2. 中国水产科学院东海水产研究所, 农业部东海与远洋渔业资源开发利用重点实验室, 上海 200090
2. Key Laboratory of East China Sea and Oceanic Fishery Resources Exploitation, Ministry of Agriculture of China, East China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai 200090, China
肠道是动物重要的消化吸收器官和最大的免疫器官,不仅负责营养物质的消化、吸收,而且可产生大量的抗体、抗菌肽及白细胞介素(IL)等活性成分,维持机体健康。据统计,猪肠道中生存着约400种各类微生物,在正常生理状况下,这些微生物的组成和种类保持相对平衡,形成稳定的微生态系统[1];平衡稳定的微生态系统能够促进肠道组织发育成熟,激活肠道免疫应答反应,抑制病原的生长繁殖,参与肠道细胞的能量、氨基酸代谢[2, 3, 4, 5, 6]。因此,有科学家将肠道微生物称为机体的一个“器官”[7]。如果肠道微生态系统失衡,有害微生物大量繁殖,就会引起机体炎症,甚至导致肥胖、糖尿病或是肿瘤等疾病[8]。近年来肠道微生态已成为国内外动物营养学界研究的热点。肠道微生物通过何种菌体成分与相关受体结合,黏附、定植在肠道黏膜细胞上?有益菌通过何种机制发挥其免疫调节作用?这2个问题更是受到广大学者关注。
磷壁酸是大多数革兰氏阳性菌细胞壁的重要成分,是一类由多聚磷酸甘油酯或多聚磷酸核糖醇构成的阴离子聚合物,因其链上羟基往往被丙氨酸(Ala)或N-乙酰葡萄糖胺(N-acetylglucosamine,GlcNAc)修饰,故通常还带有一定量的正电荷,形成正负电荷交替出现的链状聚合物结构[9, 10]。按照在菌体上的分布,磷壁酸可分为2类,一类是壁磷壁酸(wall teichoic acid,WTA),锚定到细胞壁肽聚糖上;另一类是脂磷壁酸(lipoteichoic acid,LTA),锚定到细菌细胞膜上[10, 11],见图1-A。磷壁酸对革兰氏阳性菌具有十分重要的生理功能(图1-B),主要包括:1)保护细菌免受抗生素、表面活性剂、抗菌肽、溶菌酶和热应激等的损伤;2)维持细菌细胞壁中阳离子的浓度平衡;3)参与细菌细胞分裂;4)参与细菌自溶过程;5)参与宿主细胞相关受体的识别与互作[11, 12, 13]。对宿主细胞而言,磷壁酸则是重要的细菌抗原分子,能够影响其免疫应答反应[14]。越来越多的研究表明, 磷壁酸在革兰氏阳性菌与宿主细胞的互作及免疫调节过程中发挥着十分重要的作用[11, 12, 13, 14]。
磷壁酸在革兰氏阳性菌的黏附、定植过程中发挥着重要作用。tagO是细菌体内合成磷壁酸关键酶的编码基因,Weidenmaier等[15]构建了金黄色葡萄球菌(Staphylococcus aureus)磷壁酸合成缺陷型的tagO基因突变体,利用大鼠鼻腔黏膜上皮细胞进行的黏附试验表明,该突变体的黏附率降低了90%以上。利用荧光标记的磷壁酸对HNEC和A549细胞进行黏附试验,可观察到磷壁酸直接黏附到细胞膜上[15]。Holland等[16]对表皮葡萄球菌(Staphylococcus epidermidis)的研究证明,与野生型相比,tagO突变体形成生物膜能力和黏附能力均显著降低(P<0.05)。Gao等[17]提取、纯化了丁酸梭菌(Clostridium butyricum)磷壁酸,并通过排斥试验和竞争试验证明,磷壁酸在丁酸梭菌的黏附、定植过程中发挥重要作用。上述研究表明,磷壁酸在细菌的黏附、定植过程中发挥着重要的作用。为了更加深入地研究磷壁酸黏附的分子机理,Abachin等[18]利用基因突变技术构建李斯特杆菌(Listeria monocytogenes)dltA基因突变体(dltA-),dltA-菌磷壁酸不能进行丙氨酸修饰,结果dltA- L. monocytogenes在细胞表面的黏附数量显著下降(P<0.05),这说明磷壁酸上的丙氨酸残基对L. monocytogene在细胞表面的黏附具有重要作用。对罗伊氏乳杆菌(Lactobacillus reuteri)的研究则表明,磷壁酸丙氨酸残基缺失对该菌在小鼠胃肠道中形成生物膜的能力有显著影响(P<0.05)[19]。这些研究表明,丙氨酸残基共价修饰对于磷壁酸在黏附、定植过程中发挥功能具有重要意义。需要指出的是,细菌在宿主细胞膜上的黏附、定植是一个多层次、多阶段、多因子参与的复杂过程,并不仅仅是一种因子作用的结果,还需多种因子的共同参与。对金黄色葡萄球菌黏附、定植机理的研究表明,磷壁酸在细菌与宿主互作的起始识别过程中发挥重要作用,而细菌的某些蛋白质因子则在长期、持续的黏附、定植过程中发挥关键作用[20, 21]。
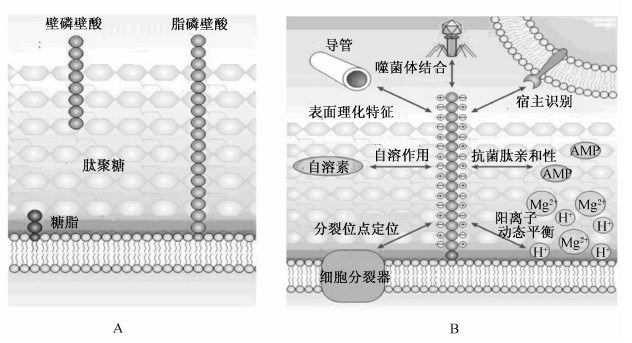 | 图1 革兰氏阳性菌的磷壁酸及其生理功能
Fig.1 The teichoic acid from Gram-positive bacteria and its physiological functions[12] |
革兰氏阳性菌磷壁酸在免疫应答过程中发挥着重要作用。肿瘤坏死因子-α(TNF-α)是机体重要的炎症因子,Morath等[22]的体外研究发现,金黄色葡萄球菌磷壁酸能够刺激肝素化全血炎症因子产生TNF-α,说明磷壁酸可以刺激机体产生炎症。Hermann等[23]的研究证明,金黄色葡萄球菌能够刺激人全血细胞TNF-α、IL-1β、IL-6和IL-10等因子的分泌。Weidenmaier等[24]的研究证明,金黄色葡萄球菌磷壁酸能够导致小鼠出现肺脓肿,并刺激人单核淋巴细胞IL-6、TNF-α等炎症因子的分泌。肺炎链球菌磷壁酸刺激机体细胞产生TNF-α的能力明显比金黄色葡萄球菌磷壁酸弱,这可能是其结构与金黄色葡萄球菌磷壁酸结构有显著不同的缘故[25]。益生菌磷壁酸的免疫调节作用亦受到广泛关注。Kim等[26]发现,植物乳杆菌(Lactobacillus plantarum)磷壁酸能够抑制金黄色葡萄球菌磷壁酸诱导的TNF-α产生。Kaji等[27]的研究表明,干酪乳酸杆菌(Lactobacillus casei)能够刺激巨噬细胞炎症因子IL-12的产生,而植物乳杆菌磷壁酸则能够改变这种作用,刺激细胞产生更多的炎症抑制因子IL-10,促进IL-10/IL-12的平衡。Kang等[28]的研究则证明,植物乳杆菌磷壁酸能够刺激巨噬细胞一氧化氮(NO)的产生,从而发挥抗炎症作用。然而也有研究指出,与致病菌一样,乳酸菌磷壁酸亦能刺激宿主细胞产生炎症反应。Mohamadzadeh等[29]利用基因敲除技术,获得了磷壁酸合成缺陷的嗜酸乳杆菌(Lactobacillus acidophilus)菌株,与野生菌株相比,该突变菌株能够增加树突状细胞(dendritic cells,DC)抗炎症因子IL-10的分泌,减少促炎症因子IL-12的分泌,这提示磷壁酸是刺激细胞炎症的因子。利用基因敲除技术,构建丙氨酸修饰缺陷的植物乳杆菌Dlt-菌株,结果发现,突变菌株能够诱导宿主细胞抗炎症因子IL-10的产生,减少TNF-α等促炎症因子的分泌,这提示植物乳杆菌磷壁酸链上的丙氨酸残基可能会引起细胞发生炎症反应[30, 31]。从上述研究可以看出,磷壁酸的免疫刺激作用,尤其是益生菌磷壁酸的免疫调节功能,尚没有形成一致的研究结论,需要更加全面深入地研究。
革兰氏阳性菌的磷壁酸通过与肠黏膜细胞膜上的蛋白质受体互作,从而黏附、定植并激活宿主免疫应答反应。利用清道夫受体A(scavenger receptor A,SR-A)特异性抑制剂多聚肌苷酸(polyinosinic acid)进行的试验表明,金黄色葡萄球菌磷壁酸可能通过与细胞膜上的SR-A蛋白互作,黏附到细胞膜上[32]。Hermann等[23]的研究指出,金黄色葡萄球菌磷壁酸可以与上皮细胞膜上的Toll样受体-2(TLR-2)、脂多糖结合蛋白(LBP)、脂多糖受体(CD14)等受体蛋白相互作用,激活免疫反应。用CD14基因敲除小鼠进行的研究证明,金黄色葡萄球菌磷壁酸可通过CD14受体激活细胞免疫应答反应[24]。另外的一些研究也发现,磷壁酸通过细胞膜上的TLR-2受体,激活宿主的免疫应答反应 。
从国内外研究进展看,尽管人们对革兰氏阳性菌磷壁酸在黏附、定植及免疫调节过程中的作用有了一定的认识,但是仍存在着以下问题:1)关于磷壁酸在黏附定植作用主要来自对金黄色葡萄球菌的研究,其在肠道益生菌黏附定植中的作用缺乏全面、深入的研究。2)在黏附定植和免疫调节过程中,宿主细胞膜上与磷壁酸直接互作的受体究竟是哪种蛋白质分子?参与2个过程的受体是否为同一蛋白质?黏附定植与免疫调节这2个过程之间有何关系?3)益生菌和致病菌2种不同来源的磷壁酸在免疫刺激过程中的作用有何不同?
| [1] | LESER T D,AMENUVOR J Z,JENSEN T K,et al.Culture-independent analysis of gut bacteria:the pig gastrointestinal tract microbiota revisited[J]. Applied and Environmental Microbiology,2002,68(2):673-690. ( 1) 1)
|
| [2] | LEBEER S,VANDERLEYDEN J,DE KEERSMAECKER S C.Genes and molecules of lactobacilli supporting probiotic action[J]. Microbiology and Molecular Biology Reviews,2008,72(4):728-764. ( 1) 1)
|
| [3] | 高权新,吴天星,王进波.肠道微生物与寄主的共生关系研究进展[J]. 动物营养学报,2010,22(3):519-526. ( 1) 1)
|
| [4] | YAO W,ZHU W Y,HAUKE S,et al.Cultivation-independent analysis of the development of the Lactobacillu spp.community in the intestinal tract of newborn piglets[J]. Agricultural Sciences in China,2011,10(3):438-447. ( 1) 1)
|
| [5] | DAI Z L,WU G Y,ZHU W Y.Amino acid metabolism in intestinal bacteria:links between gut ecology and host health[J]. Frontiers in Bioscience,2011,16:1768-1786. ( 1) 1)
|
| [6] | DAI Z L,ZHANG J,WU G Y,et al.Utilization of amino acids by bacteria from the pig small intestine[J]. Amino Acids,2010,39(5):1201-1215. ( 1) 1)
|
| [7] | ECKBURG P B,BIK E M,BERNSTEIN C N,et al.Diversity of the human intestinal microbial flora[J]. Science,2005,308(5728):1635-1638. ( 1) 1)
|
| [8] | ZHANG C H,ZHANG M H,WANG S Y,et al.Interactions between gut microbiota,host genetics and diet relevant to development of metabolic syndromes in mice[J]. ISME Journal,2010,4(2):232-241. ( 1) 1)
|
| [9] | 汪海峰,章文明,汪以真,等.乳酸杆菌与肠道黏附相关表面因子及其机制的研究进展[J]. 动物营养学报,2011,23(2):179-186. ( 1) 1)
|
| [10] | LOVERING A L,LIN L Y C,SEWELL E W,et al.Structure of the bacterial teichoic acid polymerase TagF provides insights into membrane association and catalysis[J]. Nature Structural & Molecular Biology,2010,17(5):582-589. ( 2) 2)
|
| [11] | XIA G Q,KOHLER T,PESCHEL A.The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus[J]. International Journal of Medical Microbiology,2010, 300(2/3):148-154. ( 3) 3)
|
| [12] | WEIDENMAIER C,PESCHEL A.Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions[J]. Nature Reviews Microbiology,2008,6(4):276-287. ( 3) 3)
|
| [13] | OKU Y,KUROKAWA K,MATSUO M,et al.Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells[J]. Journal of Bacteriology,2009,191(1):141-151. ( 2) 2)
|
| [14] | COT M,RAY A,GILLERON M,et al.Lipoteichoic acid in Streptomyces hygroscopicus:structural model and immunomodulatory activities[J]. PLoS One,2011,6(10):e26316. ( 2) 2)
|
| [15] | WEIDENMAIER C,KOKAI-KUN J F,KRISTIAN S A,et al.Role of teichoic acids in Staphylococcus aureus nasal colonization,a major risk factor in nosocomial infections[J]. Nature Medicine,2004,10(3):243-245. ( 2) 2)
|
| [16] | HOLLAND L M,CONLON B,O'GARA J P.Mutation of tagO reveals an essential role for wall teichoic acids in Staphylococcus epidermidis biofilm development[J]. Microbiology,2011,157(Pt 2):408-418. ( 1) 1)
|
| [17] | GAO Q X,WU T X,WANG J B,et al.Inhibition of bacterial adhesion to HT-29 cells by toteichoic acid extracted from Clostridium butyricum[J]. African Journal of Biotechnology,2011,10(39):7633-7639. ( 1) 1)
|
| [18] | ABACHIN E,POYART C,PELLEGRINI E,et al.Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes[J]. Molecular Microbiology,2002,43(1):1-14. ( 1) 1)
|
| [19] | WALTER J,LOACH D M,ALQUMBER M,et al.D-alanyl ester depletion of teichoic acids in Lactobacillus reuteri 100-23 results in impaired colonization of the mouse gastrointestinal tract[J]. Environmental Microbiology,2007,9(7):1750-1760. ( 1) 1)
|
| [20] | BURIAN M,WOLZ C,GOERKE C.Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans[J]. PLoS One,2010,5(4):e10040. ( 1) 1)
|
| [21] | EDWARDS A M,MASSEY R C,CLARKE S R.Molecular mechanisms of Staphylococcus aureus nasopharyngeal colonization[J]. Molecular Oral Microbiology,2012,27(1):1-10. ( 1) 1)
|
| [22] | MORATH S,GEYER A,HARTUNG T.Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus[J]. The Journal of Experimental Medicine,2001,193(3):393-398. ( 1) 1)
|
| [23] | HERMANN C,SPREITZER I,SCHRÖDER N W J,et al.Cytokine induction by purified lipoteichoic acids from various bacterial species—role of LBP,sCD14,CD14 and failure to induce IL-12 and subsequent IFN-γ release[J]. European Journal of Immunology,2002,32(2):541-551. ( 2) 2)
|
| [24] | WEIDENMAIER C,MCLOUGHLIN R M,LEE J C.The zwitterionic cell wall teichoic acid of Staphylococcus aureus provokes skin abscesses in mice by a novel CD4+ T-cell-dependent mechanism[J]. PLoS One,2010,56(10):e13227. ( 2) 2)
|
| [25] | HAN S H,KIM J H,MARTIN M,et al.Pneumococcal lipoteichoic acid (LTA) is not as potent as Staphylococcal LTA in stimulating Toll-like receptor 2[J]. Infection and Immunity,2003,71(10):5541-5548. ( 1) 1)
|
| [26] | KIM H G,LEE S Y,KIM N R,et al.Inhibitory effects of Lactobacillus plantarum lipoteichoic acid (LTA) on Staphylococcus aureus LTA-induced tumor necrosis factor-alpha production[J]. Journal of Microbiology and Biotechnology,2008,18(6):1191-1196. ( 1) 1)
|
| [27] | KAJI R,KIYOSHIMA-SHIBATA J,NAGAOKA M,et al.Bacterial teichoic acids reverse predominant IL-12 production induced by certain Lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages[J]. The Journal of Immunology,2010,184(7):3505-3513. ( 1) 1)
|
| [28] | KANG S S,RYU Y H,BAIK J E,et al.Lipoteichoic acid from Lactobacillus plantarum induces nitric oxide production in the presence of interferon-γ in murine macrophages[J]. Molecular Immunology,2011,48(15/16):2170-2177. ( 1) 1)
|
| [29] | MOHAMADZADEH M,PFEILER E A,BROWN J B,et al.Regulation of induced colonic infiammation by Lactobacillus acidophilus deficient in lipoteichoic acid[J]. Proceedings of the National Academy of Sciences,2011,108(Suppl.1):4623-4630. ( 1) 1)
|
| [30] | DUNCKER S C,WANG L,HOLS P,et al.The D-alanine content of lipoteichoic acid is crucial for Lactobacillus plantarum-mediated protection from visceral pain perception in a rat colorectal distension model[J]. Neurogastroenterology & Motility,2008,20(7):843-850. ( 1) 1)
|
| [31] | GRANGETTE C,NUTTEN S,PALUMBO E,et al.Enhanced anti-infiammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids[J]. Proceedings of the National Academy of Sciences,2005,102(29):10321-10326. ( 1) 1)
|
| [32] | WEIDENMAIER C,KOKAI-KUN J F,KULAUZOVIC E,et al.Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization[J]. International Journal of Medical Microbiology,2008,298(5/6):505-513. ( 1) 1)
|
| [33] | FISCHER K,STEIN K,ULMER A J,et al.Cytokine-inducing lipoteichoic acids of the allergy-protective bacterium Lactococcus lactis G121 do not activate via Toll-like receptor 2[J]. Glycobiology,2011,21(12):1588-1595. ( 1) 1)
|
| [34] | LEBEER S,CLAES I J J,VANDERLEYDEN J.Anti-infiammatory potential of probiotics:lipoteichoic acid makes a difference[J]. Trends in Microbiology,2012,20(1):5-10. ( 1) 1)
|




