中枢神经系统(CNS)能够感应机体的营养状况,并做出适当的食欲调节行为和代谢反应,以维持机体的能量稳态。能量自我平衡机制的损坏将会导致食欲和代谢异常,从而引发厌食症或肥胖症等,损害机体健康。食欲的形成机制非常复杂,其中,下丘脑是各种食欲调节信号的主要整合中枢。下丘脑促食欲神经元神经肽/豚鼠相关蛋白(NPY/AgRP)及抑食欲神经元前阿片黑皮质素原和可卡因/苯丙胺调节转录肽(POMC/CART)是中枢食欲调节的关键[1]。迄今为止,在下丘脑已发现2种具有能量感应功能的蛋白激酶。一种是腺苷酸活化蛋白激酶(AMPK),在低能量状态下整合营养和激素信号,提高食欲以维持能量的稳态[2, 3, 4];另一种是哺乳动物雷帕霉素靶蛋白(mTOR),它可以在高能量状态,尤其是ATP水平升高时被激活[5]。AMPK的激活可以抑制mTOR信号通路。AMPK和mTOR信号通路为目前熟知并互补的中枢能量感应调控机制。而近年来最新研究表明,脂类营养物质可以直接被特化的能量敏感神经元感应,充当能量的传感器,这些信号整合于下丘脑神经元回路,以维持机体能量稳态,进而影响食欲[6]。本文综述了下丘脑对脂类的营养感应及其参与食欲调控的机制。
下丘脑神经元细胞的能量来源主要是葡萄糖[7],但是神经细胞中含有的大量长链脂肪酸(LCFA)与其能量稳态及食欲调节密切相关。越来越多的证据表明,下丘脑不同区域的LCFA可以作为能量的传感器[8],脂肪酸及其代谢产物分别以非代谢依赖性和代谢依赖性营养感应2种方式在调节能量动态平衡中扮演着非常重要的角色。
下丘脑神经元的非代谢依赖性脂肪酸感应是指LCFA直接作用于神经元发挥效应。大量研究表明,LCFA可以作为CNS的信号分子,不同类型脂肪酸的作用效应和机制不尽相同。中枢注射油酸(OA)可能通过直接或间接作用于ATP敏感钾(KATP)通道,提高POMC神经元的活性,下调AgRP和NPY的表达,进而降低食欲并减少肝脏糖原的合成,而辛酸则无此效应,由此表明脂肪酸对食欲的调节作用依赖于脂肪链的长度[9, 10]。也有研究显示,给大鼠中枢注射OA和二十二碳六烯酸(DHA)等不饱和脂肪酸可以显著降低食欲和体重。而黑皮质素4受体(MC4R)拮抗剂SHU9119几乎完全消除了OA的厌食效应。由此可见,OA和DHA可以通过POMC/MC4R信号途径降低食欲[11]。有意思的是这个效应目前尚存在争议,因为脑中脂肪酸可以作为饱感信号抑制采食,而禁食后血浆中的脂肪酸的浓度不但没有降低反而升高[12]。而用棕榈酸盐处理下丘脑细胞系mHypoE-44可以显著提高神经元中NPY mRNA的表达水平,进而引起增加能量消耗的正反馈效应[13]。但也有研究表明,中枢注射棕榈酸对食欲无任何影响[11]。最新的研究发现,下丘脑的炎症发生是肥胖进程中一个重要的决定因素。大鼠中枢注射ω-3和ω-9脂肪酸可以降低食欲和体重,直接逆转食物诱导的下丘脑炎症反应并缓解机体肥胖症状[14]。由此看来,除了药理学和基因手段,营养素同样可以作为治疗肥胖的潜在手段。
下丘脑神经元的非代谢依赖性脂肪酸感应可能与某些离子通道的活性或脂肪酸膜受体有关。大量研究证明,脂肪酸可以调节许多离子通道,包括ClC-2型氯通道[15]和钙离子(Ca2+)通道[16]等。还有研究表明,KATP通道在下丘脑脂类感应中也发挥了重要的作用[17],并且抑制KATP通道可以消除中枢OA对肝脏代谢的影响[10]。此外,脂肪酸还可通过特异性膜受体调节胞内信号通路活性。比如,中枢注射ω-3和ω-9脂肪酸可以激活下丘脑的G蛋白偶联受体120(GPR120)下游信号通路,进而发挥其在下丘脑的抗炎症效应[14]。还有研究表明,抑制下丘脑神经元中的脂肪酸转位酶(FAT/CD36)可以降低OA效应约45%,该过程与细胞内脂肪酸的代谢无关[18]。因此,FAT/CD36在下丘脑腹内侧核(VMN)中的脂肪酸敏感神经元中可能是作为受体起作用,而非转运载体,其作用可能类似于口腔中的脂类感受器[19]。脂肪酸依赖FAT/CD36可能与LCFA激活舌头味觉细胞的钙库操纵性(SOC)通道相似,通过影响细胞膜电位而发挥作用[20]。但目前有关FAT/CD36作为中枢脂类感受器调节食欲和能量平衡的作用及分子机制以及其在味蕾和下丘脑中的功能存在何种联系还有待于进一步研究[21]。
下丘脑神经元的代谢依赖性脂肪酸感应是指神经元对于LCFA的效应依赖于脂肪酸的代谢。与脂肪酸代谢有关的酶,如乙酰辅酶A羧化酶(ACC)、脂肪酸合成酶(FAS)及肉毒碱棕榈酰转移酶1(CPT1)等在下丘脑弓状核(ARC)、VMH及背内侧核(DMH)的神经元均有分布[22]。细胞内的LCFA在脂酰辅酶A合成酶(ACS)的催化下迅速酯化为长链脂肪酸CoA(LCFA-CoAs),进而通过CPT1转运至线粒体进行β-氧化,如图1所示。乙酰-CoA在ACC的催化下合成丙二酰-CoA,AMPK可以抑制ACC催化乙酰-CoA生成丙二酰-CoA。丙二酰-CoA在丙二酸单酰辅酶A脱羧酶(MCD)的催化下重新生成乙酰-CoA。丙二酰-CoA可以抑制CPT1的活性,累积的LCFA-CoA可以激活调节食欲的神经元。此外,丙二酰-CoA除了作为CPT1的抑制剂,还是FAS的底物,决定了脂肪酸的生物合成。
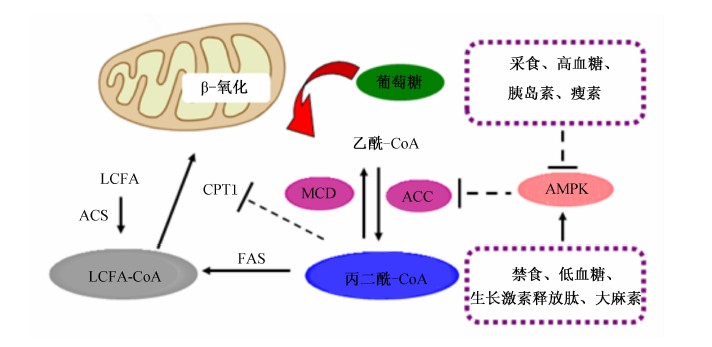 |
图1 下丘脑脂肪酸感应 Fig.1 Hypothalamic sensing of fatty acids |
遗传学和药理学证据都表明改变这些代谢酶的活性均可以调节食欲[23]。对VMN神经元能量平衡的调节机制研究表明,OA可以通过影响CPT1活性、活性氧(ROS)的形成、ACC和KATP通道的活性等多种途径来影响VMN神经元的活动[18]。细胞内的LCFA-CoA是调节能量平衡的重要代谢中间产物。中枢注射脂肪酸合成酶抑制剂C75可以形成C75-CoA,C75-CoA可通过抑制CPT1的活性,增加细胞内LCFA-CoA的聚集,从而降低食欲[24]。 LCFA-CoA可以激活下丘脑细胞膜蛋白激酶C-δ(PKC-δ),而PKC-δ可以激活KATP通道,后者对于下丘脑脂质感应和葡萄糖的合成都是必须的[25]。因此,下丘脑的LCFA-CoA/PKC-δ/KATP通道是一条潜在的下丘脑脂质感应的信号通路。此外,丙二酰-CoA也是下丘脑一个重要的能量传感器,其水平的高低可以直接影响食欲。营养过剩可以导致丙二酰-CoA累积从而降低食欲和葡萄糖的合成[26]。ACC活性直接影响丙二酰-CoA的水平。中枢注射AMPK的激动剂(AICAR)可以降低ACC的活性,减少丙二酰-CoA的积累并提高食欲[27, 28]。而中枢注射脂肪酸合成酶抑制剂C75可以下调AgRP/NPY表达和上调POMC/CART表达,提高下丘脑丙二酰-CoA的水平,起到降低食欲并减少体重的作用[29]。有趣的是,长期节食减肥可以提高丙二酰-CoA的水平,而急性饥饿状态下则情况相反,但在上述2种情况下神经肽的表达却是一致的[30]。研究还表明MCD可以调节下丘脑丙二酰-CoA的水平进而影响食欲。过氧化物酶体增殖剂活化受体α(PPARα)能通过调节MCD的表达来控制丙二酰-CoA的水平[31]。有报道称,女孩缺乏MCD极度厌食[32],而下丘脑过表达MCD的小鼠可以显著提高其食欲和体重,并最终导致肥胖[33]。在骨骼肌中,MCD可以直接被AMPK活化[34],而下丘脑的AMPK是否能直接调控MCD来控制食欲有待于进一步研究来揭示。丙二酰-CoA还可以通过与CPT1-c结合来调节食欲。CPT1-c是CPT1的同型体,在下丘脑VMH中表达并参与调节能量平衡。CPT1-c基因敲除小鼠的食欲和体重大大降低,但是在高脂饲粮条件下体重又会显著上升[35]。因此,CPT1-c可能并非丙二酰-CoA唯一的效应器,是否还存在丙二酰-CoA影响其他酶活性的具体作用途径仍需进一步揭示。所以,下丘脑的AMPK-ACC-MCD-FAS-丙二酰-CoA轴又是一条调节能量动态平衡的脂质感应的信号轴。
脂肪酸乙醇胺(FAEs)是一类广泛存在于动物体内的脂肪酸衍生物,包括N-油酰乙醇胺(OEA)和N-花生四烯酸乙醇胺(AEA)等[36]。这些物质由小肠分泌的一种活性物质N-酰基磷脂酰乙醇胺(NAPEs)在NAPE特异性磷脂酶D(NAPE-PLD)的催化下形成。新近研究表明,FAEs也可作为信号分子参与下丘脑的营养感应进而调节食欲和能量稳态[37, 38, 39]。
放射性示踪结果显示,NAPEs可以穿过血脑屏障并集中在下丘脑,直接作用于中枢神经系统而调节食欲,而且切断大鼠的迷走神经后,NAPEs降低食欲的效应并未受影响。但中枢侧脑室直接注射NAPE降低食欲的具体靶点和信号通路目前尚不明确[40, 41]。
OEA可作为动物体内G蛋白偶联受体(GPCRs)、PPARα、瞬时感受器电位离子通道香草素受体亚家族1(TRPV1)等的配体,与机体食欲调节、体内脂肪的沉积、激素的分泌等生理过程密切相关。OEA在进食后分泌明显增多以减少食物摄入,故被作为饱感信号[37];肠道上皮细胞产生OEA后作用于核受体PPARα,刺激迷走传入感觉神经,增加下丘脑室旁核(PVN)和视上核(抑采食区)的c-fos基因表达量和催产素的释放,通过延长采食间隔时间来降低食欲。脑室注射催产素受体拮抗剂L-368,899则会减弱OEA的厌食效应[39]。而且下丘脑外侧区注射OEA都能够显著降低大鼠1和4 h采食量,同时促采食区的c-fos基因表达量显著降低,而24 h的采食量无显著差异[42]。AEA是一种能够结合并激活大麻素1(CB1)受体的内源性大麻素[43]。大麻素系统在肥胖的发生中发挥了关键的作用。激活CB1受体可以显著提高食欲,药理学抑制CB1可以减轻体重并缓解肥胖相关的代谢紊乱的病症[44, 45]。饱食的大鼠下丘脑VMH区注射AEA 3 h后可以显著提高其采食量,而注射大麻素受体阻断剂SR141716则会减弱AEA诱导的促采食效应[46]。下丘脑PVN区注射AEA可以显著提高食欲和呼吸商,而注射CB1受体反向激动剂AM251则会逆转此效应[47, 48]。以上研究表明,PVN的CB1在调节食欲和能量稳态上发挥了关键作用。下丘脑CB1敲除成年小鼠饲喂标准饲粮可以减轻体重并增加能量消耗,这与褐色组织中肾上腺素能受体和解偶联蛋白的表达上升有关。但值得注意的是小鼠的采食量并无明显变化[45]。还有研究显示CB1受体具有双向调控采食的作用。激活位于皮质谷氨酸能(兴奋性递质)神经元末梢的CB1受体能够提高食欲,而激活位于γ-氨基丁酸(GABA)能神经元上的CB1受体则能够降低食欲[49]。Yoshida等[50]研究还显示甜味感受蛋白3(T1R3)受体和CB1受体在Ⅱ型味觉细胞中共表达,提示味蕾也是大麻素的作用靶点之一。由于大麻素还可以正反馈调节小肠消耗脂肪,所以大麻素如何在下丘脑-口腔-肠道这个调控食欲的功能连续系统中发挥作用需要更进一步的探索。
细胞内的脂类、糖类及碳水化合物的代谢存在相互转化,因此,下丘脑神经细胞在感应脂类营养物质的信号的同时,还与葡萄糖等营养物质感应的信号通路彼此关联。CNS根据葡萄糖和脂肪相对利用的指数,调节机体能量的状态。采用电生理学检测ARC和VMH神经元对葡萄糖和OA的应答效应也表明,中枢葡萄糖和脂类感应之间存在直接的相互作用[51]。OA可以调节ARC中3种葡萄糖依赖型神经元,表明葡萄糖与脂肪酸在调节ARC神经元感应中存在交互作用[52]。下丘脑营养感应发挥效应的同时还受到一系列因素的调节,如ROS、胰岛素、瘦素和生长激素释放肽等[53]。
葡萄糖和脂肪酸的代谢产物均要进入电子呼吸链产生ATP,线粒体呼吸的过程自然会产生ROS。新近研究表明,下丘脑ROS可以影响营养感应,具有调控食欲的作用[54, 55]。研究表明,NPY/AgRP和POMC神经元存在不同的能量底物从而形成了不同的代谢信号。POMC神经元利用葡萄糖作为主要的能量来源[7],而NPY/AgRP则将脂肪酸作为主要的能量底物[54]。糖酵解可以增强POMC神经元活性,抑制NPY/AgRP神经元活性,而β-氧化增强了NPY/AgRP神经元活性,抑制了POMC神经元活性。不论利用葡萄糖还是脂肪酸作为能量来源,其氧化产物都是ROS,而且ROS在下丘脑神经元对脂类及葡萄糖信号感应中发挥了决定性的功能(图2)[55, 56, 57]。负能量平衡状态下,NPY/AgRP神经元被激活并利用LCFA,如生长激素释放肽激活的信号途径使ROS水平上调,而ROS在NPY/AgRP神经元存在前馈缓冲机制,即通过激活解偶联蛋白2(UCP2)引起质子漏并降低AMP,从而抑制线粒体内ROS产生[58]。UCP2可以促进脂肪酸代谢,降低细胞内ROS水平,抑制POMC神经元活性,提高NPY/AgRP的活性。反之正能量平衡状态下,POMC神经元的激活依赖细胞内葡萄糖的代谢以及胰岛素和瘦素激活的信号途径,这些信号均依赖于代谢产生的ROS。ROS大量聚集,且不存在ROS缓冲机制,持续高水平ROS促使POMC神经元产生饱感信号,降低食欲[54]。因为NPY/AgRP神经元存在ROS缓冲机制,所以POMC暴露在ROS环境易受损。长此以往,POMC神经元活性的降低与随着动物和人年龄的增长体重越来越轻是一致的。因此,低能量状态使得NPY/AgRP神经元持续激活而对POMC神经元没有损伤,从而有利于健康长寿。
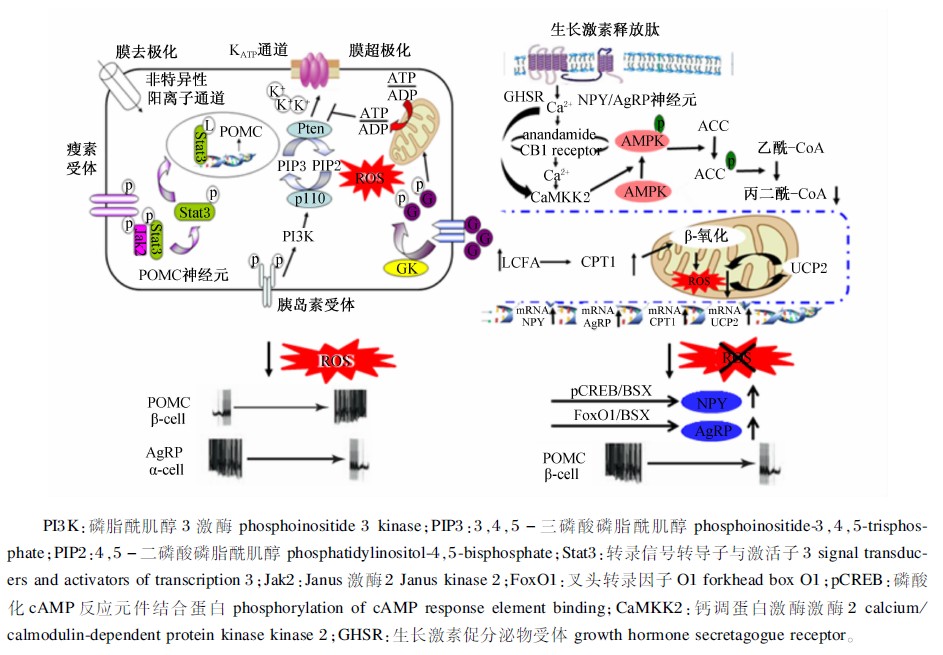 |
图2 ROS对POMC和NPY/AgRP神经元的调节 Fig.2 ROS control in POMC and NPY/AgRP neurons[57] |
胰岛素是调节食欲的饱感信号。研究表明,胰岛素与其相应受体结合可以调节下丘脑VMN中POMC神经元的KATP通道活性,进一步通过PI3K信号途径影响细胞内ROS水平(图2),以调节能量状态和食欲[59, 60]。中枢注射胰岛素可抑制下丘脑AMPK活性,进而降低食欲[61]。胰岛素在中枢不仅可以调节食欲[62],还可以调控神经元内的营养物质的代谢[63, 64, 65](图3)。中枢注射棕榈酸可能通过局部的炎症反应损害胰岛素信号途径,提高食欲并增加体重[67]。中枢注射或者口腔填饲棕榈酸均可以提高下丘脑细胞膜蛋白激酶C-θ(PKC-θ)的表达水平,这也与下丘脑胰岛素信号途径的损害有关。而ARN特异性敲除PKC-θ基因可以减弱食物诱导的肥胖,并可以改良胰岛素信号途径[68]。这些结果均表明富含棕榈酸的高脂肪食物,可以使CNS通过PKC-θ介导减弱胰岛素敏感性,对于治疗肥胖症具有重要的应用价值。
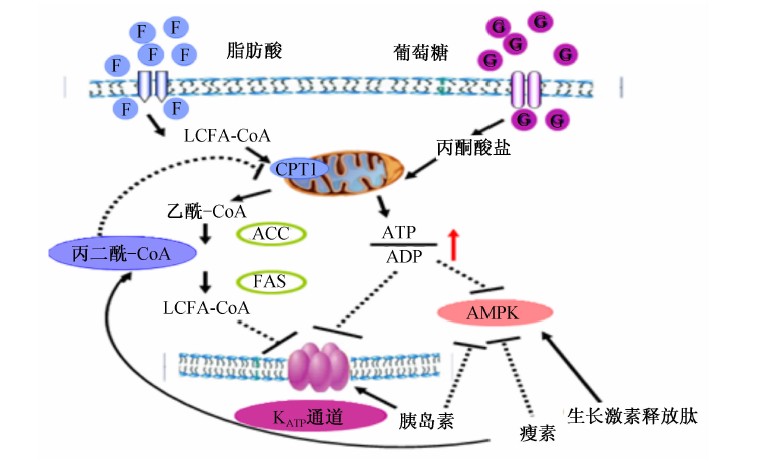 |
图3 葡萄糖和脂肪感应激素调节的交叉 Fig.3 Crosstalk and hormonal regulation of glucose and lipid sensing[66] |
瘦素是调节能量稳态的另一重要因子。在相对饱感时,瘦素打开非特异性阳离子通道使POMC神经元去极化,激活Jak2并使Stat3磷酸化(图2),进而增加POMC的表达来降低食欲[68]。此外,瘦素还可以通过Janus激酶2-腺苷酸活化蛋白激酶-电压门控钙电流(Jak2-MAPK-VGCC)通路抑制下丘脑大麻素的释放,削弱大麻素的促食欲效应[69]。瘦素可以提高下丘脑ARC的丙二酰-CoA水平,进而介导瘦素的抑食欲效应,而且这种效应并不依赖于CPT1-a活性的改变[70]。而下丘脑CPT1-c过表达却可以阻断瘦素的抑食欲效应,而且瘦素是通过改变丙二酰-CoA水平、CPT1-c的活性和神经酰胺的从头合成的信号通路来影响神经酰胺的代谢进而起到降低食欲的效果[71]。因此,调控CPT1-c和神经酰胺的代谢同样可以成为治疗肥胖等疾病的有效手段。
酰化生长激素释放肽具有促进生长激素释放、促进胰岛素分泌和调节糖脂代谢及能量稳态的作用[72, 73](图3)。中枢和外周注射生长激素释放肽均可以显著提高机体的食欲。现有的研究表明,下丘脑AMPK→脂肪酸代谢途径介导了生长激素释放肽的促采食效应[74]。生长激素释放肽与NPY/AgRP神经元上的同族膜GHSR结合,提高胞内钙离子水平并激活NPY神经元的CaMKK2,进而激活下丘脑AMPK,经ACCα→丙二酰-CoA→CPT1→脂肪酸β-氧化→ROS水平上调→UCP2表达上调途径激活AgRP/NPY神经元(图2),提高食欲 。此外,脑室注射生长激素释放肽不能提高CB1缺失小鼠的食欲,同时AMPK也不能被激活。生长激素释放肽还可以提高下丘脑大麻素的水平,阻断CB1后可逆转此效应,因此内源性大麻素→CB1→AMPK信号途径也参与了生长激素释放肽提高食欲的生理机能[76](图2)。非常有趣的是,mTOR的抑食欲作用一直以来被人们所熟知。而最新研究表明,大鼠中枢注射生长激素释放肽也能激活ARC中的mTOR,并使pCREB和FoxO1水平提高,进而提高了AgRP和NPY水平,促进了食欲[77]。因此,mTOR对食欲的调控作用也是多元化的,其分子机制同样也需要深入研究来阐明。
下丘脑的营养感应与激素信号的整合主要通过KATP通道和AMPK介导。当葡萄糖和脂类氧化程度增加时,胞内ATP的水平有所提高,从而关闭了KATP通道,影响了胞内LCFA-CoA的积聚[78],进而提高抑采食神经元的兴奋性。中枢胰岛素处理对食欲和肝脏糖异生作用的调节通过激活PI3K产生PIP3,进而调节KATP通道活性[59, 60]。因此KATP通道的激活代表下丘脑葡萄糖和脂类感应激素和营养信号整合的一种分子机制。当胞内ATP的水平降低时则可以激活AMPK,使ACC磷酸化减少,丙二酰-CoA的生成增加,促进了β-氧化。瘦素还可以抑制下丘脑AMPK活性[61],AMPK突变可以削弱瘦素的食欲调节作用,表明AMPK是瘦素影响食欲和脂类代谢最重要的介导者[79, 80, 81](图3)。中枢生长激素释放肽处理可以通过激活钙离子信号增强AMPK的活性[82]。所以下丘脑AMPK活性的调节可以作为激素和营养信号控制神经元兴奋性的更进一步的整合位点。总之,下丘脑营养感应和激素信号整合成多种信号共同维持机体能量稳态。
下丘脑的脂类营养感应与其他信号共同调节食欲并维持机体的能量稳态。越来越多的证据表明能量感应机制在中枢调节食欲和能量稳态中发挥了关键作用。其中,KATP通道、FAT/CD36(中央脂类感受器)、丙二酰-CoA和AMPK均已被证实是下丘脑葡萄糖和脂类感应中的核心能量感受器。但是,调节能量稳态的营养感应机制还有待更深入的研究。比如,FAT/CD36作为中枢脂类感受器的作用机制如何?激活或者抑制神经元最终的反应就是释放神经递质或者神经肽,那么下丘脑神经元对营养信号做出反应时释放何种神经递质或神经肽?脂肪酸感应及其代谢中间产物如何改变不同神经元的兴奋性?对上述问题的深入阐述有助于全面解析中枢食欲调节网络并寻找关键的信号整合位点,从而为治疗代谢相关疾病或者开发应用于实践的食欲调控剂提供新的依据。
| [1] | MORTON G J,CUMMINGS D E,BASKIN D G,et al.Central nervous system control of food intake and body weight[J].Nature,2006,443(7109):289-295.[本文引用:1] |
| [2] | YANG C S,LAM C K,CHARI M,et al.Hypothalamic AMP-activated protein kinase regulates glucose production[J].Diabetes,2010,59(10):2435-2443.[本文引用:1] |
| [3] | LAGE R,DIEGUEZ C,VIDALl-PUIG A,et al.AMPK:a metabolic gauge regulating whole-body energy homeostasis[J].Trends in Molecular Medicine,2008,14(12):539-549.[本文引用:1] |
| [4] | DE MORENTIN P B M,GONZALEZ C R,SAHA A K,et al.Hypothalamic AMP-activated protein kinase as a mediator of whole body energy balance[J].Reviews in Endocrine & Metabolic Disorders,2011,12(3):127-140.[本文引用:1] |
| [5] | WICZER B M,THOMAS G.The role of the mTOR pathway in regulating food intake[J].Current Opinion in Drug Discovery & Development,2010,13(5):604-612.[本文引用:1] |
| [6] | DIGEUEZ C,FRUHBECK G,LOPEZ M,et al.Hypothalamic lipids and the regulation of energy homeostasis[J].Obesity Facts,2009,2(2):126-135.[本文引用:1] |
| [7] | IBRAHIM N,BOSCH M A,SMART J L,et al.Hypothalamic proopiomelanocortin neurons are glucose responsive and express K-ATP channels[J].Endocrinology,2003,144(4):1331-1340.[本文引用:2] |
| [8] | LOPEZ M,LAGE R,SAHA A K,et al.Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin[J].Cell Metabolism,2008,7(5):389-399.[本文引用:1] |
| [9] | MORGAN K,OBICI S,ROSSETTI L.Hypothalamic responses to long-chain fatty acids are nutritionally regulated[J].Journal of Biological Chemistry,2004,279(30):31139-31148.[本文引用:1] |
| [10] | JO Y H,SU Y,GUTIERREZ-JUAREZ R,et al.Oleic acid directly regulates pomc neuron excitability in the hypothalamus[J].Journal of Neurophysiology,2009,101(5):2305-2316.[本文引用:2] |
| [11] | SCHWINKENDORF D R,TSATSOS N G,GOSNELL B A,et al.Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism[J].International Journal of Obesity,2011,35(3):336-344.[本文引用:2] |
| [12] | RUGE T,HODSON L,CHEESEMAN J,et al.Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage[J].Journal of Clinical Endocrinology and Metabolism,2009,94(5):1781-1788.[本文引用:1] |
| [13] | FICK L J,FICK G H,BELSHAM D D.Palmitate alters the rhythmic expression of molecular clock genes and orexigenic neuropeptide Y mRNA levels within immortalized,hypothalamic neurons[J].Biochemical and Biophysical Research Communications,2011,413(3):414-419.[本文引用:1] |
| [14] | CINTRA D E,ROPELLE E R,MORAES J C,et al.Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity[J].PLoS One,2012,7(1):e30571.[本文引用:2] |
| [15] | ZHENG H F,LI X L,JIN Z Y,et al.Effects of unsaturated fatty acids on calcium-activated potassium current in gastric myocytes of guinea pigs[J].World Journal of Gastroenterology,2005,11(5):672-675.[本文引用:1] |
| [16] | HONEN B N,SAINT D A,LAVER D R.Suppression of calcium sparks in rat ventricular myocytes and direct inhibition of sheep cardiac RyR channels by EPA,DHA and oleic acid[J].Journal of Membrane Biology,2003,196(2):95-103.[本文引用:1] |
| [17] | MIGRENNE S,MAGNAN C,CRUCIANI-GUGLIELMACCI C.Fatty acid sensing and nervous control of energy homeostasis[J].Diabetes & Metabolism,2007,33(3):177-182.[本文引用:1] |
| [18] | LE FOLL C,IRANI B G,MAGNAN C,et al.Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing[J].American Journal of Physiology-Regulatory Integrative and Comparative Physiology,2009,297(3):R655-R664.[本文引用:2] |
| [19] | MARTIN C,PASSILY-DEGRACE P,GAAILLARD D,et al.The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds:impact on spontaneous fat preference[J].PLoS One,2011,6(8):e24014.[本文引用:1] |
| [20] | GAILLARD D,LAUGERETTE F,DARCEL N,et al.The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse[J].The FASEB Journal,2008,22(5):1458-1468.[本文引用:1] |
| [21] | CHEVROT M,MARTIN C,PASSILLY-DEGRACE P,et al.Role of CD36 in oral and postoral sensing of lipids[J].Handbook of Experimental Pharmacology,2012,209:295-307.[本文引用:1] |
| [22] | SORENSEN A,TRAVERS M T,VEMON R G,et al.Localization of messenger RNAs encoding enzymes associated with malonyl-CoA metabolism in mouse brain[J].Brain Research Gene Experimental Patterns,2002,1(3/4):167-173.[本文引用:1] |
| [23] | LOPASCHUK G D,USSHER J R,JASWAL J S,et al.Targeting intermediary metabolism in the hypothalamus as a mechanism to regulate appetite[J].Pharmacological Reviews,2010,62(2):237-264.[本文引用:1] |
| [24] | MERA P,BENTEBIBELAL A,LOPEZ-VINAS E,et al.C75 is converted to C75-CoA in the hypothalamus,where it inhibits carnitine palmitoyltransferase 1 and decreases food intake and body weight[J].Biochemical Pharmacology,2009,77(6):1084-1095.[本文引用:1] |
| [25] | CABOU C,VACHOUX C,CAMPISTRON G,et al.Brain GLP-1 signaling regulates femoral artery blood flow and insulin sensitivity through hypothalamic PKC-delta[J].Diabetes,2011,60(9):2245-2256.[本文引用:1] |
| [26] | LANE M D,WOLFGANG M,CHA S H,et al.Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA[J].International Journal of Obesity,2008,32:S49-S54.[本文引用:1] |
| [27] | KIM E K,MILLER I,AJA S,et al.C75,a fatty acid synthase inhibitor,reduces food intake via hypothalamic AMP-activated protein kinase[J].Journal of Biological Chemistry,2004,279(19):19970-19976.[本文引用:1] |
| [28] | HU Z Y,DAI Y,PRENTKI M,et al.A role for hypothalamic malonyl-CoA in the control of food intake[J].Journal of Biological Chemistry,2005,280(48):39681-39683.[本文引用:1] |
| [29] | PROULX K,COTA D,WOODS S C,et al.Fatty acid synthase inhibitors modulate energy balance via mammalian target of rapamycin complex 1 signaling in the central nervous system[J].Diabetes,2008,57(12):3231-3238.[本文引用:1] |
| [30] | SUCAJTYS-SZULC E,TURYN J,GOYKE E,et al.Differential effect of prolonged food restriction and fasting on hypothalamic malonyl-CoA concentration and expression of orexigenic and anorexigenic neuropeptides genes in rats[J].Neuropeptides,2010,44(1):17-23.[本文引用:1] |
| [31] | LEE G Y,KIM N H,ZHAO Z S,et al.Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene:a key regulation of malonyl-CoA level[J].Journal of Biological Chemistry,2004,378:983-990.[本文引用:1] |
| [32] | DE WIT M C,DE COO I F,VERBEEK E,et al.Brain abnormalities in a case of malonyl-CoA decarboxylase deficiency[J].Molecular Genetics and Metabolism,2006,87(2):102-106.[本文引用:1] |
| [33] | HE W,LAM T K,OBICI S,et al.Molecular disruption of hypothalamic nutrient sensing induces obesity[J].Nature Neuroscience,2006,9(2):227-233.[本文引用:1] |
| [34] | SAHA A K,SCHWARSIN A J,RODUIT R,et al.Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside[J].Journal of Biological Chemistry,2000,275(32):24279-24283.[本文引用:1] |
| [35] | WOLFGANG M J,LANE M D.Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity[J].The FEBS Journal,2011,278(4):552-558.[本文引用:1] |
| [36] | KINGSLEY P J,MARNETT L J.Analysis of endocannabinoids,their congeners and COX-2 metabolites[J].Journal of Chromatography B,2009,877(26):2746-2754.[本文引用:1] |
| [37] | CAPASSO R,IZZO A.Gastrointestinal regulation of food intake:general aspects and focus on anandamide and oleoylethanolamide[J].Journal of Neuroendocrinology,2008,20:39-46.[本文引用:2] |
| [38] | DIPASQUALE P,ROMANO A,CIANCI S,et al.Oleoylethanolamide:a new player in energy metabolism control.Role in food intake[J].Drug Discovery Today:Disease Mechanisms,2010,7(3/4):169-174.[本文引用:1] |
| [39] | GAETANI S,FU J,CASSANO T,et al.The fat-induced satiety factor oleoylethanolamide suppresses feeding through central release of oxytocin[J].Journal of Neuroendocrinology,2010,30(24):8096-8101.[本文引用:2] |
| [40] | GILLUM M P,ZHANG D,ZHANG X M,et al.N-acylphosphatidylethanolamine,a gut-derived circulating factor induced by fat ingestion,inhibits food intake[J].Cell,2008,135(5):813-824.[本文引用:1] |
| [41] | QUARTA C,MAZZA R,OBICI S,et al.Energy balance regulation by endocannabinoids at central and peripheral levels[J].Trends in Molecular Medicine,2011,17(9):518-526.[本文引用:1] |
| [42] | SORIA-GOMEZ E,GUZMAN K,PECH-RUEDA O,et al.Oleoylethanolamide affects food intake and sleep-waking cycle through a hypothalamic modulation[J].Pharmacological Research,2010,61(5):379-384.[本文引用:1] |
| [43] | DE FONSECA F R,NAVARRO M,GOMEZ R,et al.An anorexic lipid mediator regulated by feeding[J].Nature,2001,414:209-211.[本文引用:1] |
| [44] | QUARTA C,BELLOCCHIO L,MANCINI G,et al.CB1 signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance[J].Cell Metabolism,2010,11(4):273-285.[本文引用:1] |
| [45] | CARDINAL P,BELLOCCHIO L,CLARK S,et al.Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice[J].Endocrinology,2012,153(9):4136-4143.[本文引用:2] |
| [46] | JAMSHIDI N,TAYLOR D A.Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats[J].British Journal of Pharmacology,2001,134(6):1151-1154.[本文引用:1] |
| [47] | DIPATRIZIO N V,PIOMELLI D.The thrifty lipids:endocannabinoids and the neural control of energy conservation[J].Trends in Neurosciences,2012,35(7):403-411.[本文引用:1] |
| [48] | CHAPMAN C D,DONO L M,FRENCH M C,et al.Paraventricular nucleus anandamide signaling alters eating and substrate oxidation[J].Neuroreport,2012,23:425-429.[本文引用:1] |
| [49] | BELLOCCHIO L,LAFENETRE P,CANNICH A,et al.Bimodal control of stimulated food intake by the endocannabinoid system[J].Nature Neuroscience,2010,13(3):281-283.[本文引用:1] |
| [50] | YOSHIDA R,OHKURI T,JYOTAKI M,et al.Endocannabinoids selectively enhance sweet taste[J].Proceedings of the National Academy of Sciences of the United States of America,2010,107(2):935-939.[本文引用:1] |
| [51] | LE FOLL C,IRANI B G,MAGNAN C,et al.Effects of maternal genotype and diet on offspring glucose and fatty acid-sensing ventromedial hypothalamic nucleus neurons[J].American Journal of Physiology:Regulatory Integrative and Comparative Physiology,2009,297(5):R1351-R1357.[本文引用:1] |
| [52] | WANG R,CRUCIANI-GUGLIELMACCI C,MIGRENNE S,et al.Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels[J].Journal of Neurophysiology,2006,95(3):1491-1498.[本文引用:1] |
| [53] | DIEGUEA C,VAZQUEZ M J,ROMERO A,et al.Hypothalamic control of lipid metabolism:focus on leptin,ghrelin and melanocortins[J].Neuroendocrinology,2011,94(1):1-11.[本文引用:1] |
| [54] | ANDREWS Z B,LIU Z W,WALLLINGFORD N,et al.UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals[J].Nature,2008,454(7206):846-851.[本文引用:3] |
| [55] | LELOUP C,CASTEILLA L,CARRIERE A,et al.Balancing mitochondrial redox signaling:a key point in metabolic regulation[J].Antioxidants & Redox Signaling,2011,14(3):519-530.[本文引用:2] |
| [56] | LELOUP C,MAGNAN C,BENANI A,et al.Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing[J].Diabetes,2006,55(7):2084-2090.[本文引用:1] |
| [57] | DIANO S,HORVATH T L.Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism[J].Trends in Molecular Medicine,2012,18(1):52-58.[本文引用:2] |
| [58] | MAILLOUX R J,HARPER M E.Uncoupling proteins and the control of mitochondrial reactive oxygen species production[J].Free Radical Biology and Medicine,2011,51(6):1106-1115.[本文引用:1] |
| [59] | COTERO V E,ROUTH V H.Insulin blunts the response of glucose-excited neurons in the ventrolateral ventromedial hypothalamic nucleus to decreased glucose[J].American Journal of Physiology Endocrinology and Metabolism,2009,29(5):1101-1109.[本文引用:2] |
| [60] | HORVATH T L,ANDREWS Z B,DIANO S.Fuel utilization by hypothalamic neurons:roles for ROS[J].Trends in Endocrinology and Metabolism,2009,20(2):78-87.[本文引用:2] |
| [61] | MINOKOSHI Y,ALQUIER T,FURUKAWA N,et al.AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus[J].Nature,2004,428(6982):569-574.[本文引用:2] |
| [62] | SCHWARTZ M W,PPRTE D.Diabetes,obesity,and the brain[J].Science,2005,307(5708):375-379.[本文引用:1] |
| [63] | KOCH L,WUNDERLICH F T,SEIBLER J,et al.Central insulin action regulates peripheral glucose and fat metabolism in mice[J].Journal of Clinical Investigation,2008,118(6):2132-2147.[本文引用:1] |
| [64] | KONNER A C,JANOSCHEK R,PLUM L,et al.Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production[J].Cell Metabolism,2007,5(6):438-449.[本文引用:1] |
| [65] | YUE J T,LAM T K.Lipid sensing and insulin resistance in the brain[J].Cell Metabolism,2012,15(5):646-655.[本文引用:1] |
| [66] | JORDAN S D,KONNER A C,BRUNING J C.Sensing the fuels:glucose and lipid signaling in the CNS controlling energy homeostasis[J].Cellular and Molecular Life Sciences,2010,67(19):3255-3273.[本文引用:1] |
| [67] | ENOIT S C,KEMP[1] MORTON G J,CUMMINGS D E,BASKIN D G,et al.Central nervous system control of food intake and body weight[J].Nature,2006,443(7109):289-295.[本文引用:1] |
| [68] | DUAN J,CHOI Y H,HARTZELL D,et al.Effects of subcutaneous leptin injections on hypothalamic gene profiles in lean and ob/ob mice[J].Obesity,2007,15(11):2624-2633.[本文引用:2] |
| [69] | JO Y H,CHEN Y J,CHUA S C,Jr,et al.Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit[J].Neuron,2005,48(6):1055-1066.[本文引用:1] |
| [70] | GAO S,KEUNG W,SERRA D,et al.Malonyl-CoA mediates leptin hypothalamic control of feeding independent of inhibition of CPT-1a[J].American Journal of Physiology:Regulatory Integrative and Comparative Physiology,2011,301(1):R209-R217.[本文引用:1] |
| [71] | GAO S,ZHU G,GAO X,et al.Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding[J].Proceedings of the National Academy of Sciences of the United States of America,2011,108(23):9691-9696.[本文引用:1] |
| [72] | GASCO V,BECCUTI G,MAROTTA F,et al.Endocrine and metabolic actions of ghrelin[M]//LOCHE S,CAPPA M,GHIZZONI L,et al.Pediatric neuroendocrinology.Cagliari:[n.s.],2010:86-95.[本文引用:1] |
| [73] | KOJIMA M,KANGAWA K.Ghrelin:from gene to physiological function[M]//REHFELD J F,BUNDGAARD H R.Cellular peptide hormone synthesis and secretory pathways.New York:Springer-Verlag Berlin Heidelberg,2010:185-205.[本文引用:1] |
| [74] | VARELA L,VAZQUEZ M J,CORDIDO F,et al.Ghrelin and lipid metabolism:key partners in energy balance[J].Journal of Molecular Endocrinology,2011,46(2):43-63.[本文引用:1] |
| [75] | KOLA B,KORBONITS M.Shedding light on the intricate puzzle of ghrelin’s effects on appetite regulation[J].Journal of Endocrinology,2009,202(2):191-198.[本文引用:0] |
| [76] | KOLA B,FARKAS I,CHRIST-CRAIN M,et al.The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system[J].PLoS One,2008,3(3):e1797.[本文引用:1] |
| [77] | YANG S B,TIEN A C,BODDUPALLI G,et al.Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons[J].Neuron,2012,75:425-436.[本文引用:1] |
| [78] | POCAI A,LAM TONY K T,GUTIERREZ-JUAREZ R,et al.Hypothalamic KATP channels control hepatic glucose production[J].Nature,2005,434:1026-1031.[JP+1][本文引用:1] |
| [79] | ANDERSSON U,FILIPSSON K,ABBOTT C R,et al.AMP-activated protein kinase plays a role in the control of food intake[J].Journal of Biological Chemistry,2004,279(13):12005-12008.[本文引用:1] |
| [80] | MINOKOSHI Y,KIM Y B,PERONI O D,et al.Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase[J].Nature,2002,415:339-343.[本文引用:1] |
| [81] | MOUNTJOY P D,BAILEY S J,RUTTER G A.Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity[J].Diabetologia,2007,50(1):168-177.[本文引用:1] |
| [82] | KOHNO D,SONE H,MINOKOSHI Y,et al.Ghrelin raises Ca2+ (i) via AMPK in hypothalamic arcuate nucleus NPY neurons[J].Biochemical and Biophysical Research Communications,2008,366(2):388-392.[本文引用:1] |




