家禽过多的腹脂沉积严重影响生产效率和胴体品质。脂肪沉积受遗传和环境因素的双重调节,营养等环境因素通过表观遗传修饰来影响目的基因表达逐渐成为热点研究。叶酸作为一碳单位代谢的重要辅助因子,因携带活性甲基基团,并作为S-腺苷甲硫氨酸(SAM)合成的甲基提供者,可通过对DNA的甲基化作用,改变表观遗传,影响传代[1]。目前,叶酸调控DNA甲基化已经得到广泛研究[2]。研究表明,小鼠饲粮叶酸严重缺乏4~6周时,肝脏整体基因组甲基化程度降低20%(P=0.032)[3]或者增加60%(P=0.1)[4]。McKay等[5]研究表明,小鼠在妊娠和泌乳期间饲喂低叶酸含量的饲粮时,显著降低肠道组织整体甲基化程度。Burdge等[6]报道大鼠生长期摄食叶酸充足的饲粮,导致肝脏及脂肪组织中与代谢稳态相关基因,如过氧化物酶体增殖激活受体(PPARα)与糖皮质激素受体等基因启动子区域甲基化程度增加。目前,大量研究集中在哺乳动物母体孕期或哺乳期间的叶酸水平对子代肥胖及慢性疾病的影响。Sinclair等[7]发现给母羊饲喂维生素B12和叶酸能增加子代体重和体型,并且90 d子代胎儿肝脏甲基化程度显著高于对照组,提示母体叶酸和维生素B12的添加能影响子代的表型和DNA甲基化程度。Venu等[8]在动物试验研究中发现,在总能量摄入相同的情况下,对母体孕期或哺乳期叶酸摄入量限制在正常水平的50%,导致子代出生后的体脂、甘油三酯含量增加。Lillycrop等[9]研究表明母体孕期饲粮添加叶酸,能逆转子代蛋白限饲引起糖皮质激素受体及PPARα基因启动子甲基化的变化。Waterland等[10]通过对刺鼠孕期饲粮添加叶酸发现,后代Avy基因甲基化程度增加,并伴随后代出现棕色小鼠出现的频率增高(正常为黄色皮肤)。以上研究证明,母代叶酸水平刺激能通过表观遗传的途径对子代的生长发育和代谢产生影响。家禽胚胎期脂肪发育是决定子代脂肪沉积的关键时期。目前,母鸡饲粮叶酸添加水平对子代胚胎期脂肪沉积的影响机制还未见报道。本试验旨在以研究母代饲粮叶酸添加水平能否影响胚胎后期肝脏基因表达为依据,探讨母体饲粮营养对子代脂肪性状影响的分子机制,为鸡脂肪性状分子营养调控机制提供参考资料。
1 材料与方法 1.1 试验动物及试验饲粮本试验采用单因子试验设计,选择90只120日龄健康北京油鸡,按遗传背景相同、体重相近原则,随机分为3组(每组30个重复,每个重复1只),分别饲喂在玉米-豆粕型饲粮基础上添加0(对照组)、2.5、5.0 mg/kg叶酸的试验饲粮,单笼饲养。同时选择种公鸡25只。在肉种鸡产蛋期产蛋率达到5%时进行饲粮的叶酸添加处理,预试期7 d,试验期90 d。组间母鸡体重平均体重[(1 848士619)、(1 921士309)、(1 938士509) g]没有显著差异(P>0.05)。基础饲粮参照NRC(1994)和NY/T 33—2004配制[11],其中基础饲粮中叶酸水平超过NRC(1994)[12]种鸡产蛋期叶酸推荐量(0.25 mg/kg),基础饲粮组成及营养水平见表1。
| 表1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) % |
试验鸡在密闭式鸡舍内饲养,采用3层全阶梯笼养,环境温度为15~27 ℃,每天光照时间为16 h,光照强度为20 lx,相对湿度50%~90%,通风方式为自然通风结合纵向负压通风。饲粮为干粉料,自由采食和饮水,每天捡蛋2次。常规防疫和免疫。
1.3 种蛋的孵化种鸡产蛋率达到50%时进行人工授精,产蛋率达到高峰时开始收集种蛋,每天捡蛋2次(10:30、16:30),剔除畸形蛋、过大过小蛋及沙壳蛋。种蛋按照种鸡分组,每组收取150枚左右,孵化机内温度设置规律为:1~6胚龄38.4 ℃,7~12胚龄38.1 ℃,13~18胚龄37.2 ℃,19~21胚龄36.9 ℃,相对湿度58%~60%。将收集的种蛋按组编号,钝端朝上分组分盘摆放。采用FT-ZF10数字微电脑全自动孵化机进行变温孵化。10胚龄照蛋,观察鸡胚发育情况。
1.4 样品采集及指标测定 1.4.1 肝脏组织提取采集16胚龄、19胚龄和1日龄时子代肝脏,每组取10枚胚重均匀的胚胎,液氮速冻后转到-80 ℃冷冻保存,用于基因表达丰度的测定及DNA甲基化的测定。
1.4.2 叶酸浓度测定饲粮原料按四分法缩分至10 g送检,重复测定3次;并于产蛋高峰期各组取15枚大小相近的新鲜种蛋,用分蛋器分离蛋黄与蛋清,蛋黄用冷冻干燥机干燥,-20 ℃保存待测叶酸浓度。
放射免疫法测定种蛋及饲粮叶酸浓度,SimulTRAC-SNB 叶酸放射分析试剂盒购于(Quantaphase Ⅱ B-12/Folate Radioassay,BioRad,Hercules CA),具体测定步骤参照试剂盒说明。
1.5 引物设计利用Primer 5.0进行引物设计,由深圳华大公司合成,引物序列见表2。按Trizol试剂盒(天根生化科技有限公司,北京)提取肝脏中总RNA,反转录成cDNA后-20 ℃保存备用。采用荧光定量试剂盒(天根生化科技有限公司,北京)进行实时荧光定量聚合酶链式反应(RT-PCR),采用20 μL的反应体系,反应程序:95 ℃ 15 min, 95 ℃ 10 s, 55 ℃ 31 s, 72 ℃ 32 s,共40个循环,每个样品重复3次,β-肌动蛋白为内参基因。用7500 Software v2.0.1软件测定每一个样品的Ct值(threshold cycles),依据Ct比较法进行相对定量分析,用2-△△Ct方法计算各组基因的相对表达量。
| 表2 荧光定量所选基因引物及固醇类调节元件结合蛋白(SREBP-1c)与脂肪酸合成酶(FAS)基因DNA甲基化引物序列
Table 2 The gene and related primers for fluorescent quantitation and for SREBP-1c and FAS promoter CpG methylation assay
|
1.6 甲基化检测
通过美国国家生物技术信息中心(NCBI)寻找基因启动子,利用http://www.cbs.dtu.dk/services/Promoter/网站对基因启动子大概位置进行预测。对基因进行启动子CpG位点分析,并设计用于重亚硫酸盐聚合酶链式反应(PCR)的特异性引物,见表2。
随机取相同日龄的8个子代的肝脏组织,基因组提取试剂盒(天根生化科技有限公司,北京)提取肝脏的全基因组DNA,紫外分光光度法鉴定DNA含量及纯度。根据测定的DNA浓度等比例混合成DNA池,取500 ng基因组DNA参照EZ DNA Methylation-GoldTM Kit甲基化试剂盒(Zymo Research Co, CA, USA)说明书进行DNA重亚硫酸氢盐修饰,-20 ℃保存备用。SREBP-1c基因PCR反应程序为:第1轮PCR(用引物SREBP-1c-F1和SREBP-1c-R1扩增),94 ℃ 5 min→(94 ℃ 30 s, 50 ℃ 30 s, 72 ℃ 1 min)×20, 72 ℃ 5 min;第2轮PCR(用引物SREBP-1c-F1和SREBP-1c-R2扩增,以第1轮PCR产物0.2 μL为模板),94 ℃ 5 min→(94 ℃ 30 s, 50 ℃ 30 s, 72 ℃ 1 min)×35, 72 ℃ 5 min。FAS基因PCR反应程序为:第1轮PCR(用引物FAS-F1和FAS-R1扩增),94 ℃ 5 min→(94 ℃ 30 s, 46 ℃ 30 s, 72 ℃ 1 min)×20, 72 ℃ 5 min;第2轮PCR(用引物FAS-F1和FAS-R2扩增,以第1轮PCR产物0.2 μL为模板),94 ℃ 5 min→(94 ℃ 30 s, 46 ℃ 30 s, 72 ℃ 1 min)×35, 72 ℃ 5 min。
PCR反应完毕后进行琼脂糖凝胶电泳。切取琼脂糖凝胶上的目的条带进行纯化,用T4连接酶将该片段连接到PMD19T克隆载体上,转化DH5α菌株,通过蓝白斑筛选与PCR鉴定,挑选8~10个阳性菌落测序,分析目的基因CpG位点的甲基化状态。
1.7 数据处理采用SPSS 16.0统计软件进行统计处理。相同组不同日龄间比较采用单因素方差分析(one-way ANONA),LSD检验平均值两两之间的差异显著性;相同日龄不同组间平均值比较采用Duncan氏法多重比较检验。甲基化比较采用Pearson X2检验或Fisher精确概率法,均为双侧检验。P<0.05为差异显著,P<0.01为差异极显著。
2 结果与分析 2.1 种鸡叶酸添加水平对种蛋叶酸沉积影响由表3可见,检测发现种蛋中5-甲基四氢叶酸含量在2.5 mg/kg叶酸添加组中高出对照组的81.92%(P<0.05),与5.0 mg/kg叶酸添加组差异不显著(P>0.05)。
| 表3 种鸡叶酸添加水平对种蛋5-甲基四氢叶酸含量的影响
Table 3 Effects of maternal folate supplemental level on content of 5-methyltetrahydrofolate in hatching egg (n=8) μg/egg
|
由表4可知,随着日龄增加,胚胎期肉鸡肝脏SREBP-1c 基因表达水平呈逐渐上升的趋势,1日龄达到峰值。组间比较,16胚龄时,组间差异不显著(P>0.05);19胚龄与1日龄时,SREBP-1c mRNA表达水平随叶酸添加水平增加均呈现显著下降(P<0.05),19胚龄各组达到显著差异(P<0.05),1日龄时5.0 mg/kg叶酸添加组与其他组差异显著(P<0.05)。
| 表4 种鸡叶酸添加水平对后代胚胎期肝脏脂肪代谢部分基因mRNA水平的影响
Table 4 Effects of maternal folate supplemental level on liver mRNA expression of partial genes in offspring during embryonic development (n=6)
|
胚胎期肉鸡肝脏FAS基因随着日龄增加表达水平呈上升趋势,胚胎期表达水平很少,而1日龄表达水平急剧增加。组间比较,胚胎期对照组和叶酸添加组基本保持一致,1日龄时,与对照组相比,叶酸添加组基因表达水平降低,5.0 mg/kg叶酸添加组降低达显著(P<0.05)。提示叶酸添加可能对胚胎后期肝脏FAS基因表达产生了影响。
胚胎期肉鸡肝脏乙酰辅酶A羧化酶(ACC)基因表达水平随日龄增加逐渐下降,1日龄时达到最小值,极显著低于其他胚龄(P<0.01)。组间比较,16胚龄时,对照组ACC基因表达水平显著高于叶 酸添加组(P<0.05),而叶酸添加组间无显著差异(P>0.05);19胚龄与1日龄时,组间没有显著性差异(P>0.05)。提示叶酸添加对胚胎期较早期肝脏ACC基因表达有显著影响。
2.3 种鸡叶酸添加水平对子代胚胎期肝脏基因启动子区甲基化的影响
由表5所示,SREBP-1c基因启动子区总体甲基化程度随日龄及叶酸添加水平的增加均呈现降低趋势(P>0.05)。SREBP-1c启动子区24个CpG位点可以发现,第7、9、12、13、18、19位点受营养的影响而发生去甲基化状态;相反,第21、22、23位点极易被甲基化,而呈现高度甲基化程度(图1A)。
| 表5 种鸡叶酸添加水平对后代胚胎期SREBP-1c及FAS启动子区总体甲基化程度的影响
Table 5 Effects of maternal folate supplemental level on total methylation of the promoter region of SREBP-1c and FAS in offspring during embryonic development
|
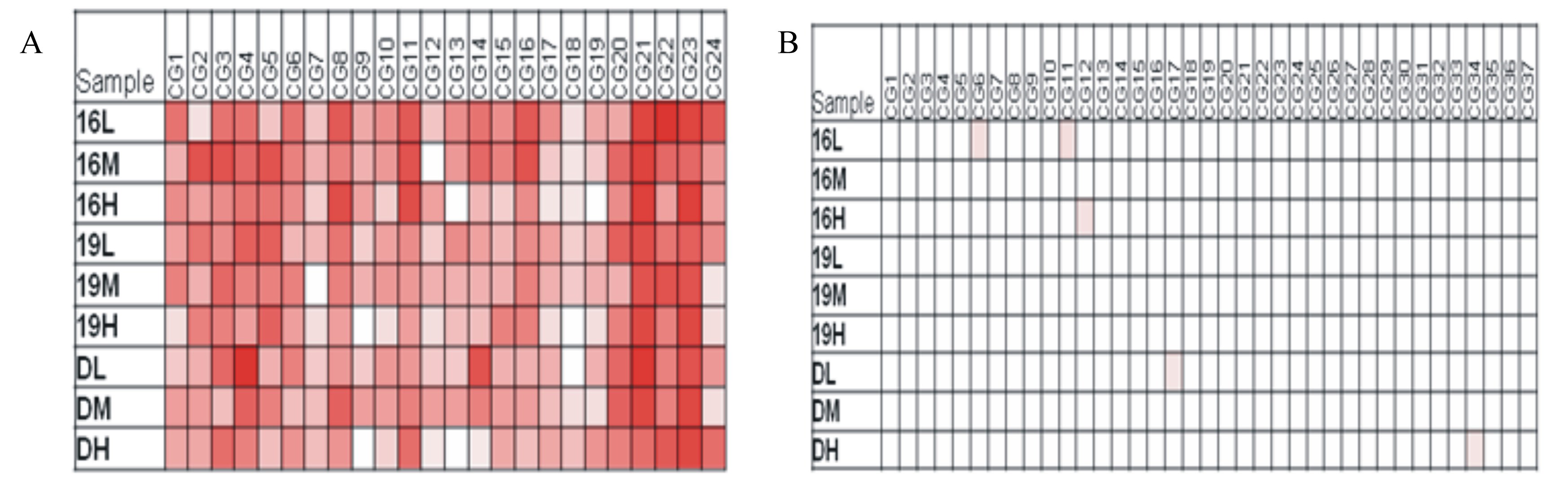 | (A)SREBP-1c每个CpG位点在各组及不同日龄中的甲基化分析,1~24代表从5′端开始的24个CpG位点;(B)FAS每个CpG位点在各组及不同日龄中的甲基化分析,1~37代表从5′端开始的37个CpG位点。16表示16胚龄,19为19胚龄,D1为1日龄。L代表对照组,M代表2.5 mg/kg叶酸添加组,H代表5.0 mg/kg叶酸添加组。 (A)Percentage of each CpG methylation of SREBP-1c in different folate groups, number 1~24 refer to the 5’-most CpG.; (B) percentage of each CpG methylation of FAS indifferent folate groups, number 1~37 refer to the 5’-most CpG. 16 represents the embryonic age of 16 days, 19 represents the embryonic age of 19 days, D1 represents 1 day of age. L represents control group, M represents 2.5 mg/kg folate supplemental level group, H represents 5.0 mg/kg folate supplemental level group. 图1 饲粮不同叶酸添加水平的子代胚胎期SREBP-1c及FAS亚硫酸盐修饰直接测序分析 Fig.1 Bisulfite sequencing analysis of SREBP-1c and FAS from different dietary folate supplemental level in offspring during embryonic development |
FAS基因启动子区总体甲基化程度在3个日龄中均是低或者未甲基化的,不同日龄及组间比较均无统计学意义。FAS启动子区37个CpG位点可以发现,基本每个位点都易发生去甲基化状态,而呈现低甲基化状态(图1B)。
综上所述,尽管SREBP-1c启动子区在组间没有显著差异,相同叶酸添加组平均甲基化率,随日龄的增大甲基化的程度是随日龄的增大总体上是降低的。而FAS启动子区在整个胚胎期均是高度去甲基化的,基本上不受日龄和叶酸添加水平的影响。 3 讨 论 3.1 种鸡叶酸添加水平对种蛋叶酸沉积的影响
种蛋中叶酸主要以5-甲基四氢叶酸的形式存在[13]。本试验结果表明,种鸡饲粮2.5 mg/kg叶酸添加组对于对照组,种蛋中叶酸沉积提高了近2倍,极显著提高了叶酸沉积,而叶酸添加组间差异不显著。Dickson等[14]研究表明,饲粮叶酸添加水平为4 mg/kg时,约128 d的产蛋期累积,能促使鸡蛋叶酸的沉积由含有0.9 mg/kg叶酸的基础饲粮的17.7~22.3 μg/egg增加到46.9~57.9 μg/egg,即与对照组比增加2~3倍的沉积。House等[15]和Sherwood等[16]研究表明,当基础饲粮叶酸添加水平在0~2 mg/kg时,鸡蛋叶酸的沉积正相关于饲粮中叶酸添加水平。House等[15]研究表明,种蛋叶酸的沉积在饲粮叶酸添加水平为4 mg/kg时达到饱和,过多添加并不会增加种蛋叶酸沉积。关于饲粮叶酸添加水平对鸡蛋叶酸沉积影响的研究还有很多报道。尽管研究方案对叶酸添加水平及作用时间各不相同,整体结果均表明饲粮添加2~8 mg/kg叶酸能有效地增加鸡蛋中约2倍的叶酸沉积,但继续增大叶酸添加水平并不能有效地增加鸡蛋叶酸沉积[17]。
3.2 种鸡叶酸添加水平对子代体脂沉积相关功能基因表达的影响关于营养对肉鸡脂肪代谢相关基因表达调控已有较多研究进展,但这些研究主要集中在母体蛋白质、脂类、能量水平对亲代脂质调控,而对于母体甲基供体叶酸改变对子代脂质代谢的研究较少。叶酸携带活性甲基化基团,并作为SAM合成的甲基提供者,是DNA甲基化反应的独特甲基供体,基因启动子区甲基化是其基因表达调控的重要途径之一。本研究试图通过改变肉种鸡饲粮叶酸水平,探讨母体营养对子代胚胎期脂肪代谢关键因子的影响。
肝脏中的脂肪代谢受多种基因控制,包括SREBP-1c、ACC、FAS基因等[18]。肉种鸡叶酸水平对子代胚胎期肝脏脂肪酸合成关键因子的调控存在基因特异性和发育特异性,这可能与各基因在脂肪酸合成代谢中各自发挥不同的功能有关。ACC是长链脂肪酸从头合成的限速酶,催化脂肪合成第1步的关键酶,主要实现对脂肪酸合成的快速调节[19]。FAS催化乙酰CoA与丙二酸单酰辅酶CoA缩合,还原等一系列反应,生成脂肪酸,决定着脂肪酸合成方向[20]。SREBP-1c是调节肝脏脂质代谢的重要核转录因子,它通过调节与脂肪生成相关酶的基因转录而调节这些酶的活性,从而控制脂肪合成,其表达水平加强能够显著促进ACC及FAS等有关脂肪合成和葡萄糖代谢的基因表达[18, 21]。本研究结果表明,种鸡子代胚胎期肝脏组织中SREBP-1c基因随胚龄的增加表达水平上升,组间比较,随饲粮叶酸添加水平增多,SREBP-1c基因的表达水平显著降低,1日龄时高叶酸组显著低于低叶酸组;FAS随胚龄的增大表达量增加,1日龄时不同叶酸添加组间基因表达水平差异显著;ACC基因表达随日龄变化与FAS基 因呈现相反的趋势,不同叶酸添加组间仅在16胚 龄时达到显著差异。提示种鸡饲粮的叶酸水平可以调控其子代胚胎期肝脏脂肪代谢关键因子的表达,高叶酸添加量能降低子代出壳时的脂肪酸合成基因的表达水平。有关母体叶酸添加对子代脂质代谢相关基因的文献报道仍少有报道,但已有研究表明叶酸对脂质代谢产生一定干预作用。Eseceli等[22]和Wang等[23]研究表明,叶酸可降低胆固醇的含量。姚英等[24]研究表明,添加5和10 mg/kg叶酸分别显著和极显著降低了甘油三脂含量。而妊娠期母大鼠饲粮补充5 mg/kg叶酸则可显著降低子代血清葡萄糖和甘油三酯浓度[25]。可以推测,叶酸为肉碱和肌酸的合成提供甲基,增强线粒体中游离脂肪酸的氧化,进而起到降低或重分配体脂的作用。与前人研究报道一致的是,艾正琳等[26]证实SREBP-1c基因和蛋白质表达水平增高均可增加FAS基因转录,引起脂肪酸合成增加。Kakuma等[27]也研究表明,fa/fa ZDF肥胖大鼠(瘦素受体突变)肝脏和胰岛SREBP-1c基因表达水平显著提高,ACC和FAS基因表达转录增多。本试验结果得出SREBP-1c基因表达水平在多个时期、不同叶酸添加组间均差异较大,可能间接影响了相应时期下游靶基因ACC和FAS基因的表达转录。
基因启动子区的甲基化是基因表达调控的重要方式之一。一般来说,DNA甲基化与基因表达呈负调控关系。本试验结果显示,SREBP-1c基因启动子区域甲基化程度随着胚龄的增大而呈现降低的趋势,相同叶酸添加水平比较,甲基化程度随日龄增加未达到显著性差异。相同日龄比较,SREBP-1c的启动子区甲基化程度随叶酸的添加水平增加,其程度呈现略微降低的趋势,但各胚龄间也未达到统计学差异。FAS基因在整个胚胎发育过程中甲基化检测也表明,其甲基化程度在胚龄及组间均差异不显著,整体上均处于去甲基化或未甲基化状态。目前关于叶酸对SREBP-1c甲基化的研究还未见其他报道。因此本试验结果表明,SREBP-1c及FAS基因mRNA表达增加可能与其启动子区甲基化程度无关。
有关营养甲基供体对FAS基因甲基化的研究已有部分报道,Xing等[28]对120日龄的种鸡饲粮添加甜菜碱,通过分析FAS启动子区域(-961 ~ -749 bp)的9个CpG位点被甲基化的情况,发现180日龄种鸡的甜菜碱添加组间甲基化未发生变化。Lomba等[29]也证实FAS基因表达水平与甲基化状态没有相关性。本试验也发现FAS基因表达水平与其甲基化程度并没有一定规律性。
总体来说,动物试验上的大量研究结果表明,叶酸对DNA甲基化调节的机制比较复杂[30, 31, 32],不仅依赖于细胞类型及组织特异性、发育阶段,而且取决于基因与位点的特异性。下一步,针对母代叶酸添加水平对子代脂肪沉积的影响研究,还有待于针对更多相关基因的进一步检测。
4 结 论① 在种鸡产蛋期基础饲粮中增加叶酸添加水平,可在一定程度上提高种蛋叶酸含量。
② 种鸡饲粮叶酸添加下调了子代胚胎部分时期脂质代谢相关基因(SREBP-1c、FAS、ACC)的表达水平。
③ 种鸡饲粮叶酸添加未改变子代SREBP-1c及FAS启动子区域的甲基化程度,且它们的表达水平改变可能不受其启动子区甲基化程度的影响。
| [1] | DURAND P,PROST M,BLACHE D.Pro-thrombotic effects of a folic acid deficient diet in rat platelets and macrophages related to elevated homocysteine and decreased n-3 polyunsaturated fatty acids[J]. Atherosclerosis,1996,121(2):231-243. ( 1) 1)
|
| [2] | MATHERS J C,FORD D.Nutrition,epigenetics and aging[J]. Nutrients and Epigenetics,2009:175-205. ( 1) 1)
|
| [3] | BALAGHI M,WAGNER C.DNA methylation in folate deficiency:use of CpG methylase[J]. Biochemical and Biophysical Research Communications,1993,193(3):1184-1190. ( 1) 1)
|
| [4] | KIM Y I,POGRIBNY I P,BASNAKIAN A G,et al.Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene[J]. The American Journal of Clinical Nutrition,1997,65(1):46-52. ( 1) 1)
|
| [5] | MCKAY J A,WALTHAM K J,WILLIAMS E A,et al.Folate depletion during pregnancy and lactation reduces genomic DNA methylation in murine adult offspring[J]. Genes & Nutrition,2011,6(2):189-196. ( 1) 1)
|
| [6] | BURDGE G C,LILLYCROP K A,PHILLIPS E S,et al.Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition[J]. The Journal of Nutrition,2009,139(6):1054-1060. ( 1) 1)
|
| [7] | SINCLAIR K D,ALLEGRUCCI C,SINGH R,et al.DNA methylation,insulin resistance,and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status[J]. Proceedings of the National Academy of Sciences of the United States of America,2007,104(49):19351-19356. ( 1) 1)
|
| [8] | VENU L,HARISHANKAR N,KRISHNA T P,et al.Maternal dietary vitamin restriction increases body fat content but not insulin resistance in WNIN rat offspring up to 6 months of age[J]. Diabetologia,2004,47(9):1493-1501. ( 1) 1)
|
| [9] | LILLYCROP K A,PHILLIPS E S,TORRENS C,et al.Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring[J]. British Journal of Nutrition,2008,100(2):278-282. ( 1) 1)
|
| [10] | WATERLAND R A,JIRTLE R L.Transposable elements:targets for early nutritional effects on epigenetic gene regulation[J]. Molecular and Cellular Biology,2003,23(15):5293-5300. ( 1) 1)
|
| [11] | 中华人民共和国农业部.NY/T33-2004鸡饲养标准[S]. 北京:中国农业出版社,2004. ( 1) 1)
|
| [12] | National Research Council.Nutrient requirements of poultry[Z]. Washington D.C.:National Academy Press,1994. ( 1) 1)
|
| [13] | SEYOUM E,SELHUB J.Properties of food folates determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action[J]. The Journal of Nutrition,1998,128(11):1956-1960. ( 1) 1)
|
| [14] | DICKSON T M,TACTACAN G B,HEBERT K,et al.Optimization of folate deposition in eggs through dietary supplementation of folic acid over the entire production cycle of Hy-Line W36,Hy-Line W98,and CV20 laying hens[J]. The Journal of Applied Poultry Research,2010,19(1):80-91. ( 1) 1)
|
| [15] | HOUSE J D,BRAUN K,BALLANCE D M,et al.The enrichment of eggs with folic acid through supplementation of the laying hen diet[J]. Poultry Science,2002,81(9):1332-1337. ( 2) 2)
|
| [16] | SHERWOOD T A,ALPHIN R L,SAYLOR W W,et al.Folate metabolism and deposition in eggs by laying hens[J]. Archives of Biochemistry and Biophysics,1993,307(1):66-72. ( 1) 1)
|
| [17] | HOEV L,MCNULTY H,MCCANN E M,et al.Laying hens can convert high doses of folic acid added to the feed into natural folates in eggs providing a novel source of food folate[J]. British Journal of Nutrition,2009,101(2):206-212. ( 1) 1)
|
| [18] | STOECKMAN A K,TOWLE H C.The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression[J]. The Journal of Biological Chemistry,2002,277(30):27029-27035. ( 2) 2)
|
| [19] | MCGARRY J D,LEATHERMAN G F,FOSTER D W.Carnitine palmitoyltransferase I.The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA.[J]. The Journal of Biological Chemistry,1978,253(12):4128-4136. ( 1) 1)
|
| [20] | BACK D W,GOLDMAN M J,FISCH J E,et al.The fatty acid synthase gene in avian liver.Two mRNAs are expressed and regulated in parallel by feeding,primarily at the level of transcription[J]. The Journal of Biological Chemistry,1986,261(9):4190-4197. ( 1) 1)
|
| [21] | BROWN M S,GOLDSTEIN J L.A proteolytic pathway that controls the cholesterol content of membranes,cells,and blood[J]. Proceedings of the National Academy of Sciences of the United States of America,1999,96(20):11041-11048. ( 1) 1)
|
| [22] | ESECELI H,DEGIRMENCIOGLU N,BILGIC M.The effect of inclusion of chromium yeast (Co-Fator Ⅱ,alltech inc.) and folic acid to the rations of laying hens on performance,egg quality,egg yolk cholesterol,folic acid and chromium levels[J]. Journal of Animal and Veterinary Advances,2010,9(2):384-391. ( 1) 1)
|
| [23] | WANG S P,YIN Y L,QIAN Y,et al.Effects of folic acid on the performance of suckling piglets and sows during lactation[J]. Journal of the Science of Food and Agriculture,2011,91(13):2371-2377. ( 1) 1)
|
| [24] | 姚英,陈代文,刘静波,等.叶酸对超早期断奶宫内发育迟缓仔猪肝脏结构和细胞凋亡相关基因表达的影响[J]. 动物营养学报,2012,24(2):271-279. ( 1) 1)
|
| [25] | BURDGE G C,LILLYCROP K A,JACKSON A A,et al.The nature of the growth pattern and of the metabolic response to fasting in the rat are dependent upon the dietary protein and folic acid intakes of their pregnant dams and post-weaning fat consumption[J]. British Journal of Nutrition,2008,99(3):540-549. ( 1) 1)
|
| [26] | 艾正琳.LXRα,SREBP-1c介导脂肪酸代谢紊乱在大鼠NAFLD形成中的作用及机制 .硕士学位论文.重庆:第三军医大学,2006:43-44. ( 1) 1)
|
| [27] | KAKUMA T,LEE Y,HIGA M,et al.Leptin,troglitazone,and the expression of sterol regulatory element binding proteins in liver and pancreatic islets[J]. Proceedings of the National Academy of Sciences of the United States of America,2000,97(15):8536-8541. ( 1) 1)
|
| [28] | XING J Y,KANG L,JIANG Y L.Effect of dietary betaine supplementation on lipogenesis gene expression and CpG methylation of lipoprotein lipase gene in broilers[J]. Molecular Biology Reports,2011,38(3):1975-1981. ( 1) 1)
|
| [29] | LOMBA A,MARTINEZ J A,GARCIA-DIAZ D F,et al.Weight gain induced by an isocaloric pair-fed high fat diet:a nutriepigenetic study on FASN and NDUFB6 gene promoters[J]. Molecular Genetics and Metabolism,2010,101(2/3):273-278. ( 1) 1)
|
| [30] | KOTSOPOULOS J,SOHN K J,KIM Y I.Postweaning dietary folate deficiency provided through childhood to puberty permanently increases genomic DNA methylation in adult rat liver[J]. The Journal of Nutrition,2008,138(4):703-709. ( 1) 1)
|
| [31] | LILLYCROP K A,SLATER-JEFFERIES J L,HANSON M A,et al.Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications[J]. British Journal of Nutrition,2007,97(6):1064-1073. ( 1) 1)
|
| [32] | STEEGERS-THEUNISSEN R P,OBERMANN-BORST S A,KREMER D,et al.Periconceptional maternal folic acid use of 400 μg per day is related to increased methylation of the IGF2 gene in the very young child[J]. PLoS One,2009,4(11):7845. ( 1) 1)
|




