1.5 微生物与代谢组联合分析利用Python软件对差异代谢物和差异微生物进行PCoA和普氏分析(Procrustes analysis),分析差异代谢物和差异微生物之间的相似和变异情况,使用R语言包(ggplot2)可视化作图。利用R语言包(psych、caret)计算差异代谢物和属水平的差异微生物的皮尔森相关系数(Pearson correlation coefficient)和P值,并构建相关性热图。
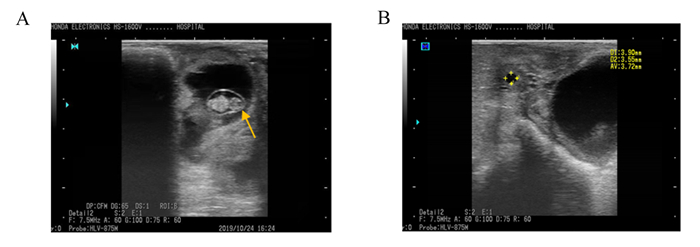
2 结果 2.1 妊娠诊断应用直肠B超可准确区分妊娠与空怀母羊,妊娠母羊B超图像中紧挨膀胱的微亮区域为孕囊切面,孕囊内有一较强的回声区域(图 1-A);空怀母羊没有看到孕囊结构,可见卵泡(清晰的无回声圆形结构)(图 1-B)。
|
A:妊娠组,箭头所指为孕囊;B:空怀组,星号为卵泡。 A: pregnant group, the arrow refers to the pregnant sac; B: non-pregnant group, the asterisk shows follicle. 图 1 母羊B超妊娠诊断图 Fig. 1 B-ultrasound pregnancy diagnostic images of ewes |
在对12个样本质控过滤后共获得556 983条高质量序列,其中,最小长度为213 bp,最大长度为511 bp,平均长度为413 bp。根据97%相似性的阈值,将序列聚类为2 222个OTU,每个样本OTU数量在1 105~1 392个。
2.3 粪便微生物群落丰富度和多样性使用Chao、Ace、Shannon、Simpson和Coverage指数来估计粪便微生物群落的丰富度和多样性。由表 1可知,与妊娠组相比,空怀组的Shannon指数显著降低(P < 0.05),而Chao、Ace、Coverage和Simpson指数无显著差异(P>0.05)。
|
|
表 1 α多样性指数分析 Table 1 α diversity index analysis |
由表 2可知,在门水平上,相对丰度≥1%的优势菌群在妊娠组和空怀组分别有6和4个。其中厚壁菌门(Firmicutes)和拟杆菌门(Bacteroidota)是妊娠组和空怀组母羊粪便微生物群落中共有优势菌群,二者相对丰度之和分别为90%和92%;其他菌群相对丰度较低,妊娠组螺旋菌门(Spirochaetota)、疣微菌门(Verrucomicrobiota)、Patescibacteria和放线菌门(Actinobacteriota)相对丰度分别为3.74%、2.56%、1.04%和1.00%,空怀组螺旋菌门和疣微菌门相对丰度分别为3.41%、1.13%。其中,妊娠组放线菌门相对丰度显著高于空怀组(P < 0.05)。
|
|
表 2 门水平下的优势菌群分布 Table 2 Distribution of dominant microflora at phylum level |
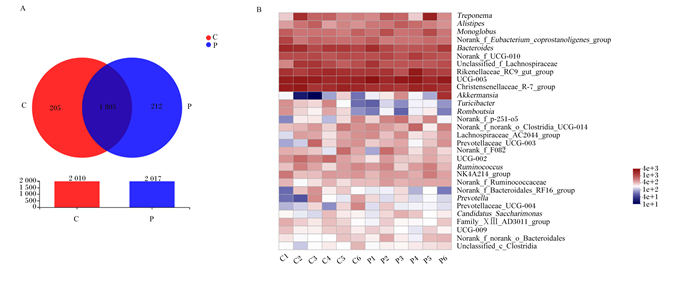
在妊娠组和空怀组共检出2 222个OTU,2组共有的OTU有1 835个,妊娠组特有的OTU有212个,空怀组特有的OTU有205个(图 2-A)。OTU分类分析中相对丰度前30菌属的热图见图 2-B,在属水平上,UCG-005(10.70%)、克里斯滕菌科R-7群(Christensenellaceae_R-7_group)(7.39%)、理研菌科RC9肠道群(Rikenellaceae_RC9_gut_group)(6.54%)和拟杆菌属(Bacteroides)(4.97%)依次是妊娠组中相对丰度前4的菌属;UCG-005(9.64%)、克里斯滕菌科R-7群(8.87%)、理研菌科RC9肠道群(6.76%)和拟杆菌属(6.24%)依次是空怀组中相对丰度前4的菌属。
|
Treponema:密螺旋体属;Alistipes:阿利斯泰伯属;Monoglobus:单球虫属;Norank_f_Eubacterium_coprostanoligenes_group:未分类的粪真杆菌群;Bacteroides:拟杆菌属;Norank_f_UCG-010:未分类的UCG-010科;Unclassified_f_Lachnospiraceae:未分类的毛螺菌科;Rikenellaceae_RC9_gut_group:理研菌科_RC9_肠道群;Christensenellaceae_R-7_group:克里斯滕菌科_R-7群;Turicibacter:尿道杆菌属;Norank_f_p-251-o 5:未分类的p-251-o5科;Norank_f_norank_o_Clostridia_UCG-014:未分类的未分类科_梭状芽孢杆菌目_UCG-014;Lachnospiraceae_AC2044_group:毛螺菌科_AC2044群;Prevotellaceae_UCG-003:普氏菌科_UCG-003;Norank_f_F082:未分类的F082科;Ruminococcus:瘤胃球菌属;NK4A214_group:NK4A214群;Norank_f_Ruminococcaceae:未分类的瘤胃球菌科;Norank_f_Bacteroidales_RF16_group:未分类的拟杆菌目_RF16群;Prevotella:普氏菌属;Prevotellaceae_UCG-004:普氏菌科_UCG-004;Candidatus_Saccharimonas:糖单胞菌属;Family_ⅩⅢ_AD3011_group:ⅩⅢ科_AD3011群;Norank_f_norank_o_Bacteroidales:未分类的未分类科拟杆菌;Unclassified_c_Clostridia:未分类的梭状芽孢杆菌纲。 C:空怀组non-pregnant group;P:妊娠组pregnant group。下图同the same as below。 A:OTU水平的韦恩图;B:属水平微生物组成热图。 A: Venn diagram at the OTU level; B: Heatmap of microbial composition at genus level. 图 2 妊娠组和空怀组粪便微生物群落组成 Fig. 2 Composition of fecal microbial community in pregnant group and non-pregnant group |
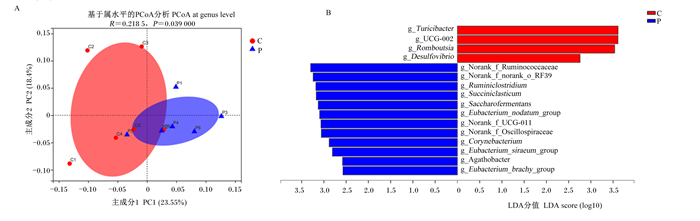
基于bray-curtis算法的PCoA显示,在属水平上,大多数样本与同一组的其他样本聚在一起,妊娠组与空怀组样品明显分开(图 3-A),ANOSIM表明妊娠组与空怀组属水平菌群组成差异显著(P < 0.05)。在属水平进行LEfSe多级差异物种判别分析,筛选出LDA值大于2.5的属水平菌群,其中妊娠组具有显著作用的菌属有12个,包括瘤胃梭菌属(Ruminiclostridium)、琥珀酸菌属(Succiniclasticum)、Saccharofermentans等;空怀组具有显著作用的菌属有4个,包括Turicibacter、UCG-002、Romboutsia和脱硫弧菌属(Desulfovibrio)(图 3-B)。
|
Eubacterium_siraeum_group:惰性真杆菌群;Eubacterium_brachy_group:短优杆菌群;Succiniclasticum:琥珀酸菌属;Desulfovibrio:脱硫弧菌属;Corynebacterium:棒状杆菌属;Norank_f_UCG-011:未分类的UCG-011科;Ruminiclostridium:瘤胃梭菌属;Norank_f_Oscillospiraceae:未分类的颤螺菌科;Norank_f_norank_o_RF39:未分类的科_未分类RF39目;Eubacterium_nodatum_group:缠结真杆菌群;Saccharofermentans:产乙酸糖发酵菌属。 A:基于bray-curtis算法的PCoA;B:2组LDA柱形图,LDA值大于2.5。 A: PCoA based on bray-curtis algorithm; B: LDA histogram of two groups, LDA value was more than 2.5. 图 3 妊娠组与空怀组之间属水平微生物组成差异分析 Fig. 3 Difference analysis of microbial composition at genus level between pregnant group and non-pregnant group |
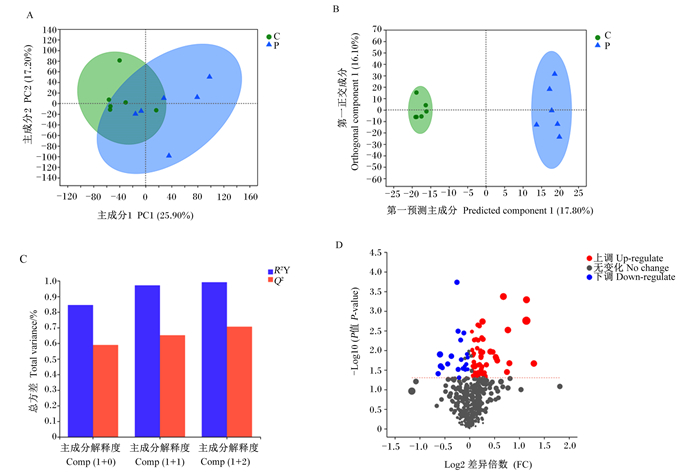
对差异代谢物进行PCA,主成分1和主成分2对样本差异贡献值分别为25.90%和17.20%,2组样品明显分开(图 4-A)。去除与分组无关的信息,通过OPLS-DA进一步分析,妊娠组与空怀组各组样品很好地聚集在一起并且完全分开(图 4-B),OPLS-DA模型的R2Y和Q2的累计值(cumulative values)均大于0.5,表明OPLS-DA具有良好的预测能力(图 4-C)。妊娠组与空怀组之间共发现78种血清差异代谢物,其中58种差异代谢物表达上调,20种差异代谢物表达下调(图 4-D)。
|
A:PCA;B:OPLS-DA;C:OPLS-DA模型验证,R2Y表示对Y矩阵解释率,Q2表示模型的预测能力,R2Y和Q2越接近1表示模型越可靠;D:差异代谢物火山图。 A: PCA; B: OPLS-DA; C: OPLS-DA model verification, R2Y represents the interpretation rate of Y matrix, Q2 represents the prediction ability of the model, and the closer the R2Y and Q2 to 1, the model is more reliable; D: volcano map of differential metabolites. 图 4 妊娠组和空怀组母羊血清差异代谢物分析 Fig. 4 Analysis of serum differential metabolites of ewes in pregnant group and non-pregnant group |
如图 5所示,根据HMDB数据库,共有66个差异代谢物被标注为8个超类(图 5-A),脂质和类脂分子28个、有机酸及衍生物12个、有机氧化合物9个、有机杂环化合物8个、核苷和核苷酸类似物4个、苯环型化合物2个、糖类多酮类化合物2个和有机硫化合物1个。差异代谢物的聚类热图(图 5-B)显示了VIP值前30的差异代谢物表达谱和VIP值,可以看出代谢物被分为上下2个群集,上群集代谢物中LysoPC(O-18 ∶ 0)含量在空怀组极显著上调(P < 0.01);下群集代谢物中脱氧肌苷(deoxyinosine)含量在空怀组显著下调(P < 0.05),berteroin和C16二氢(神经)鞘氨醇(C16 sphinganine)含量在空怀组极显著下调(P < 0.01)。
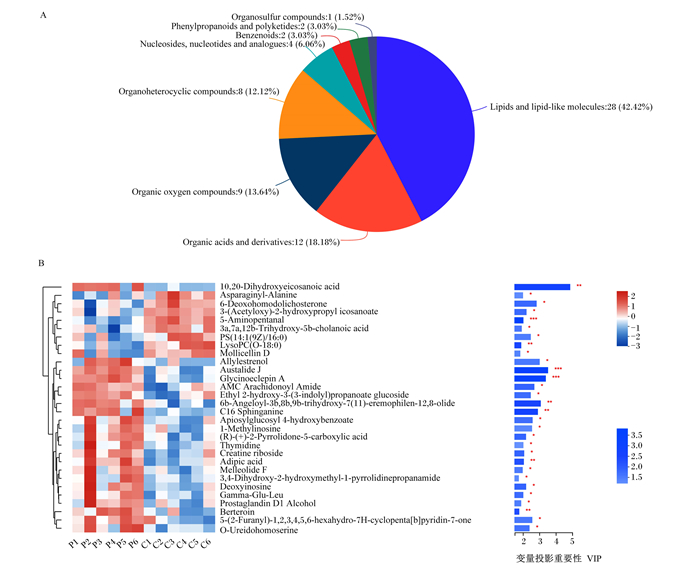
|
A:HMBD超类化合物饼图HMBD superclass pie chart。Organosulfur compounds:有机硫化合物;Phenylpropanoids and polyketides:糖类多酮类化合物;Benzenoids;苯环型化合物;Nucleosides, nucleotides and analogues:核苷和核苷酸类似物;Organoheterocyclic compounds:有机杂环化合物;Organic oxygen compounds:有机氧化合物;Organic acids and derivatives:有机酸及衍生物;Lipids and lipid-like molecules;脂质和类脂分子。 B:差异代谢物的聚类热图,VIP值>1的前30种差异代谢物Clustering heat map of differential metabolites, the first 30 differential metabolites with VIP value>1。10, 20-Dihydroxyeicosanoic acid:10,20-二羟基二十烷酸;Asparaginyl-Alanine:天冬酰胺-丙氨酸;6-Deoxohomodolichosterone:6-去氧羟孕酮;3-(Acetyloxy)-2-hydroxypropyl icosanoate:3-(乙酰氧基)-2-羟丙基烟酸酯;5-Aminopentanal:5-氨基戊醛;3a, 7a, 12b-Trihydroxy-5b-cholanoic acid:3a,7a,12b-三羟基-5b-胆酸;Mollicellin D:莫利瑟林D;Allylestrenol:烯丙雌醇;Austalide J:奥司他林J;Glycinoeclepin A:糖胞苷A;AMC Arachidonoyl Amide:AMC花生酰胺;Ethyl 2-hydroxy-3-(3-indolyl)propanoate glucoside:2-羟基-3-(3-吲哚基)丙酸乙酯葡萄糖苷;6b-Angeloyl-3b, 8b, 9b-trihydroxy-7(11)-eremophilen-12, 8-olide:6b-当归酰-3b,8b,9b-三羟基-7(11)-嗜热菌素-12,8-内酯;C16 Sphinganine:C16二氢(神经)鞘氨醇;Apiosylglucosyl 4-hydroxybenzoate:4-羟基苯甲酸阿皮糖苷;1-Methylinosine:1-甲基次黄嘌呤核苷;(R)-(+)-2-Pyrrolidone-5-carboxylic acid:(R)-(+)-2-吡咯烷酮-5-羧酸;Thymidine:胸苷;Creatine riboside:肌酸核苷;Adipic acid:己二酸;Melleolide F:米洛里德F;3, 4-Dihydroxy-2-hydroxymethyl-1-pyrrolidinepropanamide:3,4-二羟基-2-羟甲基-1-吡咯烷丙酰胺;Deoxyinosine:脱氧肌苷;Gamma-Glu-Leu:γ-谷氨酸-亮氨酸;Prostaglandin D1 Alcohol:前列腺素D1酒精;5-(2-Furanyl)-1, 2, 3, 4, 5, 6-hexahydro-7H-cyclopenta[b]pyridin-7-one:1,2,3,4,5,6-六氢化-7H-环戊烷[b]吡啶-7-1;O-Ureidohomoserine:尿素高丝氨酸。 * * *表示P < 0.001,* *表示P < 0.01,*表示P < 0.05。 * * * indicated P < 0.001, * * indicated P < 0.01, and * indicated P < 0.05. 图 5 妊娠组和空怀组母羊血清差异代谢物分类 Fig. 5 Classification of serum differential metabolites of ewes in pregnant group and non-pregnant group |
差异代谢物在11条KEGG通路上显著富集(P < 0.05),分别是哮喘、醚类脂代谢、矿物质吸收、β-丙氨酸代谢、十字孢碱生物合成、苯丙氨酸代谢、氨基酰-tRNA生物合成、蛋白质消化吸收、癌症中的胆碱代谢、癌症中的中心碳代谢和ABC转运蛋白(图 6)。
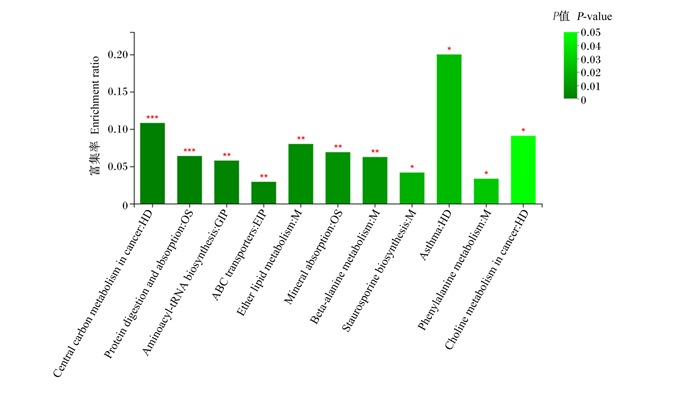
|
Central carbon metabolism in cancer:癌症中的中心碳代谢;Protein digestion and absorption;蛋白质消化吸收;Aminoacyl-tRNA biosynthesis:氨基酰-tRNA生物合成;ABC transporters:ABC转运蛋白;Ether lipid metabolism:醚类脂代谢;Mineral absorption:矿物质吸收;Beta-alanine metabolism:β-丙氨酸代谢;Staurosporine biosynthesis:十字孢碱生物合成;Asthma:哮喘;Phenylalanine metabolism:苯丙氨酸代谢;Choline metabolism in cancer:癌症中的胆碱代谢。 图 6 KEGG富集分析柱形图 Fig. 6 KEGG enrichment analysis column chart |
普氏分析将微生物和代谢物PCoA排序构型连接起来,分析结果显示妊娠组与空怀组样本的微生物组学相对丰度和代谢组学表达量趋势显著一致(P < 0.05)(图 7-A)。为近一步挖掘在微生物多样性和代谢组分析结果中差异菌群(属水平)和差异代谢物之间的关系,采用皮尔逊分析2组的相关性关系,对具有显著相关性关系的微生物和代谢物建立热图,图中共包含有22种代谢物和14种微生物。微生物被分为2个群集(上和下),代谢物被分为2个群集(左和右)(图 7-B)。图中上群集微生物都在妊娠组显著上调并且几乎都属于厚壁菌门,这些菌属与左群集代谢物呈正相关,提示这些菌属和代谢物可能与成功妊娠相关;下群集微生物中脱硫弧菌属(Desulfovibrio)在空怀组中显著上调,并且和3a,7a,12b-三羟基-5b-胆烷酸(3a, 7a, 12b-trihydroxy-5b-cholanoic acid)含量呈显著正相关(P < 0.05),提示这些菌属和代谢物可能与空怀相关。对相关性热图分析表明血清代谢物和肠道微生物的相互作用能够影响母羊妊娠。
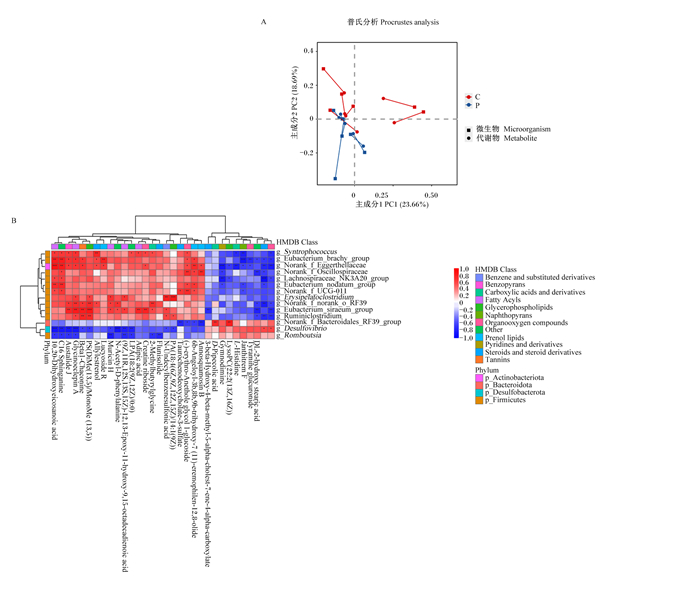
|
Actinobacteriota:放线菌门;Bacteroidota:拟杆菌门;Desulfobacterota:脱硫菌门;Firmicutes:厚壁菌门;Syntrophococcus:同性球菌属;Norank_f_Eggerthellaceae:未分类的伊格尔兹氏菌科;Lachnospiraceae_NK3A20_group:毛螺菌科_NNK3A20群;Erysipelatoclostridium:丹毒梭菌属;Beta1-chaconine:Beta1-查茄碱;Lucyoside R:丝瓜皂甙R;Muricin H:木犀草素H;N-Acetyl-D-phenylalanine:N-乙酰-D-苯丙氨酸;(9Z, 11R, 12S, 13S, 15Z)-12, 13-Epoxy-11-hydroxy-9, 15-octadecadienoic acid:(9Z,11R,12S,13S,15Z)-12,13-环氧-11-羟基-9,15-十八二烯酸;2-Methylbutyrylglycine:2-甲基丁酰甘氨酸;Flunisolide:氟尼缩松;N-undecylbenzenesulfonic acid:N-十一烷基苯磺酸;Taurochenodeoxycholate-3-sulfate:牛磺鹅去氧胆酸盐-3-硫酸盐;(-)-erythro-anethole glycol 1-glucoside:(-)-红-茴香脑二醇1-葡萄糖苷;Annosquamosin B:番红花素B;3-beta-hydroxy-4-beta-methyl-5-alpha-cholest-7-ene-4-alpha-carboxylate:3-β-羟基4β-甲基5-α-胆甾-7-烯-4-α-羧酸盐;D-Pipecolic acid:D-哌啶酸;Gymnodimine:环亚胺毒素;LysoPC(22 ∶ 2(13Z, 16Z)):LysoPC(22 ∶ 2(13Z, 16Z));L-histidine:L-组氨酸;Tyramine glucuronide:酪胺葡萄糖醛酸苷;DL-2-hydroxy stearic acid:DL-2-羟基硬脂酸;Benzene and substituted derivatives:苯和取代衍生物;Benzopyrans:苯骈吡喃类;Carboxylic acids and derivatives:羧酸及衍生物;Fatty Acyls:脂肪酰基;Glycerophospholipids:甘油磷脂;Naphthopyrans:萘并吡喃;Organooxygen compounds:有机氧化合物;Other:其他;Prenol lipids:异戊二烯脂类;Pyridines and derivatives:吡啶及衍生物;Steroids and steroid derivatives:甾体和甾体衍生物;Tannins:鞣质。 A:妊娠组和空怀组母羊粪便菌群和血清代谢物普氏分析图,P值表示微生物相对丰度和代谢物表达量趋势显著一致(P < 0.05);B:代谢物和属水平微生物的相关性热图,皮尔森相关性系数值在图中以不同颜色表示,右侧为不同颜色范围的系数值大小,越接近1表示相关性越大,* * *表示P < 0.001,* *表示P < 0.01,*表示P < 0.05。 A: Procrustes analysis of fecal microflora and serum metabolites of ewes in pregnant group and non-pregnant group. P-value indicated that the trend of microbial relative abundance was significantly consistent with that of metabolite expression (P < 0.05). B: Correlation heat map of metabolites and microorganisms at genus level, the Pearson correlation coefficient values were expressed in different colors on the right, and the closer to 1 indicated greater correlation, * * * indicated P < 0.001, * * indicated P < 0.0l, and * indicated P < 0.05. 图 7 妊娠组和空怀组母羊粪便微生物与血清差异代谢物关联性分析 Fig. 7 Correlation of fecal microbiota and serum differential metabolites of ewes in pregnant group and non-pregnant group |
肠道微生物影响宿主的生长、新陈代谢和免疫功能,宿主肠道微生物的组成不但与宿主本身遗传有关,还受到环境的影响[19-20]。粪便微生物可以反映肠道微生物的组成,并且越来越多研究者通过新鲜粪便微生物组成来研究肠道微生物区系[21-23]。妊娠是一个复杂的生物学过程,母体会发生一系列变化,包括激素水平、新陈代谢和肠道黏膜免疫。本研究中,妊娠组和空怀组母羊粪便微生物结构与相对丰度存在明显变化。有研究报道,不孕与健康女性的子宫[24]、阴道[25-26]微生物结构组成存在显著差异,而关于肠道微生物的研究报道较少。本研究中,与妊娠组相比,空怀组母羊粪便微生物的α多样性指数有所降低,这与Komiya等[27]研究结果相似,其中Shannon指数显著降低。α多样性指数可以反映微生物群落的稳定性和功能,并且已有报道提议其作为一种健康指标[28],推测低多样性的肠道微生物可能不利于母体维持妊娠稳态,这需要进一步研究论证。
本研究表明,妊娠组和空怀组母羊微生物相对丰度存在差异。在门水平上,与妊娠组相比,空怀组母羊粪便放线菌门相对丰度显著减少。子宫内膜容受性和胚胎着床是妊娠2个主要过程,调节性T细胞在这2个过程中都发挥重要作用[29],其可以抑制母体对胎儿的免疫反应,如果调节性T细胞数量缺少会导致子宫炎症和纤维化,可能导致胚胎着床失败[30]。放线菌门可以增加调节性T细胞数量,并且可以通过诱导调节性T细胞来调节免疫炎症和免疫反应,减少炎症因子释放,避免过度炎症[31]。此外,放线菌门还与短链脂肪酸(SCFA)的产量增加有关[32],SCFA参与维持妊娠期的葡萄糖稳态和能量平衡[33],这对发育中的胎儿和母体的健康至关重要[34]。推测放线菌门的减少可能与调节性T细胞数量和脂质代谢变化相关,导致母羊空怀。
属水平LDA表明瘤胃梭菌属、棒状杆菌属(Corynebacterium)、脱硫弧菌属和Turicibacter是妊娠和空怀母羊的差异微生物。瘤胃梭菌属在妊娠组中显著富集,瘤胃梭菌属的纤维素分解功能已经被广泛研究,直到近几年,瘤胃梭菌属对宿主生理的影响才被广泛报道[35]。Song等[36]研究发现,瘤胃梭菌属与血脂、血糖水平呈显著负相关。虽然脂质和葡萄糖可以给母羊和胎儿提供营养,满足胚胎植入和胎儿发育需要的大量能量[37-38],但是也有研究表明,脂质代谢和葡萄糖代谢出现紊乱,可能会影响妊娠率[39-40]。所以推测瘤胃梭菌属可能通过参与调解血脂、血糖的稳态,影响到母羊的早期妊娠发育。炎症及其引起的氧化应激可以影响受精和卵母细胞正常发育[41],且炎症或炎症介质引起的类固醇合成改变导致子宫受损,继而影响妊娠的建立和维持[42]。棒状杆菌属与肠道通透性和炎症呈负相关,而脱硫弧菌属能够产生脂多糖诱发肠道炎症[43]。有研究表明,在妊娠早期肠道通透性的增加与脂多糖含量升高相关,会增加妊娠失败的风险[44]。Turicibacter是空怀组中对组间相对丰度差异影响最大的菌属,Shang等[45]通过建立葡聚糖硫酸钠结肠炎小鼠模型,发现Turicibacter是结肠炎中的靶标微生物,而且与炎症呈正向相关。还有研究证明,孕妇妊娠期Turicibacter相对丰度的增加与肝内胆汁淤积症有关,可能导致不良妊娠结局[46]。本研究中,与妊娠组相比,空怀组粪便脱硫弧菌属和Turicibacter相对丰度显著增加,棒状杆菌属相对丰度显著减少,所以推测这些微生物导致的异常炎症反应可能与绵羊的空怀有关,需进一步研究证实。
3.2 母羊妊娠与血清代谢物的关系为了研究母羊空怀生理状态下血清代谢组的变化特征,通过LC-MS/MS技术发现了妊娠组与空怀组间存在78种差异代谢物,这些代谢物被分类为脂质和类脂分子、有机酸及衍生物、有机氧化合物、有机杂环化合物、核苷和核苷酸类似物、苯环型化合物、糖类多酮类化合物和有机硫化合物。基于OPLS-DA计算VIP值,并根据VIP值大小排列的前30种差异代谢物(VIP值>1,P < 0.05),有可能是母羊妊娠潜在的生物标志物。
溶血磷脂酰胆碱(lysophosphatidylcholines,LysoPC)是磷脂酰胆碱(phosphatidylcholines,PC)通过磷脂酶A2水解的产物[47],有研究报道,在体外受精流产组孕妇的卵泡液中LysoPC相对丰度显著升高。在本研究中,LysoPC(O-18 ∶ 0)相对丰度在空怀组中增加,并且显著富集在醚脂代谢通路,提示脂质代谢异常可能是导致空怀的原因之一。此外,LysoPC(O-18 ∶ 0)相对丰度的增加可能与氧化应激相关[47-48]。氧化应激会影响胎盘发育[49]和成功受精[41],是女性不孕症发病的因素之一[50-51]。
本研究中脱氧肌苷含量在空怀组中显著减少,KEGG分析结果表明脱氧肌苷显著富集于ABC转运蛋白通路,ABC转运体是胎盘屏障功能和关键生殖过程的重要调节因子[52],其利用ATP水解参与运输多种底物(类固醇激素、胆固醇和细胞因子等),而这些底物都参与胚胎植入、胎盘发育等过程[53]。Gao等[54]研究发现,补充外源性核苷酸可能通过哺乳动物雷帕霉素靶蛋白-过氧化物酶体增殖物激活受体(mammalian target of rapamycin-peroxisome proliferators-activated receptors,mTORC1-PPARs)通路调节胎盘营养物质的转运。此外,脱氧肌苷的增加能提供更多的能量[55],促进卵母细胞发育[56]。研究结果提示脱氧肌苷含量减少可能与母羊空怀相关。
本研究中berteroin含量在空怀组中显著下调,研究表明,berteroin具有很强的抗炎活性,并能抑制巨噬细胞M1标志物(CD80和CD86)的表达和促炎介质(一氧化氮和前列腺素E2)、促炎细胞因子(肿瘤坏死因子和白细胞介素)的分泌[57]。成功妊娠需要促炎因子与抗炎因子的平衡,过度的炎症反应可能导致妊娠失败[58-59],与能正常繁殖的绵羊相比,受孕失败绵羊的血清中炎症因子含量显著升高[60]。结合本研究结果,推测Berteroin可能通过调节母羊的炎症因子平衡发挥促进妊娠建立和维持的作用。
3.3 微生物组与代谢组学联合分析本研究中,妊娠组与空怀组母羊差异粪便微生物相对丰度和血清代谢物表达量趋势一致。其中C16-鞘氨醇(C16-sphinganine)含量在妊娠组显著增加,C16-鞘氨醇属于鞘脂,鞘脂是真核细胞膜的普遍组成部分,可作为信号分子调节包括细胞增殖、生长和存活等多种细胞过程[61]。通过联合分析热图发现与C16-鞘氨醇含量显著相关的肠道菌群属大多数是厚壁菌门。厚壁菌门是空怀组和妊娠组的共同优势菌群,且不存在显著差异,这与前人研究结果[62]一致。Sun等[63]研究发现,假肠膜明串珠菌饮食干预组相比高脂饮食组,肝脏C16-鞘氨醇含量升高,肠道微生物与肝脏代谢物的相关性分析表明厚壁菌门相对丰度与C16-鞘氨醇含量呈负相关。而在本研究中,血清C16-鞘氨醇含量与厚壁菌门相对丰度呈显著正相关,可能与不同的饲料组成和生理状态等有关,需进一步研究。
本研究中,空怀组粪便脱硫弧菌属相对丰度显著增加,在联合分析中与脱硫弧菌属相对丰度显著负相关的代谢物大部分是脂质。脱硫弧菌能通过增强CD36表达来上调宿主对肠道内脂质的吸收[64],空怀组的脱硫弧菌属相对丰度增加可能与脂质代谢有关;此外脱硫弧菌属还能促进炎症反应[65],Yi等[66]研究表明,与正常组相比,孕妇流产组血清炎症因子含量显著增加,且血清3a, 7a, 12b-三羟基-5b-胆烷酸含量显著增加。本研究中,血清3a, 7a, 12b-三羟基-5b-胆烷酸含量与脱硫弧菌属相对丰度呈显著正相关,基于这些结果推测3a, 7a, 12b-三羟基-5b-胆烷酸与脱硫弧菌属可能通过增加炎症反应共同导致母羊的空怀。
4 结论与妊娠组相比,空怀组母羊粪便菌群组成和血清代谢物发生了明显改变,差异菌群(属水平)和差异代谢物之间的相互作用也是影响母羊妊娠与否的因素之一。
| [1] |
NEUMAN H, KOREN O. The pregnancy microbiome[J]. Nestle Nutrition Institute Workshop Series, 2017, 88: 1-9. |
| [2] |
MEI C L, YANG W N, WEI X, et al. The unique microbiome and innate immunity during pregnancy[J]. Frontiers in Immunology, 2019, 10: 2886. DOI:10.3389/fimmu.2019.02886 |
| [3] |
SHEN G P, LI Z S, ZHANG Y, et al. 1H NMR-based metabolomics study on the physiological variations during the rat pregnancy process[J]. Molecular and Cellular Endocrinology, 2016, 423: 40-50. DOI:10.1016/j.mce.2016.01.003 |
| [4] |
MANUCK T A, LAI Y J, RU H Y, et al. Metabolites from midtrimester plasma of pregnant patients at high risk for preterm birth[J]. American Journal of Obstetrics & Gynecology MFM, 2021, 3(4): 100393. |
| [5] |
LIANG L, RASMUSSEN M L H, PIENING B, et al. Metabolic dynamics and prediction of gestational age and time to delivery in pregnant women[J]. Cell, 2020, 181(7): 1680-1692. DOI:10.1016/j.cell.2020.05.002 |
| [6] |
DUNLOP A L, MULLE J G, FERRANTI E P, et al. Maternal microbiome and pregnancy outcomes that impact infant health: a review[J]. Advances in Neonatal Care, 2015, 15(6): 377-385. DOI:10.1097/ANC.0000000000000218 |
| [7] |
AULT T B, CLEMMONS B A, REESE S T, et al. Uterine and vaginal bacterial community diversity prior to artificial insemination between pregnant and nonpregnant postpartum cows[J]. Journal of Animal Science, 2019, 97(10): 4298-4304. DOI:10.1093/jas/skz210 |
| [8] |
AULT T B, CLEMMONS B A, REESE S T, et al. Bacterial taxonomic composition of the postpartum cow uterus and vagina prior to artificial insemination1[J]. Journal of Animal Science, 2019, 97(10): 4305-4313. DOI:10.1093/jas/skz212 |
| [9] |
SINGER M, BORG M, OUBURG S, et al. The relation of the vaginal microbiota to early pregnancy development during in vitro fertilization treatment—a Meta-analysis[J]. Journal of Gynecology Obstetrics and Human Reproduction, 2019, 48(4): 223-229. DOI:10.1016/j.jogoh.2019.01.007 |
| [10] |
VASUNDHARA D, RAJU V N, HEMALATHA R, et al. Vaginal & gut microbiota diversity in pregnant women with bacterial vaginosis & effect of oral probiotics: an exploratory study[J]. The Indian Journal of Medical Research, 2021, 153(4): 492-502. DOI:10.4103/ijmr.IJMR_350_19 |
| [11] |
RAVEL J, MORENO I, SIMÓN C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease[J]. American Journal of Obstetrics and Gynecology, 2021, 224(3): 251-257. DOI:10.1016/j.ajog.2020.10.019 |
| [12] |
GOHIR W, RATCLIFFE E M, SLOBODA D M. Of the bugs that shape us: maternal obesity, the gut microbiome, and long-term disease risk[J]. Pediatric Research, 2015, 77(1/2): 196-204. |
| [13] |
TILG H, MOSCHEN A R. Microbiota and diabetes: an evolving relationship[J]. Gut, 2014, 63(9): 1513-1521. DOI:10.1136/gutjnl-2014-306928 |
| [14] |
TADDEI C R, CORTEZ R V, MATTAR R, et al. Microbiome in normal and pathological pregnancies: a literature overview[J]. American Journal of Reproductive Immunology, 2018, 80(2): e12993. DOI:10.1111/aji.12993 |
| [15] |
SUN T Y X, LEE B, KINCHEN J, et al. Differences in first-trimester maternal metabolomic profiles in pregnancies conceived from fertility treatments[J]. The Journal of Clinical Endocrinology and Metabolism, 2019, 104(4): 1005-1019. DOI:10.1210/jc.2018-01118 |
| [16] |
WANG Q, WVRTZ P, AURO K, et al. Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence[J]. BMC Medicine, 2016, 14(1): 205. DOI:10.1186/s12916-016-0733-0 |
| [17] |
DAI Z L, WU Z L, HANG S Q, et al. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction[J]. Molecular Human Reproduction, 2015, 21(5): 389-409. DOI:10.1093/molehr/gav003 |
| [18] |
PHILLIPS K M, READ C C, KRIESE-ANDERSON L A, et al. Plasma metabolomic profiles differ at the time of artificial insemination based on pregnancy outcome, in Bos taurus beef heifers[J]. Scientific Reports, 2018, 8(1): 13196. DOI:10.1038/s41598-018-31605-0 |
| [19] |
AMATO K R. Incorporating the gut microbiota into models of human and non-human primate ecology and evolution[J]. American Journal of Physical Anthropology, 2016, 159(Suppl.61): S196-S215. |
| [20] |
FUHLER G M. The immune system and microbiome in pregnancy[J]. Best Practice & Research.Clinical Gastroenterology, 2020, 44/45: 101671. |
| [21] |
CHANG J J, YAO X T, ZUO C X, et al. The gut bacterial diversity of sheep associated with different breeds in Qinghai province[J]. BMC Veterinary Research, 2020, 16(1): 254. DOI:10.1186/s12917-020-02477-2 |
| [22] |
LIAO R R, XIE X H, LV Y H, et al. Ages of weaning influence the gut microbiota diversity and function in Chongming white goats[J]. Applied Microbiology and Biotechnology, 2021, 105(9): 3649-3658. |
| [23] |
MAMUN M A A, SANDEMAN M, RAYMENT P, et al. The composition and stability of the faecal microbiota of Merino sheep[J]. Journal of Applied Microbiology, 2020, 128(1): 280-291. DOI:10.1111/jam.14468 |
| [24] |
CHEN W J, WEI K H, HE X, et al. Identification of uterine microbiota in infertile women receiving in vitro fertilization with and without chronic endometritis[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 693267. DOI:10.3389/fcell.2021.693267 |
| [25] |
ZHAO C Y, WEI Z F, YANG J J, et al. Characterization of the vaginal microbiome in women with infertility and its potential correlation with hormone stimulation during in vitro fertilization surgery[J]. mSystems, 2020, 5(4): e00450-20. |
| [26] |
KITAYA K, NAGAI Y, ARAI W, et al. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure[J]. Mediators of Inflammation, 2019, 2019: 4893437. |
| [27] |
KOMIYA S, NAITO Y, OKADA H, et al. Characterizing the gut microbiota in females with infertility and preliminary results of a water-soluble dietary fiber intervention study[J]. Journal of Clinical Biochemistry and Nutrition, 2020, 67(1): 105-111. DOI:10.3164/jcbn.20-53 |
| [28] |
CLARKE S F, MURPHY E F, O'SULLIVAN O, et al. Exercise and associated dietary extremes impact on gut microbial diversity[J]. Gut, 2014, 63(12): 1913-1920. DOI:10.1136/gutjnl-2013-306541 |
| [29] |
GALLINO L, HAUK V, FERNÁNDEZ L, et al. VIP promotes recruitment of Tregs to the uterine-placental interface during the peri-implantation period to sustain a tolerogenic microenvironment[J]. Frontiers in Immunology, 2020, 10: 2907. DOI:10.3389/fimmu.2019.02907 |
| [30] |
TELES A, SCHUMACHER A, KÜHNLE M C, et al. Control of uterine microenvironment by foxp3(+) cells facilitates embryo implantation[J]. Frontiers in Immunology, 2013, 4: 158. |
| [31] |
O'MAHONY C, SCULLY P, O'MAHONY D, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation[J]. PLoS Pathogens, 2008, 4(8): e1000112. DOI:10.1371/journal.ppat.1000112 |
| [32] |
BINDA C, LOPETUSO L R, RIZZATTI G, et al. Actinobacteria: a relevant minority for the maintenance of gut homeostasis[J]. Digestive and Liver Disease, 2018, 50(5): 421-428. DOI:10.1016/j.dld.2018.02.012 |
| [33] |
FULLER M, PRIYADARSHINI M, GIBBONS S M, et al. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis[J]. American Journal of Physiology: Endocrinology and Metabolism, 2015, 309(10): E840-E851. DOI:10.1152/ajpendo.00171.2015 |
| [34] |
ZIETEK M, CELEWICZ Z, SZCZUKO M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy[J]. Nutrients, 2021, 13(4): 1244. DOI:10.3390/nu13041244 |
| [35] |
LOMAN B R, JORDAN K R, HAYNES B, et al. Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice[J]. Scientific Reports, 2019, 9(1): 16490. DOI:10.1038/s41598-019-52893-0 |
| [36] |
SONG H Z, SHEN X C, CHU Q, et al. Vaccinium bracteatum Thunb. fruit extract reduces high-fat diet-induced obesity with modulation of the gut microbiota in obese mice[J]. Journal of Food Biochemistry, 2021, 45(7): e13808. |
| [37] |
LU Y F, JIA Z X, SU S F, et al. Establishment of trimester-specific reference intervals of serum lipids and the associations with pregnancy complications and adverse perinatal outcomes: a population-based prospective study[J]. Annals of Medicine, 2021, 53(1): 1632-1641. DOI:10.1080/07853890.2021.1974082 |
| [38] |
DE BRUN V, LOOR J J, NAYA H, et al. The embryo affects day 14 uterine transcriptome depending on nutritional status in sheep.A.Metabolic adaptation to pregnancy in nourished and undernourished ewes[J]. Theriogenology, 2020, 145: 14-19. |
| [39] |
JAMRO E L, BLOOM M S, BROWNE R W, et al. Preconception serum lipids and lipophilic micronutrient levels are associated with live birth rates after IVF[J]. Reproductive BioMedicine Online, 2019, 39(4): 665-673. DOI:10.1016/j.rbmo.2019.06.004 |
| [40] |
ZHANG X Q, ZHAO D, MA Y D, et al. Impact of disturbed glucose homeostasis regulated by AMPK in endometrium on embryo implantation in diabetes mice[J]. Reproductive Sciences, 2020, 27(9): 1752-1757. DOI:10.1007/s43032-020-00169-8 |
| [41] |
DA BROI M G, GIORGI V S I, WANG F, et al. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications[J]. Journal of Assisted Reproduction and Genetics, 2018, 35(5): 735-751. DOI:10.1007/s10815-018-1143-3 |
| [42] |
GILBERT R O. Symposium review: mechanisms of disruption of fertility by infectious diseases of the reproductive tract[J]. Journal of Dairy Science, 2019, 102(4): 3754-3765. DOI:10.3168/jds.2018-15602 |
| [43] |
YANG Y H, ZHANG Y H, XU Y C, et al. Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice[J]. Food & Function, 2019, 10(9): 5952-5968. |
| [44] |
DI SIMONE N, SANTAMARIA ORTIZ A, SPECCHIA M, et al. Recent insights on the maternal microbiota: impact on pregnancy outcomes[J]. Frontiers in Immunology, 2020, 11: 528202. DOI:10.3389/fimmu.2020.528202 |
| [45] |
SHANG L J, LIU H B, YU H T, et al. Core altered microorganisms in colitis mouse model: a comprehensive time-point and fecal microbiota transplantation analysis[J]. Antibiotics, 2021, 10(6): 643. DOI:10.3390/antibiotics10060643 |
| [46] |
ZHAN Q T, QI X C, WENG R P, et al. Alterations of the human gut microbiota in intrahepatic cholestasis of pregnancy[J]. Frontiers in Cellular and Infection Microbiology, 2021, 11: 635680. DOI:10.3389/fcimb.2021.635680 |
| [47] |
HOU Y R, CAO C, BAO W, et al. The plasma metabolic profiling of chronic acephate exposure in rats via an ultra-performance liquid chromatography-mass spectrometry based metabonomic method[J]. Molecular BioSystems, 2015, 11(2): 506-515. DOI:10.1039/C4MB00523F |
| [48] |
CHI L, TU P C, LIU C W, et al. Chronic arsenic exposure induces oxidative stress and perturbs serum lysolipids and fecal unsaturated fatty acid metabolism[J]. Chemical Research in Toxicology, 2019, 32(6): 1204-1211. DOI:10.1021/acs.chemrestox.9b00039 |
| [49] |
BURTON G J, JAUNIAUX E. Oxidative stress[J]. Best Practice & Research Clinical Obstetrics & Gynaecology, 2011, 25(3): 287-299. |
| [50] |
FERREIRA E M, GIORGI V S I, RODRIGUES J K, et al. Systemic oxidative stress as a possible mechanism underlying the pathogenesis of mild endometriosis-related infertility[J]. Reproductive BioMedicine Online, 2019, 39(5): 785-794. DOI:10.1016/j.rbmo.2019.06.011 |
| [51] |
HAYASHI S, NAKAMURA T, MOTOOKA Y, et al. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice[J]. Redox Biology, 2020, 37: 101726. DOI:10.1016/j.redox.2020.101726 |
| [52] |
AYE I L M H, KEELAN J A. Placental ABC transporters, cellular toxicity and stress in pregnancy[J]. Chemico-Biological Interactions, 2013, 203(2): 456-466. DOI:10.1016/j.cbi.2013.03.007 |
| [53] |
BLOISE E, ORTIGA-CARVALHO T M, REIS F M, et al. ATP-binding cassette transporters in reproduction: a new frontier[J]. Human Reproduction Update, 2016, 22(2): 164-181. |
| [54] |
GAO L M, ZHOU T T, CHEN Z P, et al. Maternal yeast-based nucleotide supplementation decreased stillbirth by regulating nutrient metabolism[J]. Journal of the Science of Food and Agriculture, 2021, 101(10): 4018-4032. DOI:10.1002/jsfa.11037 |
| [55] |
CARTA M C, MATTANA A, CAMICI M, et al. Catabolism of exogenous deoxyinosine in cultured epithelial amniotic cells[J]. Biochimica et Biophysica Acta, 2001, 1528(2/3): 74-80. |
| [56] |
CHEN M X, ZHANG B, CAI S, et al. Metabolic disorder of amino acids, fatty acids and purines reflects the decreases in oocyte quality and potential in sows[J]. Journal of Proteomics, 2019, 200: 134-143. DOI:10.1016/j.jprot.2019.03.015 |
| [57] |
JUNG Y J, JUNG J I, CHO H J, et al. Berteroin present in cruciferous vegetables exerts potent anti-inflammatory properties in murine macrophages and mouse skin[J]. International Journal of Molecular Sciences, 2014, 15(11): 20686-20705. DOI:10.3390/ijms151120686 |
| [58] |
CHEN C S, SHI L, LI Y P, et al. Disease-specific dynamic biomarkers selected by integrating inflammatory mediators with clinical informatics in ARDS patients with severe pneumonia[J]. Cell Biology and Toxicology, 2016, 32(3): 169-184. DOI:10.1007/s10565-016-9322-4 |
| [59] |
LIN Y H, CHEN Y H, CHANG H Y, et al. Chronic niche inflammation in endometriosis-associated infertility: current understanding and future therapeutic strategies[J]. International Journal of Molecular Sciences, 2018, 19(8): 2385. DOI:10.3390/ijms19082385 |
| [60] |
NASRELDIN N, ALI F A Z, ABD-ELHAFEEZ H H, et al. Characterization of immunological, biochemical and inflammatory response of clinical and subclinical endometritis in ewes in the subtropics[J]. Animal Reproduction Science, 2020, 219: 106541. DOI:10.1016/j.anireprosci.2020.106541 |
| [61] |
LEE J Y, JIN H K, BAE J S. Sphingolipids in neuroinflammation: a potential target for diagnosis and therapy[J]. BMB Reports, 2020, 53(1): 28-34. DOI:10.5483/BMBRep.2020.53.1.278 |
| [62] |
WANG X M, MA Z Y, SONG N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis[J]. European Review for Medical and Pharmacological Sciences, 2018, 22(9): 2513-2518. |
| [63] |
SUN M Z, WANG Q Y, ZHANG M M, et al. Leuconostoc pseudomesenteroides improves microbiota dysbiosis and liver metabolism imbalance and ameliorates the correlation between dihydroceramide and strains of Firmicutes and Proteobacteria in high fat diet obese mice[J]. Food & Function, 2020, 11(8): 6855-6865. |
| [64] |
PETERSEN C, BELL R, KLAG K A, et al. T cell-mediated regulation of the microbiota protects against obesity[J]. Science, 2019, 365(6451): eaat9351. DOI:10.1126/science.aat9351 |
| [65] |
SONG J, ZHANG J J, SU Y, et al. Monascus vinegar-mediated alternation of gut microbiota and its correlation with lipid metabolism and inflammation in hyperlipidemic rats[J]. Journal of Functional Foods, 2020, 74: 104152. DOI:10.1016/j.jff.2020.104152 |
| [66] |
YI Z W, LIU X F, LIANG L H, et al. Antrodin a from Antrodia camphorata modulates the gut microbiome and liver metabolome in mice exposed to acute alcohol intake[J]. Food & Function, 2021, 12(7): 2925-2937. |




