2. 中国农业大学动物科学技术学院, 动物营养学国家重点实验室, 北京 100193
2. State Key Laboratory of Animal Nutrition, College of Animal Science and Technology, China Agricultural University, Beijing 100193, China
在实际生产中为提高家畜生产性能如产奶量、日增重等,饲粮中精料添加水平普遍较高。长期高精料饲养会对家畜采食与营养物质消化产生不利影响,甚至会导致瘤胃酸中毒等代谢疾病,造成严重经济损失[1]。研究表明,酵母益生菌在平衡瘤胃微生物群落,稳定瘤胃液pH及促进瘤胃微生物纤维降解方面均可发挥积极调节作用[2]。产朊假丝酵母(Candida utilis,CU)作为一种假丝酵母属真菌,在农业上与饲用酿酒酵母(Saccharomyces cerevisiae)同为农业部批准使用畜禽酵母益生菌[3]。有研究发现,CU可通过将农副产品中植物蛋白质转化为优质微生物蛋白质(microbial protein,MCP)[4]与降低糠麸饲料中粗脂肪含量[5]等方式改善饲料营养价值,提高畜禽饲料利用率,具有很好的饲用潜力。但与酿酒酵母相比,目前有关CU饲用价值的研究报道十分有限,并主要集中于改善水产动物生长性能[6]及生猪育肥[7]领域,在反刍动物上鲜有应用报道。由于活性酵母益生菌在改善反刍动物胃肠道功能上的作用效果因菌株不同呈现差异[8],探究CU在瘤胃中的益生作用具有较强的实际指导意义。本试验采用体外瘤胃发酵结合动态产气实时记录技术,以我国北方大多数规模化奶牛场常用的全混合日粮(total mixed ration,TMR)为底物,通过分析不同CU添加水平对72 h累计产气量、产气动力学参数和瘤胃发酵特性的影响,评价CU对瘤胃消化代谢的益生功效,以期为今后开展动物饲养试验提供参考依据。
1 材料与方法 1.1 瘤胃液采集选用5头体况相近且装有永久性瘤胃瘘管的平均日产奶量为(18.47±0.77) kg的荷斯坦奶牛作为瘤胃液供体动物。奶牛每日于04:30和16:30共饲喂2次,每次饲喂2.0 kg苜蓿干草、2.0 kg全株玉米青贮以及3.0 kg精料。瘤胃液于晨饲前1 h内采集,经4层纱布过滤后等体积混匀置于39 ℃恒温水浴锅中备用。 1.2 试验设计
整个试验分4个发酵批次完成。每一批次中,选择如表1所示TMR作为发酵底物,粗料来源为玉米青贮与苜蓿干草,该TMR可代表我国北方规模化集约饲养牛场常用的饲粮模式。试验分为6组,各组CU(4×109 CFU/g)添加水平分别为0(对照组)、0.53×106、1.07×106、1.60×106、2.13×106、2.67×106 CFU/mL,每组4个重复。另设空白组(4个重复)用于校正数据。根据上述试验设计,称取500 mg发酵底物与相应质量的CU于配有几丁质胶塞和亨氏旋盖的140 mL厌氧发酵瓶中。使用100 mL移液器向各瓶中加入50 mL pH 6.85的缓冲液[9],预热至39 ℃,然后向各瓶内接种25 mL瘤胃液。自瓶口通入氮气3~5 s以驱除空气后,立即盖上胶塞并旋紧瓶盖,将发酵瓶按照预先排列好的接种顺序,逐一与AGRS-Ⅲ型微生物发酵微量产气自动记录仪[10]相应气路通道连接后,自动进行实时产气量数据记录和存储。所有发酵瓶在39 ℃恒温生化培养箱中连续培养72 h。
1.3 样品收集与处理发酵结束后,各发酵瓶内容物经孔径200目的尼龙袋过滤,测定滤液pH后采集1.0 mL滤液3份冻存于-20 ℃以备分析测试。尼龙袋残渣采用自来水进行漂洗直至无色,将收集到的残渣连同尼龙袋置于65 ℃烘箱中连续烘干72 h后,置于干燥器中室温冷却后称重,利用差减法计算底物的体外营养物质消失率,并用空白组数据对其进行校正。随后将每批次中相同试验组的底物残渣混匀并保存。
1.4 产气动力学模型分析参照Groot等[11]的数学模型运用SAS 9.0软件NLIN过程对各发酵时间(t,h)及对应累积产气量(GPt,mL)进行非线性拟合:
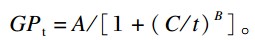
式中:A为理论最大产气量(mL),B为曲线平滑参数,C为达到1/2理论最大产气量的时间(h)。
| 表1 全混合日粮组成与营养水平(干物质基础) Table 1 Composition and nutrient levels of the TMR (DM basis) % |
达到最大产气速率的时间(TRmaxG)和最大产气速率(RmaxG)计算公式分别为:
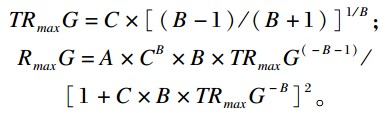
1.5 测定指标
底物干物质与粗蛋白质含量参照AOAC(1999)[12]中的方法测定。参照Van Soest[13]的方法测定底物与残渣中中性洗涤纤维(NDF)与酸性洗涤纤维(ADF)含量,并利用差减法计算底物NDF与ADF体外消失率。发酵液样品经10 000×g 4 ℃离心15 min后,上清液中氨态氮(ammonia nitrogen,NH3-N)浓度测定采用靛酚比色法[14],MCP浓度测定采用Makkar等[15]的方法,总挥发性脂肪酸(total volatile fatty acids,tVFA)浓度各挥发性脂肪酸(volatile fatty acids,VFA)含量采用气相色谱法[16]测定,支链脂肪酸(branch-chained fatty acids,BCFA)含量为异丁酸和异戊酸含量之和。非生糖与生糖脂肪酸之比计算公式[17]为:

1.6 统计分析
使用SAS 9.0软件中GLM过程对数据单因素方差分析,采用Duncan氏法对组间平均值进行多重比较,利用LSMEANS语句输出各测定指标最小二乘法均值的标准误(SEM)。显著水平设定为P<0.050,差异极显著水平为P<0.001。
2 结 果 2.1 营养物质体外消失率、产气量及产气动力学参数由表2可知,与对照组相比,试验组底物干物质、NDF、ADF体外消失率均随CU添加水平的增加而呈显著的线性升高(P<0.050),72 h累积产气量、理论最大产气量、达到1/2理论最大产气量的时间也呈显著的线性升高(P<0.050)。由图1可知,各组各时间点产气量与随CU添加水平的增加而增加。在此底物条件下,CU添加水平为2.67×106 CFU/mL时作用最强。
| 表2 产朊假丝酵母添加水平对体瘤胃外发酵营养物质体外消失率、产气量及产气动力学参数的影响Table 2 Effects of CU supplemental level on disappearance rate of nutrients, gas production and its kinetic parameters after in vitro ruminal fermentation |
由表3可知,发酵液pH随CU添加水平升高而呈极显著的线性降低(P<0.001),而tVFA浓度随CU添加水平升高而极显著增高(P<0.001),NH3-N和MCP浓度与CU添加水平不呈线性关系(P>0.050),而呈显著的二次曲线相关(P<0.050),试验组MCP浓度显著高于对照组(P<0.050),1.07×106和1.60×106 CFU/mL CU添加水平的试验组显著高于其他试验组(P<0.050)。乙酸与丁酸含量均随CU添加水平升高而呈极显著的线性降低(P<0.001),丙酸、戊酸与BCFA含量则呈极显著的线性升高(P<0.001)。此外试验组NGR随CU添加水平的升高而极显著降低(P<0.001),甲烷生成量亦极显著下降(P<0.001)。在此底物条件下,CU添加水平为2.67×106 CFU/mL时作用最强。
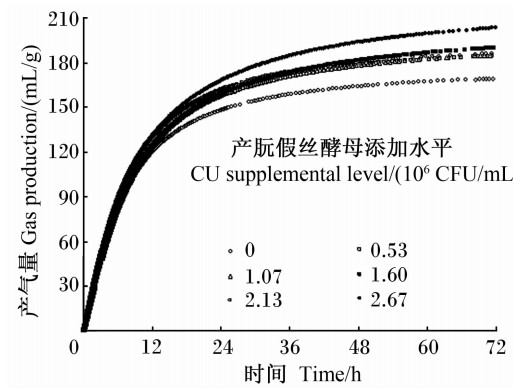 | 图1 产朊假丝酵母添加水平对体外瘤胃发酵产气量动态变化的影响 Fig.1 Effects of CU supplemental level on dynamic changes of gas production after in vitro ruminal fermentation |
已有研究表明,体外试验中底物干物质体外消失率与发酵总产气量呈高度正相关[19],本试验中底物的干物质体外消失率与72 h累积产气量相关系数R=0.924 1(P=0.036),变化趋势符合前人描述。瘤胃微生物具有优先降解饲料中易消化营养物质而非结构性碳水化合物的倾向[20]。本试验中添加CU后底物NDF与ADF体外消失率分别提升了5.7%~18.6%与4.9%~28.9%,表明CU可能提高了瘤胃纤维分解微生物活性。尽管有关CU瘤胃益生作用的报道极少,但大量体内外试验证实其他酵母菌种,如酿酒酵母,可通过消耗瘤胃内氧气[21]及分泌有机酸等促生长因子[22]刺激琥珀酸丝状杆菌等纤维分解细菌生长[23]。Dawson等[24]在体外试验中发现向奶牛饲粮中添加活酵母菌后纤维分解菌数量提高了5~40倍,Bitencourt等[25]则发现向奶牛饲粮日添加1×1010 CFU活性酿酒酵母可使饲粮NDF降解率提高11.3%。但在丁洪涛等[26]的24 h短期体外试验中,相较于本试验(0.53×106~2.67×106 CFU/mL)更高添加水平的CU(3.3×106 ~3.3×108 CFU/mL)并未显著提升发酵底物中NDF与ADF降解率,这可能由于培养时间过短无法准确评价CU对底物纤维降解程度的影响。早期体外研究表明,在评价高精料底物瘤胃降解能力时,依据瘤胃食糜外流速率所推算的体外培养时间应不小于72 h;当底物粗料水平较高时培养时间则应大于96 h[27]。本试验中所测得产气动力学参数则证实各试验组在发酵后期仍能继续保持微生物活性并提高产气量,从而提高饲料底物的最大发酵潜能。
3.2 产朊假丝酵母对体外瘤胃发酵特性的影响瘤胃液pH低于6.4时将会对采食量、瘤胃微生物生长及饲粮营养物质消化吸收产生负面影响,甚至导致瘤胃酸中毒等代谢疾病[28]。有试验证实,活酵母益生菌可稳定瘤胃液pH[29]。在静态发酵中随着瘤胃发酵VFA等有机酸生成和累积,尤其在高精料条件下常会导致发酵液pH急剧下降[30]。但在本试验中饲料底物精料比例虽高达64%,与对照组相比添加CU后发酵液pH仅降低了1.3%~2.5%,这表明CU有助于维持瘤胃液pH稳定,防止酸中毒发生。
NH3-N是饲料含氮物质瘤胃消化代谢中间产物,也是瘤胃微生物合成自身MCP的氮源,其浓度可反映瘤胃中蛋白质降解与合成间的平衡情况。体外发酵消除了瘤胃壁对NH3-N的吸收及随唾液尿素再循环的影响,本试验中虽然干物质体外消失率升高,但NH3-N浓度各组间差异不显著,且MCP浓度提高了4.2%~7.6%,表明添加CU可以提高瘤胃微生物对饲料蛋白质利用效率,进而促进瘤胃微生物的生长。
碳水化合物在瘤胃微生物作用下分解并生成VFA,可为宿主提供所需总能量的70%~80%[31]。研究表明,添加酿酒酵母可增加瘤胃tVFA生成量[32],本试验发现,CU可提高tVFA生成量达8.4%~23.5%。此外,乙酸是乳脂合成的重要前体物,丙酸则是肝糖原合成中的主要前体物,可用来合成乳糖[33]。本试验中添加CU后乙酸含量降低丙酸含量升高,表明CU可正向调控瘤胃发酵丙酸生成量,预示可为宿主肝脏糖异生提供更多能量和前体物。Cummins等[34]证实瘤胃中BCFA含量与MCP合成能力呈显著正相关,因此本试验中BCFA含量的升高符合MCP变化规律。
| 表3 产朊假丝酵母添加水平对体外瘤胃发酵特性的影响
Table 3 Effects of CU supplemental level on in vitro fermentation characteristics
|
瘤胃中VFA生成与温室气体甲烷的生成密切相关,其中乙酸与丁酸含量升高有助于甲烷生成,而丙酸生成过程则会与甲烷竞争性利用瘤胃发酵所生成中的氢,进而减少甲烷生成[18]。Lila等[35]发现,向奶牛饲粮中添加活性酿酒酵母可显著提高瘤胃中产丙酸细菌(如埃氏巨球形菌)数量,从而抑制甲烷生成。单安山等[36]也证实,活酵母菌株可提高奶牛瘤胃微生物活性并降低甲烷生成量。本试验中依据VFA测算出的甲烷生成量随CU添加水平增加也呈现出显著下降,但此估测值今后仍需通过实测进行进一步验证。
4 结 论① 添加CU可提高瘤胃微生物纤维降解与发酵效率。
② 此TMR条件下,CU最适添加水平为2.67×106 CFU/mL。
| [1] | STONE W C.The effect of subclinical acidosis on milk components[M]//Cornell nutrition conference for feed manufacturers.Ithaca:Cornell University,1999:40-46. ( 1) 1)
|
| [2] | BACH A,IGLESIAS C,DEVANT M.Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation[J]. Animal Feed Science and Technology,2007,136(1/2):156-163. ( 1) 1)
|
| [3] | 中华人民共和国农业部.中华人民共和国农业部公告第1126号[EB/OL]. (2008-12-11)[2008-12-25].http://www.moa.gov.cn/zwllm/zcfg/nybgz/200812/t20081225_1196468.htm. ( 1) 1)
|
| [4] | KALRA M S,SINGH A.Recycling of sugarcane byproducts and agro-industrial waste for food and feed[M]. New Delhi:Oxford & IBH Publishing Co. Pvt. Ltd.,1995:229-243. ( 1) 1)
|
| [5] | ANDO S,NISHIGUCHI V,HAYASAKA K,et al.Effects of Candida utilis treatment on the nutrient value of rice bran and the effect of Candida utilis on the degradation of forages in vitro[J]. Asian-Australian Journal of Animal Science,2006,19(6):806-810. ( 1) 1)
|
| [6] | 崔敏,郭冉,夏辉,等.饲料酵母对大菱鲆生长及肠道显微结构的影响[J]. 江西农业大学学报,2011,33(5):0976-0981. ( 1) 1)
|
| [7] | CASTILLO A J,DUARTE A J,SOSA M E.Effect of yeast (Candida utilis) on pigs feed with restaurant wastes during the finishing phase[J]. Revista Chapingo Serie Zootecnia,1995,1(1):83-86. ( 1) 1)
|
| [8] | DUTTA T K,KUNDU S S,KUMAR M.Potential of direct-fed microbials on lactation performance in ruminants—a critical review[J]. Livestock Research for Rural Development,2009,21(10):219-227. ( 1) 1)
|
| [9] | 杨红建,宋正河,祝仕平,等.一种发酵微量气体产生量数据自动采集存储装置及方法:中国,ZL200610011301.X[P]. 2007-12-19. ( 1) 1)
|
| [10] | MENKE K H,STEINGASS H.Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid[J].Animal Research Development,1988,28(1):7-55. ( 1) 1)
|
| [11] | GROOT J C,CONE J W,WILLIAMS B A,et al.Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds[J]. Animal Feed Science and Technology,1996,64(1):77-89. ( 1) 1)
|
| [12] | AOAC.Official methods of analysis[M]. 16th ed.Washington,D.C.:AOAC,1999. ( 1) 1)
|
| [13] | VAN SOEST P J.Nutritional ecology of ruminants[M]. 2nd ed.Ithaca:Comstock Pub. Co.,1994. ( 1) 1)
|
| [14] | VERDOUW H,VAN ECHTELD C,DEKKERS E.Ammonia determination based on indophenol formation with sodium salicylate[J]. Water Research,1978,12(6):399-402. ( 1) 1)
|
| [15] | MAKKAR H P S,SHARMA O P,DAWRA R K,et al.Simple determination of microbial protein in rumen liquor[J]. Journal of Dairy Science,1982,65(11):2170-2173. ( 1) 1)
|
| [16] | YUE Q,YANG H J,CAO Y C,et al.Feruloyl and acetyl esterase production of an anaerobic rumen fungus Neocallimastix sp. YQ2 effected by glucose and soluble nitrogen supplementations and its potential in the hydrolysis of fibrous feedstuffs[J]. Animal Feed Science and Technology,2009,153(3):263-277. ( 1) 1)
|
| [17] | ØRSKOV E R.Manipulation of rumen fermentation for maximum food utilization[J]. World Review of Nutrition and Dietetics,1975,22:152-182. ( 1) 1)
|
| [18] | MOSS A R,JOUANY J,NEWBOLD J.Methane production by ruminants:its contribution to global warming [C]//Annales de Zootechnie.Paris: Institut national de la recherche agronomique,2000.( 2) 2)
|
| [19] | MENKE K H,STEINGASS H.Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid[J]. Animal Research and Development,1988,28:7-55. ( 1) 1)
|
| [20] | BOLSEN K K,LIU C,BRENT B E,et al.Effect of silage additives on the microbial succession and fermentation process of alfalfa and corn silages[J]. Journal of Dairy Science,1992,75(11):3066-3083. ( 1) 1)
|
| [21] | MARDEN J P,JULIEN C,MONTEILS V,et al.How does live yeast differ from sodium bicarbonate to stabilize ruminal pH in high-yielding dairy cows[J]. Journal of Dairy Science,2008,91(9):3528-3535. ( 1) 1)
|
| [22] | JOUANY J P.Optimizing rumen functions in the close-up transition period and early lactation to drive dry matter intake and energy balance in cows[J]. Animal Reproduction Science 2006,96(3/4):250-264. ( 1) 1)
|
| [23] | CHAUCHEYRAS-DURAND F,AMEILBONNE A,WALKER N D,et al.Effect of a live yeast, Saccharomyces cerevisiaeⅠ-1077 on in situ ruminal degradation of alfalfa hay and fibre-associated microorganisms[J]. Journal of Animal Science,2010,88(E-Suppl.2):145. ( 1) 1)
|
| [24] | DAWSON K A,NEWMAN K E,BOLING I A.Effects of microbial supplements containing yeast and lactobacilli on roughage fed ruminal microbial activities[J]. Journal of Animal Science,1990,68(3):392-398. ( 1) 1)
|
| [25] | BITENCOURT L L,PEREIRA M N,DE OLIVERIA B M L,et al.Response of lactating cows to the supplementation with live yeast[J]. Journal of Dairy Science,2008,91(E-suppl.1):264. ( 1) 1)
|
| [26] | 丁洪涛,刘星,夏冬华,等.产朊假丝酵母对奶牛体外瘤胃发酵参数及日粮营养物质消化率的影响[J]. 中国畜牧杂志,2012,9(4):56-59. ( 1) 1)
|
| [27] | SPANGHERO M,BOCCALON S,GRACCO L,et al.NDF degradability of hays measured in situ and in vitro[J]. Animal Feed Science and Technology,2003,104(1/2/3/4):201-208. ( 1) 1)
|
| [28] | ERDMAN R A,HEMKEN R W,BULL L S.Dietary sodium bicarbonate and magnesium oxide for early postpartum lactating dairy cows: effects on production, acid-base metabolism, and digestion[J]. Journal of Dairy Science,1988,71(5):754-761. ( 1) 1)
|
| [29] | DESNOYERS M,GIGER-REVERDIN S,BERTIN G,et al.Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants[J]. Journal of Dairy Science,2009,92(4):1620-1632. ( 1) 1)
|
| [30] | NEWBOLD C J,WALLACE R J,CHEN X B,et al.Different strains of Saccharomyces cerevisiae differ in their effects on ruminal bacterial numbers in vitro and in sheep[J]. Journal of Animal Science,1995,73(6):1811-1818. ( 1) 1)
|
| [31] | BERGMAN E N.Glucose metabolism in ruminants as related to hypoglycemia and ketosis[J]. Cornell Veterinarian,1973,63(3):341-382. ( 1) 1)
|
| [32] | LASCANOA G J,HEINRICHS A J.Rumen fermentation pattern of dairy heifers fed restricted amounts of low, medium, and high concentrate diets without and with yeast culture[J]. Livestock Science,2009,124(1-3):48-57. ( 1) 1)
|
| [33] | HERDT T H.Metabolic diseases of ruminant livestock: fuel homeostasis in the ruminants[J]. The Veterinary Clinics of North America,1988,4:213-231. ( 1) 1)
|
| [34] | CUMMINS K A,PAPAS A H.Effect of isocarbon-4 and isocarbon-5 volatile fatty acids on microbial protein synthesis and dry matter digestibility in vitro[J]. Journal of Dairy Science,1985,68(10):2588-2595. ( 1) 1)
|
| [35] | LILA Z A,MOHAMMED N,TAKAHASHI T,et al.Increase of ruminal fiber digestion by cellobiose and a twin of Saccharomyces cerevisiae live cells in vitro[J]. Animal Science Journal,2006,77(4):407-413. ( 1) 1)
|
| [36] | 单安山,乔国华.直接饲喂微生物培养物对奶牛瘤胃发酵产甲烷及生产性能的影响[J]. 中国畜牧杂志,2006,33(5):11-13. ( 1) 1)
|




