2. 农业部饲料生物技术重点开放实验室, 北京 100081
2. Key Laboratory of Aquaculture Nutrition and Feed, Ministry of Agriculture, Beijing 100081, China
花鲈(Lateolabrax japonicus)是一种典型的肉食广盐性鱼类,既可在海水中养殖,也可经过淡化在淡水中养殖,是我国重要的海水养殖品种之一。随着集约化养殖的快速发展,花鲈的病害频繁发生且日趋严重。多年来,抗生素被广泛应用于水产饲料中,以促进鱼体生长及预防某些疾病的发生。但是,抗生素的滥用引起诸多问题,如抗药菌株的出现、动物产品中抗生素的残留等。因此,筛选抗生素替代品成为科研人员的重要工作之一。
此外,随着水产养殖对鱼粉需求的不断增加,鱼粉短缺已成为一个全球性问题。近年来,水产饲料中植物饲料的使用受到高度的关注。豆粕由于其蛋白质含量高、氨基酸相对平衡、价格合理[1,2]而成为重要的鱼粉替代蛋白质源。但是,豆粕中的抗营养因子会导致饲料适口性降低、鱼体发生肠炎、抗病力降低等,这也是大多数肉食性鱼类对鱼粉依赖性较高的原因。本实验室前期研究表明:对于花鲈来说,至少需要25%的高质量鱼粉来维持饲料的适口性、消化率以及生长[3,4]。
壳寡糖(chitosan oligosaccharide,COS)、百泰A(GroBiotic -A)、酵母细胞壁(yeast cell wall,YCW)、牛磺酸均为重要的免疫调节剂。壳寡糖是一种由甲壳素或壳聚糖经过化学或酶解得到的碱性寡糖。Luo等[5]的研究表明40 mg/kg壳寡糖能够提高虹鳟的免疫力和抗病力。百泰A是一种益生元,由部分自溶性啤酒酵母、奶组分、晒干的发酵产物组成[6]。Gibson等[6]认为益生元是食物中不可消化的成分,能够选择性地促进肠道有益菌群的生长或特异性提高它们的活性,从而调节宿主肠道菌群平衡,促进生长并提高抗病力。研究表明,在饲料中添加1%~2%百泰A能够显著提高水产动物对营养物质的消化率[7]、生长性能[8]以及细菌攻毒后的存活率(survival rate,SR)[8,9,10]。酵母细胞壁的主要活性成分为β-葡聚糖和甘露聚糖。β-葡聚糖能够通过直接与免疫细胞结合而激活它们[11,12],从而提高水产动物的抗病力和一些先天性免疫反应。而甘露聚糖则能够竞争性地与肠道致病菌表面的外源凝集素结合,从而阻止致病菌在肠上皮上的黏附。此外,甘露聚糖还具有抗氧化、抗突变、抗癌等活性[13,14]。本实验室前期研究也发现500~2 000 mg/kg酵母细胞壁能够提高花鲈的生长性能和抗病力[15]。牛磺酸是一种半条件性必需氨基酸[16],不参与机体蛋白质的合成。作为一种重要的免疫调节剂,牛磺酸在免疫系统的发育、抗炎、抗氧化等多种生理过程中均发挥着重要作用[17]。Qi等[18]发现0.5%牛磺酸能够显著提高大菱鲆的生长性能。而抗生素类促生长剂(如黄霉素、喹乙醇等)长期使用可能造成鱼类免疫抑制[19,20],产生耐药性等问题[21]。本试验拟对几种免疫调节剂与抗生素(黄霉素)的应用效果进行比较,研究适量壳寡糖、百泰A、酵母细胞壁、牛磺酸对摄食低鱼粉饲料花鲈生长性能、免疫力及细菌感染后存活率的影响,为抗生素替代品的筛选及豆粕在水产饲料中的合理使用提供理论参考。 1 材料与方法 1.1 试验设计
试验所用花鲈购自山东威海,将花鲈暂养于养殖系统中,逐渐从海水鱼种淡化为淡水鱼种。正式试验开始前,对试验鱼进行为期4周的驯养。试验选用体质健康、个体大小均匀[平均体重(18.3±0.01) g]的花鲈840尾,随机分为7组,每组4个重复,每个重复30尾鱼。养殖周期为72 d,每天表观饱食投喂2次,分别为09:00和16:00。养殖试验在国家水产饲料安全评价基地室内循环养殖系统中进行。试验期间水温24~27 ℃,溶氧浓度>7 mg/L,pH 7.5~8.5,氨氮浓度<0.3 mg/L。首先设计1个含38.500%鱼粉(低温干燥鱼粉)的饲料作为正对照组饲料,然后以豆粕替代正对照组饲料中13.500%的鱼粉作为对照组饲料,并在此基础上分别添加0.010%预混级黄霉素(黄霉素含量为4%;湖北康宝泰精细化工有限公司;负对照组)、0.004%壳寡糖(壳寡糖含量为85%;大连中科格莱克生物科技有限公司)、1.000%百泰A[由部分自溶性啤酒酵母、奶组分、晒干的发酵产物组成,粗蛋白质含量为35.2%,粗脂肪含量为1.7%,单糖和多糖(包括寡聚糖)含量为53.0%;美国国际原料公司]、0.050%酵母细胞壁(葡聚糖含量为28%,甘露聚糖含量为24%;广西一品鲜生物科技有限公司)、0.500%牛磺酸(牛磺酸含量为98%;国药集团化学试剂北京有限公司)制成5种试验饲料。将上述7个组依次命名为FM、SBM、Fla、COS、GA、YCW、Tau组。各受试物的添加量均为其最适添加量[5, 8, 18, 22]。各饲料原料按照添加量从小到大顺序逐级搅拌混匀,制成粒径为2 mm的挤压膨化沉性料(EXT50A,北京现代洋工机械科技发展有限公司),自然晾干(温度:室温;时间:48 h)后于-20 ℃保存。饲料组成及营养水平见表1。
| 表1 饲料组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of diets (DM basis) % |
试验72 d后,试验鱼禁食24 h,分别对各桶鱼称重并统计存活率、增重率(weight gain rate,WGR)、特定生长率(specific growth rate,SGR)、饲料系数(feed conversion ratio,FCR)、摄食率(feeding rate,FR)。各指标计算公式如下:
存活率(%)=100×Nt/N0;
增重率(%)=100×(Wt-W0+Wd)/W0;
特定生长率(%/d)=100×[lnFBW-lnIBW]/t;
饲料系数=C/(Wt-W0+Wd);
摄食率(%/d)=100×C/[(W0+Wt+Wd)/2]/t。
式中:N0为终末鱼数(尾);Nt为初始鱼数(尾);IBW为鱼体初始均重(g);FBW为终末均重(g);W0为鱼体初始总重(g);Wt为终末总重(g);Wd为死亡鱼体总重(g);C为摄食量(g);t为试验天数(d)。 1.2.2 形体指标
每桶随机取3尾鱼测量体长、体重、肝脏重、内脏重、脾脏重,计算形体指标。各指标计算公式如下:
肥满度(condition factor,CF,g/cm3)=100×体重(g)/[体长(cm)]3;
肝体指数(hepato-somatic index,HSI,%)=100×肝脏重(g)/体重(g);
脏体指数(viscera-somatic index,VSI,%)=100×内脏重(g)/体重(g);
脾脏指数(spleen-somatic index,SSI,%)=100×脾脏重(g)/体重(g)。
每桶随机取3~4尾鱼,三氯叔丁醇麻醉后,尾静脉取血,血液经抗凝剂(2% NaF+4%草酸钾)抗凝后,取0.5 mL全血用于呼吸爆发强度的测定,剩余血液在4 ℃条件下离心10 min(1 500×g),取血浆分装并存于-80 ℃待测。
血浆髓过氧化物酶(myeloperoxidase,MPO)活性的测定参考文献[23]中描述的方法进行,具体如下:取15 μL血浆,加入135 μL Hank’s平衡盐溶液(HBSS,Sigma,美国)后摇匀,加入52.5 μL四甲基联苯胺(TMB,Sigma,美国),振荡2 min后,加入52.5 μL 0.5 mmol/L H2SO4终止反应,摇匀后450 nm波长下,用酶标仪(PowerWave XS2,BioTek,美国)读出光密度(optical density,OD)值。MPO活性以OD值表示。
全血呼吸爆发强度的测定参考文献[24]中描述的硝基四氮唑蓝(nitroblue tetrazolium,NBT)方法进行,具体如下:取100 μL抗凝全血,加入100 μL 0.2% NBT染液,25 ℃孵育30 min后,取15 μL反应液,加入300 μL N,N-二甲基甲酰胺,混匀后在750×g下离心5 min,取上清液200 μL在540 nm波长下读取OD值,以200 μL N,N-二甲基甲酰胺为空白对照。呼吸爆发强度以NBT反应的OD值表示。
血浆中一氧化氮(nitric oxide,NO)含量及抗超氧阴离子自由基(anti-superoxide anion free radical,Anti-O-2·)活性采用试剂盒测定,所用试剂盒购自南京建成生物工程研究所。血浆中补体3(complement 3,C3)、免疫球蛋白M(immunoglobulin M,IgM)含量采用酶联免疫吸附法(ELISA)测定,所用试剂盒购自上海博耀生物科技有限公司。 1.2.4 细菌攻毒试验 1.2.4.1 细菌培养
维氏气单胞菌(菌种编号:CGMCC No.4274)由北京市水产科学研究所提供,在普通营养琼脂培养基上28 ℃过夜培养,然后用0.7%生理盐水洗脱,稀释到试验所需菌液浓度。 1.2.4.2 预试验
使用浓度梯度为107、106、105 CFU/mL菌液进行攻毒预试验,每个浓度3个重复,每个重复10尾鱼,注射剂量为每100 g体重200 μL,注射方法为背鳍基部肌肉注射,养殖周期为7 d,最后确定的半致死菌液浓度为2×105 CFU/mL。 1.2.4.3 正式试验
生长试验结束后,每组取40尾鱼,均分为2个重复,每个重复20尾鱼。攻毒时水温为(26±1) ℃,攻毒菌液浓度为2×105 CFU/mL,剂量为每100 g体重200 μL(即每100 g体重4×104 CFU),采用背鳍基部肌肉注射。攻毒期间不投喂饲料。攻毒2 d后,其中1个重复取血样测定攻毒后免疫指标,另1个重复用来计算攻毒后7 d的累计存活率。 1.3 数据统计与分析
试验数据以平均值±标准误(mean±SE)表示,使用SPSS 17.0软件对花鲈的生长指标、形体指标进行单因素方差分析(one-way ANOVA),对攻毒前后的免疫指标进行双因素方差分析(two-way ANOVA),差异显著时通过Duncan氏法进行多重比较检验,以P<0.05为差异显著性标准。 2 结 果 2.1 生长指标
几种免疫调节剂对花鲈生长指标的影响见表2。与对照组相比,免疫调节剂的添加对花鲈各生长指标的影响均不显著(P>0.05)。FM组的增重率、特定生长率最高,显著高于其他各组(P<0.05)。FM组的饲料系数最低,显著低于其他各组(P<0.05)。YCW组的摄食率显著高于FM组(P<0.05)。72 d的生长试验后,FM、COS、GA组的存活率均为100.0%,显著高于Tau组的96.7%(P<0.05)。
| 表2 几种免疫调节剂对花鲈生长指标的影响 Table 2 Effects of several immunomodulators on growth indices of Japanese seabass |
几种免疫调节剂对花鲈形体指标的影响见表3。各组间CF、HSI无显著差异(P>0.05)。YCW组的VSI最高,显著高于SBM组(P<0.05)。SSI以Fla组最高,而COS组则最低,并显著低于除FM组外的其他各组(P<0.05)。
| 表3 几种免疫调节剂对花鲈形体指标的影响 Table 3 Effects of several immunomodulators on body indices of Japanese seabass |
攻毒2 d后,Fla组所有花鲈全部死亡,因此未测定该组攻毒后的免疫指标。几种免疫调节剂对花鲈攻毒前后免疫指标的影响见表4。攻毒前,GA、YCW组的血浆C3含量较高,显著高于COS组(P<0.05);Fla组的血浆IgM含量最高,显著高于除SBM、Tau组外的其他各组(P<0.05);SBM组的血浆MPO活性显著高于其他各组(P<0.05),其他各组间无显著差异(P>0.05);全血呼吸爆发强度以FM组最高,YCW组最低,显著低于其他各组(P<0.05);Fla组的血浆NO含量最高,显著高于SBM组(P<0.05);Tau组的血浆Anti-O-2·活性最高,显著高于YCW组(P<0.05)。相较于攻毒前,攻毒后各组花鲈的非特异性免疫指标血浆MPO活性、NO含量及全血呼吸爆发强度均显著升高(P<0.05),血浆C3含量、Anti-O-2·活性则 无显著变化(P>0.05),而特异性免疫指标血浆IgM含量仅在FM、YCM和Tau组显著升高(P<0.05)。攻毒后,Tau组的血浆IgM含量、MPO活性、、NO含量、Anti-O-2·活性及全血呼吸爆发强度均处于最高水平。YCW组攻毒7 d后的存活率最高,为85%;其次是Tau组,为65%;随后依次是GA、FM、COS组,分别为60%、50%、50%;SBM组最低,仅为40%(图1)。
| 表4 几种免疫调节剂对花鲈攻毒前后免疫指标的影响 Table 4 Effects of several immunomodulators on immune indices before and after challenge of Japanese seabass |
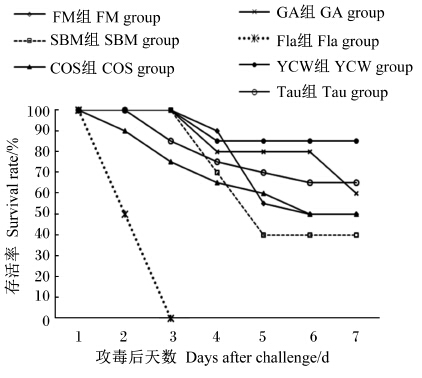 | 图1 几种免疫调节剂对维氏气单胞菌 攻毒后花鲈7 d累计存活率的影响 Fig.1 Effects of several immunomodulators on 7-day cumulative survival rate of Japanese seabass challenged with Aeromonas veronii |
植物蛋白质替代鱼粉时,摄食率降低是肉食性鱼类生长性能下降的重要原因之一[25,26]。花鲈也是如此,即便是在补充了必需氨基酸和矿物质的情况下[3,27]。本试验中,用豆粕替代13.500%的鱼粉并未影响花鲈的摄食率,可能是由于正式试验开始前4周的暂养使花鲈适应了SBM组的饲料[3,27]。研究表明。虹鳟[28]和异育银鲫[29]在投喂4周低鱼粉饲料后也表现出了适应。尽管SBM组摄食率未降低,但是其生长性能显著低于FM组,说明除了蛋氨酸、磷外,豆粕中还缺乏一些其他的必需生长因子,比如核苷酸[30]、甘氨酸[31]等,或是豆粕中的抗营养因子导致[1]SBM组的生长性能降低。
本试验中,添加0.004%壳寡糖后对花鲈的生长指标、免疫指标均未产生显著影响。这与前人的研究结果不同。Lin等[32]研究表明,500 mg/kg壳寡糖能够显著提高锦鲤的生长性能,2或4 g/kg壳寡糖能够显著提高卵形鲳鲹(Trachinotus ovatus)的生长、免疫反应及对细菌的抵抗力[33]。Luo等[5]和蔡雪峰等[34]均证明40 mg/kg壳寡糖能够提高虹鳟的免疫反应及细菌攻毒后的存活率。尽管有效剂量不同,但是在这些研究中,壳寡糖都显现出了改善免疫性能的作用。本试验得到不同的结果可能是试验动物不同、剂量不同所致。
百泰A是一种益生元,主要的活性成分是半乳寡糖。Buentello等[10]在以豆粕为基础的饲料中添加1%百泰A,投喂4周后,美国红鱼(Sciaenops ocellatus)的饲料系数显著降低。Li等[8]也发现2%百泰A能够显著提高杂交条纹鲈12周后的增重率。生长性能的提高可能主要与百泰A提高了水产动物对蛋白质、有机物和能量的消化率有关[7]。但是本实验室前期的研究结果表明饲料中添加0.2%、0.4%、0.8%、2.0%的百泰A对花鲈的生长性能均无显著影响[9],与本试验(饲料中添加1.000%的百泰A)结果相同。百泰A对生长性能的作用结论不一致的主要原因是试验动物、条件及时间不同。百泰A的另一个重要作用是提高免疫力和细菌攻毒后的存活率。与生长性能不同,关于百泰A对免疫性能的作用研究结果相对一致。Buentello等[10]在美国红鱼的饲料中添加1%百泰A,投喂4周后,显著提高了卵圆鞭毛虫(Amyloodinium ocellatum)攻毒后的存活率。Li等[8]研究表明2%的百泰A能够显著提高杂交条纹鲈攻毒后12、16、21周的存活率。Burr等[7]也证明1%的百泰A能够显著提高美国红鱼攻毒后的存活率。本试验中,GA组细菌攻毒后7 d的存活率为60%,高于SBM组的40%,再次证明了百泰A能够提高水产动物的抗病力。
在水产动物上,关于酵母细胞壁及其组分的研究主要集中在生长、免疫及肠道健康上。β-葡聚糖广泛分布于真菌、细菌和植物中,是目前研究最为广泛的免疫促进剂之一。关于β-葡聚糖对水产动物生长和免疫的研究结果不尽相同。有研究认为酵母细胞壁既能提高生长性能也能提高抗病力[35];而另有研究认为酵母细胞壁能够提高抗病力,但是对生长性能没有改善作用[36],甚至会降低生长性能[37]。本试验中,0.050%的酵母细胞壁对花鲈的生长性能无显著影响,但是提高了细菌攻毒7 d后的存活率(85%)。研究结果的不同主要归因于动物种类、投喂剂量及投喂时间的不同。目前,关于甘露聚糖的研究较少,研究的热点主要集中在甘露寡糖(mannan oligosaccharide,MOS)。甘露寡糖作为一种益生元,不仅能提高水产动物的免疫性能[38],还能够改善肠道形态[39],调节肠道菌群[40]。
牛磺酸是一种含硫的非蛋白质氨基酸,在体内主要以游离状态存在,具有多种生物功能[41]。对于一些肉食性鱼类来说,在高植物蛋白质饲料中,牛磺酸是条件性必需氨基酸[16]。研究表明,在全植物蛋白质饲料中添加0.5%牛磺酸能够提高虹鳟[42]、真鲷[43]的生长性能。Kim等[44]也发现,在去除鱼粉中的牛磺酸后,添加0.5%牛磺酸能够显著提高牙鲆的生长性能。然而,在本试验中,饲料中添加0.500%牛磺酸并未对花鲈的生长性能产生显著影响,可能是因为不同鱼种合成牛磺酸的能力不同或是因为本试验中并不是用植物蛋白质替代全部鱼粉,25%鱼粉中含有的牛磺酸能够满足花鲈的需求。虽然本试验中0.500%牛磺酸未能显著提高花鲈的生长性能,但是无论在攻毒前还是攻毒后,Tau组的免疫指标均处于相对最高水平,且攻毒后7 d的存活率为65%,高于SBM组的40%,说明在相对低鱼粉(25%)饲料中添加0.500%牛磺酸仍然能够显著提高花鲈的免疫力和细菌攻毒后的存活率。牛磺酸是白细胞胞质中含量最丰富的游离氨基酸,能够与白细胞中产生的过多次卤酸反应生成毒性较弱的牛磺酸氯胺(taurine chloramine,Tau-Cl)),从而达到保护白细胞的作用。此外,生成的Tau-Cl还具有抗炎和抗菌特性[45,46],而且能够非常有力的调节免疫系统,在人类和啮齿动物中,Tau-Cl能够下调白细胞中多种促炎介质的产生,还能够通过氧化核转录因子抑制蛋白来抑制炎性细胞因子的信号转换器(核转录因子)的激活[17]。
在本试验中,黄霉素的添加并未提高花鲈的生长性能,尽管攻毒前花鲈的免疫指标处于高水平,但是攻毒2 d后,Fla组花鲈全部死亡,说明黄霉素不仅没能真正提高花鲈的免疫力,还对花鲈的抗病力产生了负面影响。 4 结 论
① 豆粕替代13.500%的鱼粉后,各组花鲈摄食率没有差异,但生长性能显著下降。
② 试验所选几种免疫调节剂的添加对花鲈的生长性能均无显著影响,但是百泰A、酵母细胞壁、牛磺酸显著提高了花鲈的免疫力和对细菌感染的抵抗力。
③ 黄霉素的添加对花鲈的生长无促进作用,且对花鲈的抗病力产生了负面影响。
| [1] | GATLIN D M, BARROWS F T, BROWN P, et al.Expanding the utilization of sustainable plant products in aquafeeds:a review[J]. Aquaculture Research, 2007, 38(6):551-579. ( 2) 2)
|
| [2] | HARDY R W.Utilization of plant proteins in fish diets:effects of global demand and supplies of fishmeal[J]. Aquaculture Research, 2010, 41(5):770-776. ( 1) 1)
|
| [3] | HU L, YUN B, XUE M, et al.Effects of fishmeal quality and fishmeal substitution by animal protein blend on growth performance, flesh quality and liver histology of Japanese seabass (Lateolabrax japonicus)[J]. Aquaculture, 2013, 372/373/374/375:52-61. ( 3) 3)
|
| [4] | WANG J, YUN B, XUE M, et al.Apparent digestibility coefficients of several protein sources, and replacement of fishmeal by porcine meal in diets of Japanese seabass, Lateolabrax japonicus, are affected by dietary protein levels[J]. Aquaculture Research, 2012, 43(1):117-127. ( 1) 1)
|
| [5] | LUO L, CAI X F, HE C, et al.Immune response, stress resistance and bacterial challenge in juvenile rainbow trouts Oncorhynchus mykiss fed diets containing chitosan-oligosaccharides[J]. Current Zoology, 2009, 55(6):1-14. ( 3) 3)
|
| [6] | GIBSON G R, ROBERFROID M B.Dietary modulation of the human colonie microbiota:introducing the concept of prebiotics[J]. The Journal of Nutrition, 1995, 125:1401-1412. ( 1) 1)
|
| [7] | BURR G, HUME M, NEILL W H, et al.Effects of prebiotics on nutrient digestibility of a soybean-meal-based diet by red drum Sciaenops ocellatus (Linnaeus)[J]. Aquaculture Research, 2008, 39(15):1680-1686. ( 3) 3)
|
| [8] | LI P, GATLIN Ⅲ D M.Evaluation of the prebiotic GroBiotic-A and brewer's yeast as dietary supplements for sub-adult hybrid striped bass (Morone chrysops×M.saxatilis) challenged in situ with Mycobacterium marinum[J]. Aquaculture, 2005, 248(1/2/3/4):197-205. ( 4) 4)
|
| [9] | 葛红云.饲料中半乳寡糖和棉子糖对花鲈生长、免疫以及肠道菌群的影响[D]. 硕士学位论文.北京:中国农业科学院, 2010:8-14. ( 2) 2)
|
| [10] | BUENTELLO J A, NEILL W H, GATLIN Ⅲ D M.Effects of dietary prebiotics on the growth, feed efficiency and non-specific immunity of juvenile red drum Sciaenops ocellatus fed soybean-based diets[J]. Aquaculture Research, 2010, 41(3):411-418. ( 3) 3)
|
| [11] | HERRE J, GORDON S, BROWN G D.Dectin-1 and its role in the recognition of beta-glucans by macrophages[J]. Molecular Immunology, 2004, 40(12):869-876. ( 1) 1)
|
| [12] | ROBERTSEN B.Modulation of the non-specific defence of fish by structurally conserved microbial polymers[J]. Fish & Shellfish Immunology, 1999, 9(4):269-290. ( 1) 1)
|
| [13] | KRIŽKOVÁ L, ĎURAČKOVÁ Z, ŠANDULA J, et al.Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro[J]. Mutation Research:Genetic Toxicology and Environmental Mutagenesis, 2001, 497(1):213-222. ( 1) 1)
|
| [14] | MIADOKOVÁ E, SVIDOVÁ S, VLĎKOVÁ V, et al.Diverse biomodulatory effects of glucomannan from Candida utilis[J]. Toxicology in vitro, 2006, 20(5):649-657. ( 1) 1)
|
| [15] | YU H H, HAN F, XUE M, et al.Efficacy and tolerance of yeast cell wall as an immunostimulant in the diet of Japanese seabass (Lateolabrax japonicus)[J]. Aquaculture, 2014, 432:217-224. ( 1) 1)
|
| [16] | TAKEUCHI T.A review of feed development for early life stages of marine finfish in Japan[J]. Aquaculture, 2001, 200(1):203-222. ( 2) 2)
|
| [17] | SCHULLER-LEVIS G B, PARK E.Taurine and its chloramine:modulators of immunity[J]. Neurochemical Research, 2004, 29(1):117-126. ( 2) 2)
|
| [18] | QI G S, AI Q H, MAI K S, et al.Effects of dietary taurine supplementation to a casein-based diet on growth performance and taurine distribution in two sizes of juvenile turbot (Scophthalmus maximus L.)[J]. Aquaculture, 2012, 358:122-128. ( 2) 2)
|
| [19] | 蔡春芳, 宋学宏.几种抗病促生长剂对银孵生长和免疫力的影响[J]. 水利渔业, 2002, 22(2):20-22. ( 1) 1)
|
| [20] | 刘立鹤, 刘辉宇, 董爱华, 等.饲料中添加黄霉素对凡纳滨对虾生长和非特异性免疫力的影响[J]. 淡水渔业, 2006, 36(6):25-28. ( 1) 1)
|
| [21] | MCPHEARSON R M, DEPAOLA A, ZYWNO S R, et al.Antibiotic resistance in Gram-negative bacteria from cultured catfish and aquaculture ponds[J]. Aquaculture, 1991, 99(3):203-211. ( 1) 1)
|
| [22] | 罗莉, 梁金权, 唐显虹, 等.国产与进口黄霉素对草鱼生产性能影响的比较[J]. 饲料博览, 2005(6):43-45. ( 1) 1)
|
| [23] | QUADE M J, ROTH J A.A rapid, direct assay to measure degranulation of bovine neutrophil primary granules[J]. Veterinary Immunology and Immunopathology, 1997, 58(3):239-248. ( 1) 1)
|
| [24] | ANDERSON D P, SIWICKI A K.Basic haematology and serology for fish health programs[J]. Diseases in Asian Aquaculture Ⅱ, 1995, 2:185-202. ( 1) 1)
|
| [25] | HILL H A, TRUSHENSKI J T, KOHLER C C.Utilization of soluble canola protein concentrate as an attractant enhances production performance of sunshine bass fed reduced fish meal, plant-based diets[J]. Journal of the World Aquaculture Society, 2013, 44(1):124-132. ( 1) 1)
|
| [26] | BLAUFUSS P, TRUSHENSKI J.Exploring soy-derived alternatives to fish meal:using soy protein concentrate and soy protein isolate in hybrid striped bass feeds[J]. North American Journal of Aquaculture, 2012, 74(1):8-19. ( 1) 1)
|
| [27] | 张志勇, 薛敏, 王嘉, 等.混合植物蛋白质替代鱼粉对花鲈和西伯利亚鲟生长和肉质影响的比较研究[J]. 动物营养学报, 2013, 25(6):1260-1275. ( 2) 2)
|
| [28] | REFSTIE S, HELLAND S J, STOREBAKKEN T.Adaptation to soybean meal in diets for rainbow trout, Oncorhynchus mykiss[J]. Aquaculture, 1997, 153(3):263-272. ( 1) 1)
|
| [29] | XUE M, XIE S Q, CUI Y B.Effect of a feeding stimulant on feeding adaptation of gibel carp Carassius auratus gibelio (Bloch), fed diets with replacement of fish meal by meat and bone meal[J]. Aquaculture Research, 2004, 35(5):473-482. ( 1) 1)
|
| [30] | ABTAHI B, YOUSEFI M, KENARI A A.Influence of dietary nucleotides supplementation on growth, body composition and fatty acid profile of Beluga sturgeon juveniles (Huso huso)[J]. Aquaculture Research, 2013, 44(2):254-260. ( 1) 1)
|
| [31] | MCGOOGAN B B, GATLIN D M.Effects of replacing fish meal with soybean meal in diets for red drum Sciaenops ocellatus and potential for palatability enhancement[J]. Journal of the World Aquaculture Society, 1997, 28(4):374-385. ( 1) 1)
|
| [32] | LIN S M, MAO S H, GUAN Y, et al.Effects of dietary chitosan oligosaccharides and Bacillus coagulans on the growth, innate immunity and resistance of koi (Cyprinus carpio koi)[J]. Aquaculture, 2012, 342:36-41. ( 1) 1)
|
| [33] | LIN S M, MAO S H, GUAN Y, et al.Dietary administration of chitooligosaccharides to enhance growth, innate immune response and disease resistance of Trachinotus ovatu[J]. Fish & Shellfish Immunology, 2012, 32(5):909-913. ( 1) 1)
|
| [34] | 蔡雪峰, 罗琳, 战文斌, 等.壳寡糖对虹鳟幼鱼肠道菌群影响的研究[J]. 中国海洋大学学报:自然科学版, 2006, 36(4):606-610. ( 1) 1)
|
| [35] | MISRA C K, DAS B K, MUKHERJEE S C, et al.Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings[J]. Aquaculture, 2006, 255(1):82-94. ( 1) 1)
|
| [36] | AI Q H, MAI K S, ZHANG L, et al.Effects of dietary β-1, 3 glucan on innate immune response of large yellow croaker, Pseudosciaena crocea[J]. Fish & Shellfish Immunology, 2007, 22(4):394-402. ( 1) 1)
|
| [37] | SEALEY W M, BARROWS F T, HANG A, et al.Evaluation of the ability of barley genotypes containing different amounts of β-glucan to alter growth and disease resistance of rainbow trout Oncorhynchus mykiss[J]. Animal Feed Science and Technology, 2008, 141(1):115-128. ( 1) 1)
|
| [38] | LIU B, XU L, GE X P, et al.Effects of mannan oligosaccharide on the physiological responses, HSP70 gene expression and disease resistance of allogynogenetic crucian carp (Carassius auratus gibelio) under Aeromonas hydrophila infection[J]. Fish & Shellfish Immunology, 2013, 34(6):1395-1403. ( 1) 1)
|
| [39] | TORRECILLAS S, MAKOL A, BETANCOR M B, et al.Enhanced intestinal epithelial barrier health status on European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides[J]. Fish & Shellfish Immunology, 2013, 34(6):1485-1495. ( 1) 1)
|
| [40] | DIMITROGLOU A, MERRIFIELD D L, SPRING P, et al.Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata)[J]. Aquaculture, 2010, 300(1):182-188. ( 1) 1)
|
| [41] | STEPHEN W S, CHIAN J J, RAMIL A K, et al.Physiological roles of taurine in heart and muscle[J]. Biomedical Science, 2010, 17(Suppl.1):2. ( 1) 1)
|
| [42] | GAYLORD T G, TEAGUE A M, BARROWS F T.Taurine supplementation of all-plant protein diets for rainbow trout (Oncorhynchus mykiss)[J]. Journal of the World Aquaculture Society, 2006, 37(4):509-517. ( 1) 1)
|
| [43] | TAKAGI S, MURATA H, GOTO T, et al.Necessity of dietary taurine supplementation for preventing green liver symptom and improving growth performance in yearling red sea bream Pagrus major fed nonfishmeal diets based on soy protein concentrate[J]. Fisheries Science, 2010, 76(1):119-130. ( 1) 1)
|
| [44] | KIM S K, MATSUNARI H, TAKEUCHI T, et al.Effect of different dietary taurine levels on the conjugated bile acid composition and growth performance of juvenile and fingerling Japanese flounder Paralichthys olivaceus[J]. Aquaculture, 2007, 273(4):595-601. ( 1) 1)
|
| [45] | MARCINKIEWICZ J.Taurine bromamine:a new therapeutic option in inflammatory skin diseases[J]. Polish Archives of Internal Medicine, 2009, 119(10):673-676. ( 1) 1)
|
| [46] | NAGL M, HESS M W, PFALLER K, et al.Bactericidal activity of micromolarn-chlorotaurine:evidence for its antimicrobial function in the human defense system[J]. Antimicrobial Agents and Chemotherapy, 2000, 44(9):2507-2513. ( 1) 1)
|




