2. 动物抗病营养教育部重点实验室, 雅安 625014;
3. 通威股份有限公司, 成都 610000;
4. 广西商大科技有限公司, 南宁 530021
2. Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Ya'an 625014, China;
3. TongweiCo., Ltd., Chengdu 610000, China;
4. Guangxi Shangda Technology Co., Ltd., Nanning 530021, China
母猪的繁殖活动是一项具有典型周期循环的系统工程,适时启动发情周期是这项工程的枢纽。据报道,规模化猪场后备母猪不发情或发情推迟的比例达20%~30%[1]。同时,因情期启动失败导致经产母猪淘汰的比例高达30%~40%,50%的母猪仅提供30~40头断奶仔猪就被淘汰[2, 3, 4]。雌性动物的情期启动受复杂的神经-内分泌系统调控,情期启动体现在3个层面[5]:1)下丘脑促性腺激素释放激素(gonadotropin-releasing hormone,GnRH)神经元脉冲分泌;2)垂体腺在GnRH刺激下脉冲分泌促卵泡素(follicle-stimulating hormone,FSH)和促黄体素(luteinizing hormone,LH);3)性腺轴接收来自FSH和LH的脉冲信号,刺激排卵,由卵巢分泌的性腺激素(雌二醇)对下丘脑GnRH神经元进行正、负反馈调控。作为动物情期启动的关键因素,GnRH的分泌调控是深入揭示动物情期启动奥秘的关键组成部分。营养是动物繁殖活动的物质基础,但营养代谢信号并不直接作用于GnRH神经元,这表明下丘脑存在介导营养调控GnRH分泌的中间信号途径。Kisspeptin(最初名为metastin)是由Kiss-1基因编码的神经内分泌肽类激素,由Kisspeptin神经元分泌,为G蛋白偶联受体54(GPR54)的内源性配体[9]。大量研究揭示,由下丘脑Kiss-1基因编码的蛋白质Kisspeptin,与其受体GRP54结合启动的信号途径是下丘脑GnRH脉冲分泌的关键信号[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33]。下丘脑Kisspeptin神经元表达营养代谢信号如胰岛素(insulin)、瘦素(leptin)、胰岛素样生长因子-1(insulin like growth factor-1,IGF-1)的受体[28],表明Kisspeptin神经元是营养调控动物情期启动及卵泡发育的关键组成部分。
营养调控母猪情期启动主要有2种代谢途径:1)营养改变激素分泌(如瘦素、胰岛素、胰岛素样生长因子),调控情期启动;2)营养改变血液代谢底物(metabolites)浓度,参与情期启动调控。因此,本文重点讨论营养调控母猪情期启动的理论假设及分子机制。
1 营养调控后备母猪情期启动的主要理论假设总结前人研究,营养调控后备母猪情期启动主要存在2种理论假设。
理论假设一:组织器官发育理论假说。母猪各组织和器官发育到一定阈值后才能启动情期(图1)。该理论认为母猪体重和体组成是营养累 积的综合效应,后备母猪在达到最低阈值的体重 或者体组成之后才能启动情期。“最低脂肪假说”提出雌性动物只有沉积一定比例的脂肪才会进入青春期[34]。研究发现,瘦肉组织沉积对后备母猪情期启动亦非常重要[35, 36, 37]。Oury等[38]证实骨骼处于合成代谢时性腺才能正常发育。依据此理论假设,下丘脑存在响应外周组织发育信号的组织和细胞,当机体组织的生长和发育达到一定标准后,触发下丘脑中Kisspeptin表达和GnRH、LH脉冲分泌,动物由生长转向繁殖。
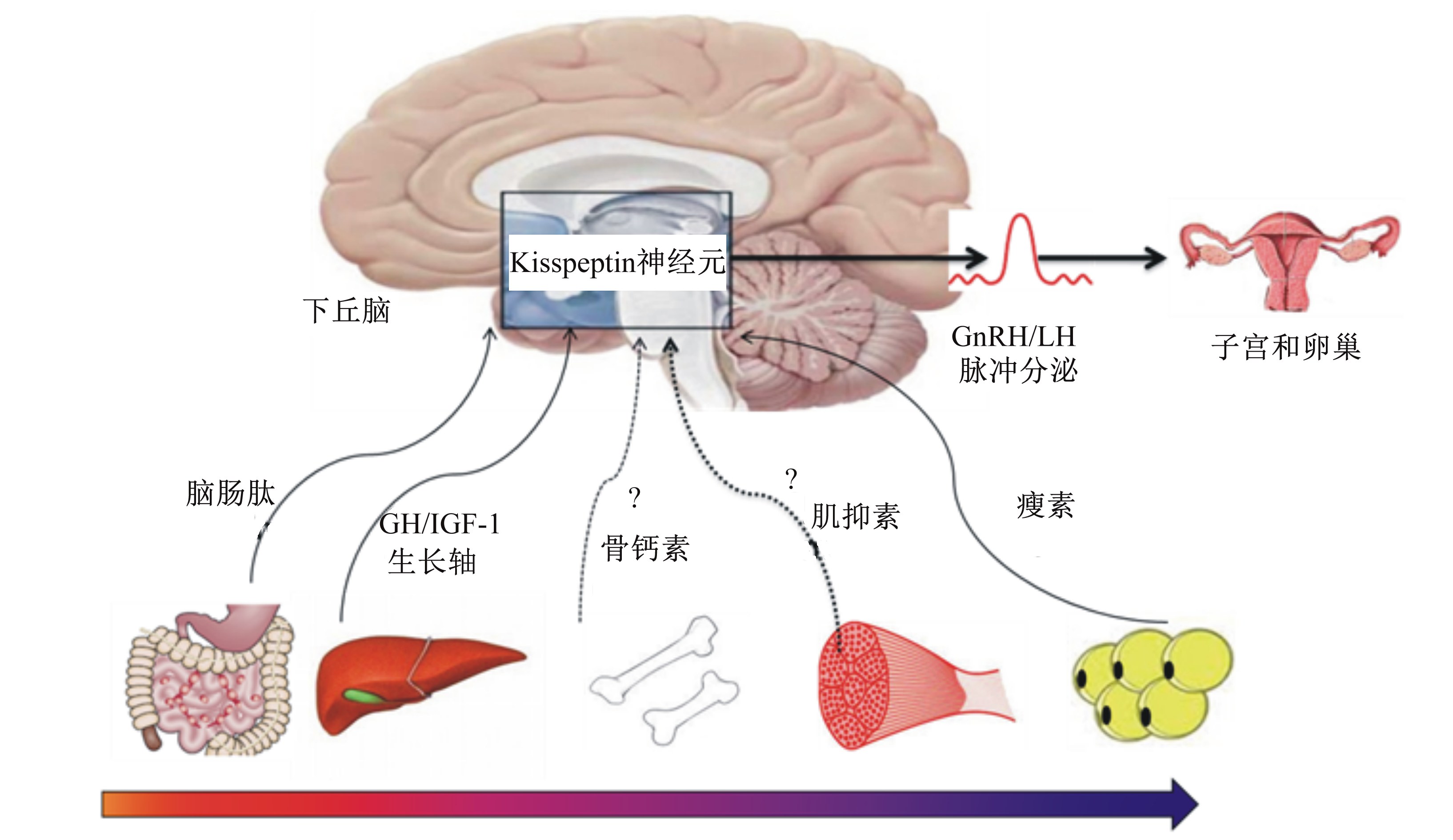 | GH:生长激素 growth hormone;IGF-1:胰岛素样生长因子-1 insulin like growth factor-1;GnRH:促性腺激素释放激素 gonadotropin-releasing hormone;LH:促黄体素 luteinizing hormone。
外周组织(如消化道、骨骼、肌肉、脂肪、肝脏等)发育成熟信号传递至下丘脑Kisspeptin神经元,触发GnRH分泌及性成熟,动物由生长转向繁殖。 Developmental maturation signals originating from peripheral tissues (e.g., gastrointestinal tract, bone, muscle, adipocytes and liver, et al) can be sensed by hypothalamic Kisspeptin neurone, and then triggers GnRH secretion and sexual maturation, which promotes the switch from growth to reproduction. 图1 组织器官发育与情期启动 Fig. 1 Tissue organ development and estrus onset |
理论假设二:雌激素回馈理论(gonadostat hypothesis)(图2)。生长期体组织尚未发育完善时,雌激素主要通过下丘脑弓状核(arcuate nucleus,ARC)区域Kisspeptin神经元产生负反馈效应,抑制性腺发育,防止早熟;当体组织发育完善时,雌激素通过下丘脑前腹侧室旁核(anteroventral periventricular nucleus,AVPV)Kisspeptin神经元产生正反馈效应,动物启动情期,加速卵泡发育[39],雌激素回馈理论是确保动物体成熟和性成熟同步性的关键。下丘脑ARC是营养代谢信号作用靶点,生长期动物血液中营养代谢因子刺激ARC区域Kisspeptin的分泌,以保证对雌激素的负反馈抑制;当动物体组织逐渐发育完善,营养储备足够时,雌激素负反馈抑制减弱,正反馈作用加强,动物性腺加速发育并启动情期。
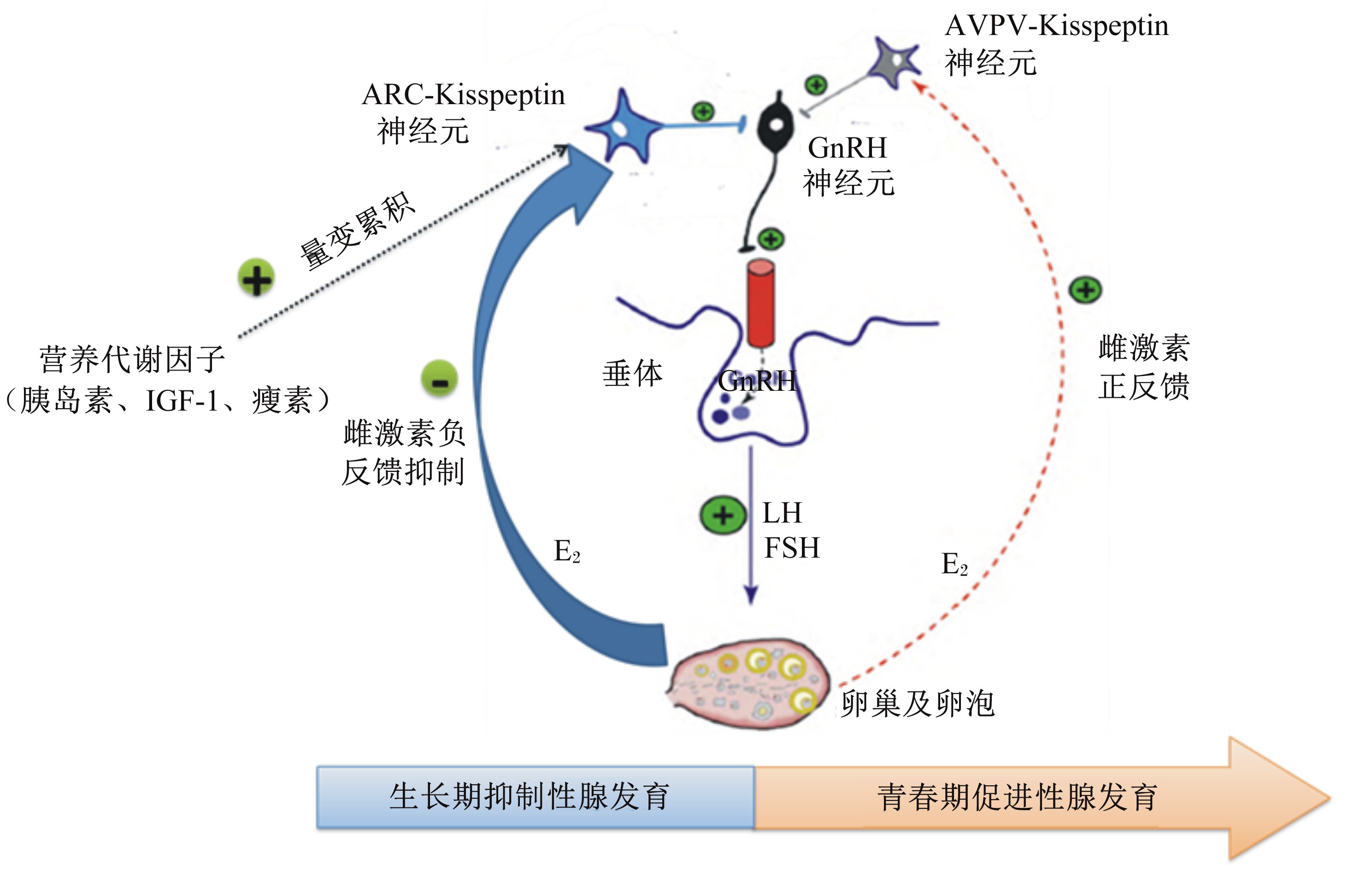 | ARC:弓状核 arcuate nucleus;AVPV:前腹侧室旁核 anteroventral periventricular nucleus;IGF-1:胰岛素样生长因子-1 insulin like growth factor-1;GnRH:促性腺激素释放激素 gonadotropin-releasing hormone;LH:促黄体素 luteinizing hormone;FSH:促卵泡素 follicle-stimulating hormone;E-2:雌二醇 estradiol。 生长期营养累积效应刺激ARC区域Kisspeptin神经元,雌激素通过ARC区域Kisspeptin神经元负反馈抑制性腺发育,促进体组织发育。青春期来临时,下丘脑AVPV的Kisspeptin神经元介导雌激素正反馈效应,促进性腺成熟。 Nutrition accumulation influence can stimulate the activation of Kisspeptin neurons. Kisspeptin neurons in ARC mediate the negative feedback inhibition of estrogen action on gonadal maturation to allow sufficient peripheral body tissue development. Kisspeptin neurons in AVPV during pubertal phase, on the contrary, mediate the positive feedback acceleration of estrogen action on gonadal maturation. 图2 雌激素回馈理论与情期启动 Fig. 2 Estrogen feedback hypothesis and estrus onset |
能量负平衡导致后备母猪发情推迟甚至乏情。Zhou等[40]将已有2个正常发情周期的长大二杂后备母猪分别饲喂1.00和2.86 kg/d饲粮,连续限饲4个发情周期后,母猪出现营养性乏情。下丘脑ARC和AVPV区域上Kiss-1、GPR54和GnRH mRNA表达量,以及垂体和卵巢上IGF-1R、FSH和LH mRNA表达量均发生了显著变化,表明Kisspeptin/GPR54信号系统参与营养调控后备母猪情期启动。Castellano等[41]发现禁食小鼠的情期启动严重紊乱,血液中GnRH和LH等繁殖激素的水平下降,对小鼠中枢灌注Kisspeptin后,小鼠血液中GnRH和LH的水平显著提高,并重新表现正常情期循环。繁殖系统处于养分分配末端,营养不足时养分优先用于维持需要,并抑制繁殖活动。Owen等[42]研究了营养缺乏情况下繁殖活动受到抑制的分子机理,发现成纤维细胞生长因子21(fibroblast growth factor 21,FGF21)是营养不足情况下肝脏分泌的关键信号,营养不足时肝脏分泌的FGF21作用于视交叉上核(suprachiasmatic nucleus,SCN)神经元,抑制Kisspeptin分泌,推迟雌性动物的情期启动及卵泡发育。同时,FGF21增加外周组织如骨骼肌、脂肪组织的胰岛素敏感性,有利于机体在营养不足情况下优先保证生存需要[43]。
母猪泌乳期情期循环终止,卵泡发育受到抑制,有2个方面的原因(图3):1)Kisspeptin神经元存在催乳素受体(PRL-R),泌乳期高浓度催乳素通过其受体抑制Kisspeptin分泌[44],抑制下丘脑-垂体-性腺轴活性;2)泌乳母猪采食量低,泌乳量大,机体处于分解代谢,血液中瘦素、胰岛素样生长因子-1浓度降低,情期启动和卵泡发育受阻。泌乳母猪能量负平衡导致实际生产中断奶母猪不发情、受胎率低,增加母猪的淘汰率。有学者将泌乳期血液瘦素、胰岛素等浓度恢复至正常生理水平,但是并未发现下丘脑Kiss-1基因表达量及卵泡发育恢复[45, 46]。上述结果表明泌乳期乏情是一 个复杂且多因素综合作用的结果,只有动物机体组织恢复到“标准”体况后动物的情期启动才能恢复。目前有关营养对泌乳母猪代谢状态、雌激素正负反馈途径、断奶-发情分子机理的研究较少,待进一步探索。
 | NEFA:非酯化脂肪酸 nonesterified fatty acids;AA:氨基酸 amino acids;mTOR:哺乳动物雷帕霉素靶蛋白 mammalian target of rapamycin;ERα:雌激素受体α estrogen receptor α;CRTC-1:环AMP响应元件结合蛋白-1转录调控因子 cyclic AMP responsive element-binding protein-1 (Creb1)-regulated transcription coactivator-1;FGF21:成纤维细胞生长因子21 fibroblast growth factor 21;IGF-1:胰岛素样生长因子-1 insulin like growth factor-1;IGF-1R:胰岛素样生长因子-1受体insulin-like growth factor-1 receptor;SCN:视交叉上核 suprachiasmatic nucleus;GnRH:促性腺激素释放激素 gonadotropin-releasing hormone;LH:促黄体素 luteinizing hormone;FSH:促卵泡素 follicle-stimulating hormone。 肝脏解读能量负平衡时血液代谢底物(如非酯化脂肪酸和氨基酸组成)等变化,调整内分泌因子释放,抑制Kisspeptin神经元活性及下丘脑-垂体-性腺轴活性。 Liver can sense the negative energy balance through changes in blood metabolites (e.g., nonesterified fatty acid and amino acid composition), and adjusts the hormone secretions to inhibit the activaties of Kisspeptin neurons and the hypothalamus-pituitary-gonadal axis. 图3 泌乳或营养限制导致母猪生理性乏情机制 Fig. 3 Mechanisms of the phvsiological anestrus of sows in lactation or nutrient restriction |
动物机体发展了一套精准的适应性机制,让下丘脑能够准确地感知外周组织的营养代谢状态。能量负平衡时,外周代谢信号能够快速、准确地传递至下丘脑,养分分配转向生存需要,繁殖轴活性抑制,说明下丘脑存在一套能量负平衡响应机制感知并调控机体的繁殖活动(图4)。Roland等[47]研究表明,GnRH神经元细胞中的能量感受器腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)能够感知细胞内葡萄糖浓度,低葡萄糖浓度通过AMPK途径抑制GnRH分泌。Zhang等[48]发现下丘脑ATP敏感型钾离子通道可能参与机体能量负平衡对LH浓度的调控。哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)是机体广泛表达的一种蛋白质,通过中枢雷帕霉素处理抑制mTOR活性,下丘脑ARC区域的Kiss-1基因表达量受到显著抑制,卵巢和子宫萎缩,小鼠的初情日龄显著推迟[49]。Altarejos等[50]通过基因敲除模型,特异性敲除下丘脑环AMP响应元件结合蛋白-1转录调控因子[cyclic AMP responsive element-binding protein-1 (Creb1)-regulated transcription coactivator-1,CRTC-1],阻断下丘脑对外周代谢状态响应通路,发现小鼠表现出肥胖且不育。 进一步研究证实,CRTC-1对繁殖活动的调控主要依赖Kisspeptin信号途径,当瘦素处理增加Kiss-1基因表达量的同时,CRTC-1与Kiss-1基因启动子区域出现非常紧密的结合[50]。
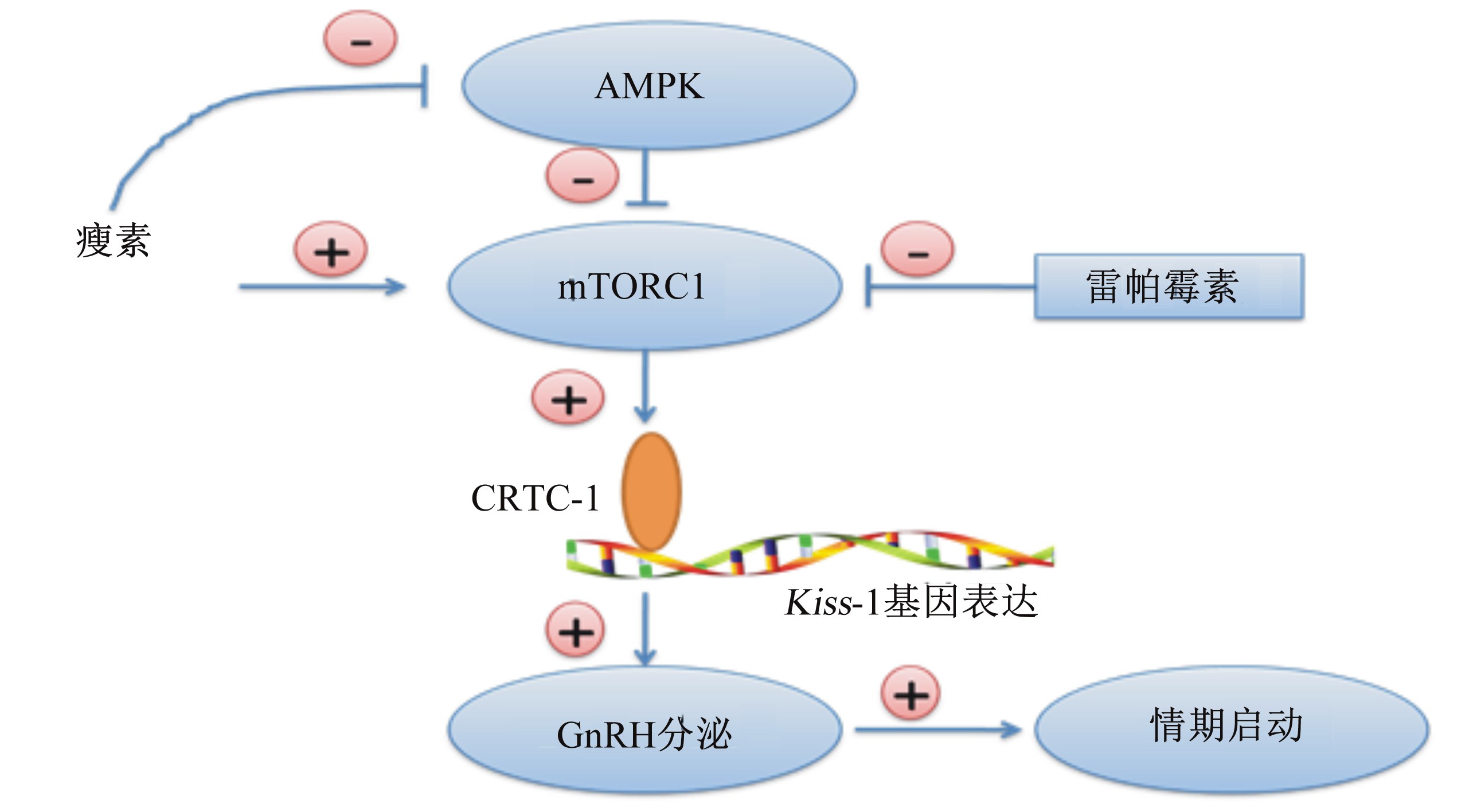 | AMPK:腺苷酸活化蛋白激酶 AMP-activated protein kinase;mTORC1:哺乳动物雷帕霉素靶蛋白复合体1 mammalian target of rapamycin complex 1;CRTC-1:环AMP响应元件结合蛋白-1转录调控因子 cyclic AMP responsive element-binding protein-1 (Creb1)-regulated transcription coactivator-1;GnRH:促性腺激素释放激素gonadotropin-releasing hormone。 下丘脑存在解读外周能量代谢的分子机制,维持或抑制繁殖轴活性。 Molecular mechanisms exists in hypothalamus could sense the energy balance to maintain or inhibit the activities of reproductive axis. 图4 下丘脑能量响应机制对动物情期启动的影响 Fig. 4 Influence of hypothalamic energy sensing mechanism on estrus onset |
2.1.2 能量正平衡
本课题组研究表明,后备母猪饲粮中添加脂肪,可提高血液中瘦素浓度,增强下丘脑ARC区域瘦素信号途径,后备母猪的初情日龄提前[51]。瘦素是反映能量代谢的关键代谢信号,对母猪的繁殖轴存在非常广泛的影响[52]。体外培养大鼠的ARC神经元,培养基中添加生理水平瘦素可通过蛋白酪氨酸激酶2(janus kinase 2,JAK2)/磷酸化信号转导子与激活子3(signal transducer and activator of transcription 3,STAT3)信号途径促进Kisspeptin表达,诱导GnRH的体外分泌[53]。随着瘦素对Kisspeptin信号途径影响研究的深入,发现瘦素可直接作用于Kiss-1基因促进Kisspeptin表达[54]。
过度饲喂或肥胖对会动物繁殖活动产生负面影响。本课题组体外培养下丘脑ARC神经元后发现(图5),培养基中瘦素浓度过高时会抑制Kiss-1基因的表达丰度。高脂饲粮诱导DBA/2J小鼠肥胖[55],下丘脑ARC神经元Kiss-1的基因表达量显著下调,且小鼠表现出不育。目前有关肥胖对动物情期启动的分子机理的报道较少,肥胖者血液中FGF21浓度显著提高,因此可能通过FGF21抑制下丘脑Kisspeptin表达抑制动物的下丘脑-垂体-性腺轴活性,但该假设尚待进一步证实。
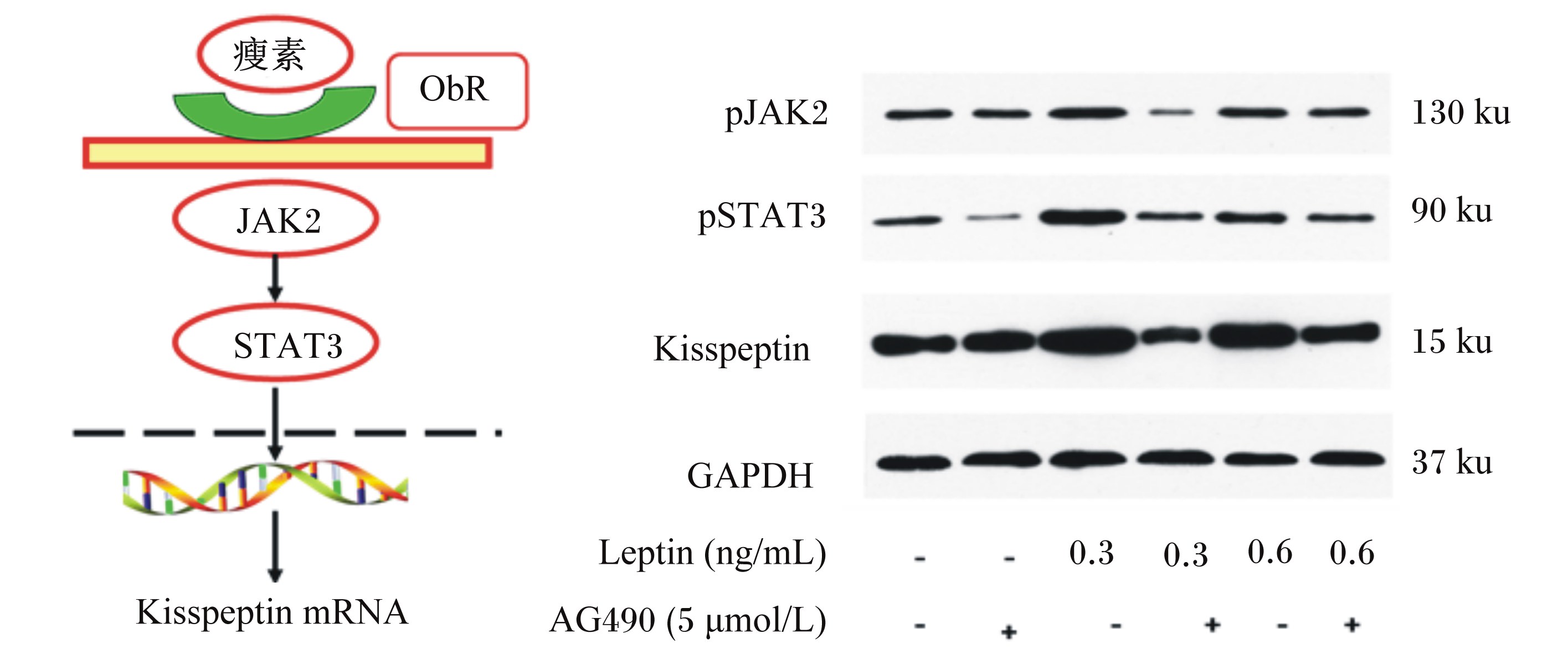 | ObR:瘦素受体 leptin receptor;JAK2:蛋白酪氨酸激酶2 janus kinase 2;STAT3:磷酸化信号转导子与激活
子3 signal transducer and activator of transcription 3;pJAK2:磷酸化蛋白酪氨酸激酶2 phosphorylated janus kinase 2;pSTAT3:磷酸化信号转导子与激活子3 phosphorylated signal transducer and activator of transcription 3;GAPDH:甘油醛-3-磷酸脱氢酶glyceraldehyde phosphate dehydrogenase。 通过添加JAK2的抑制剂AG490,可阻断leptin对Kisspeptin的表达。 AG490, an inhibitor of JAK2, blocks the expression of Kisspeptin stimulated by leptin. 图5 瘦素信号调控下丘脑ARC区域Kisspeptin表达的机制 Fig. 5 Mechanism of Kisspeptin expression in ARC area regulated by leptin signal |
由于遗传选育更趋向于选择瘦肉型猪种,后备母猪蛋白质沉积对情期启动更为重要。后备母猪饲喂10%蛋白质水平组比14%蛋白质水平组的初情日龄推迟18.7 d[56]。研究发现,后备母猪瘦肉沉积量与初情日龄呈显著负相关关系[57]。小鼠限制采食量40%诱导营养性乏情后,分别给予富含碳水化合物、脂肪、蛋白质的饲粮,结果发现饲喂富含蛋白质饲粮的小鼠能更快地恢复情期循环[58]。饲粮中单一氨基酸缺乏同样导致情期启动紊乱,研究发现,某一特定的氨基酸如苏氨酸、赖氨酸、色氨酸、蛋氨酸、缬氨酸缺乏将导致发情周期紊乱[59]。由于氨基酸或者蛋白质缺乏会导致机体蛋白质处于分解代谢,当Kisspeptin神经元感知 这种分解代谢时,繁殖轴活性减低,繁殖活动受到抑制(图6)。
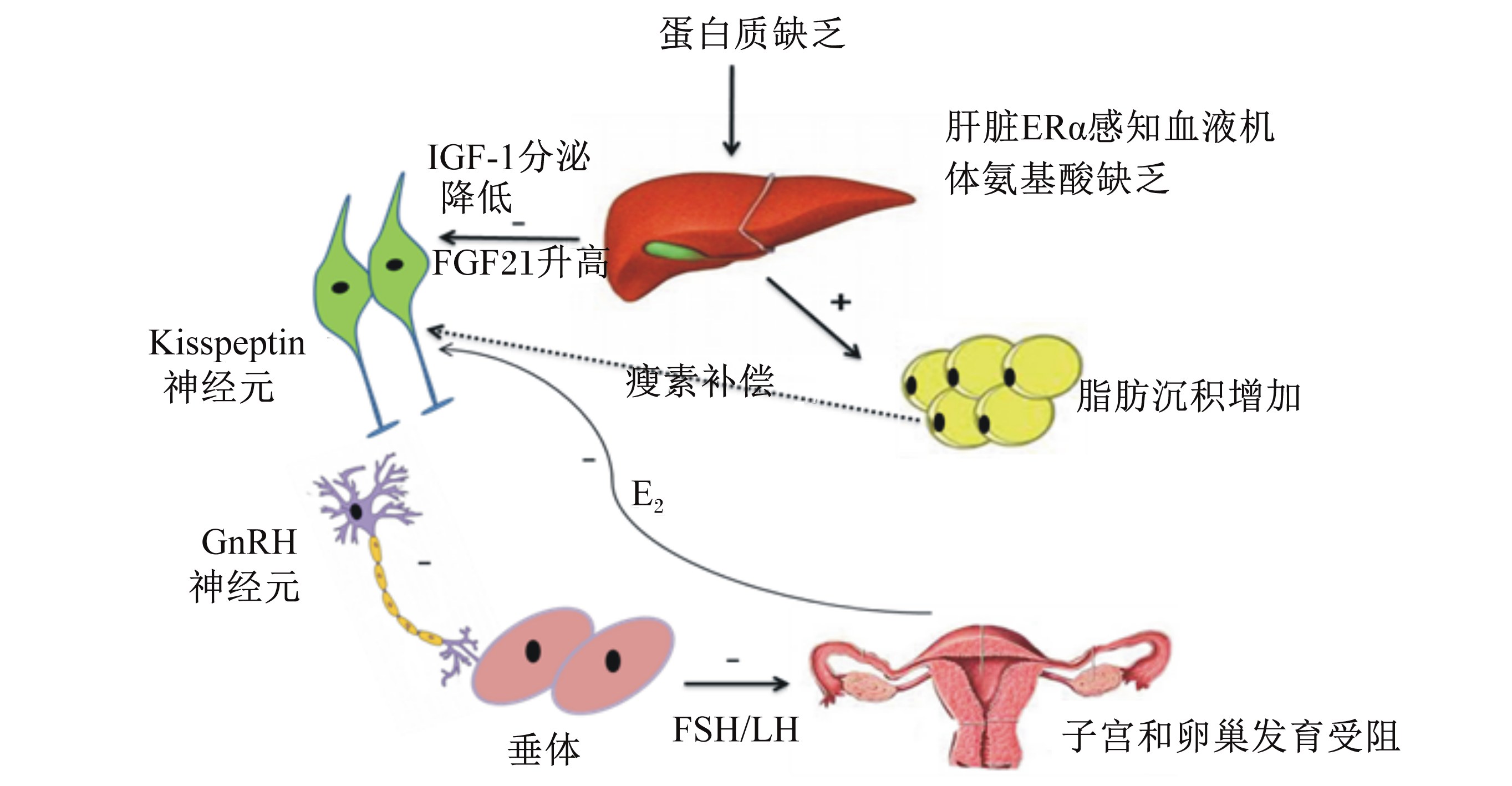 | ERα:雌激素受体α estrogen receptor α;FGF21:成纤维细胞生长因子21 fibroblast growth factor 21;IGF-1:胰岛素样生长因子-1 insulin like growth factor-1;GnRH:促性腺激素释放激素 gonadotropin-releasing hormone;LH:促黄体素 luteinizing hormone;FSH:促卵泡素 follicle-stimulating hormone;E-2:雌二醇 estradiol。 蛋白质或氨基酸缺乏导致脂肪沉积增加,抑制下丘脑Kisspeptin表达及下丘脑-垂体-性腺轴活性。 Protein or amino acid restriction induces lipidosis increase, andinhibits the expression of hypothalamic Kisspeptin and the activation of hypothalamus-pituitary-gonadal axis. 图6 蛋白质限制推迟情期启动的理论假设 Fig. 6 Theory hypothesis of estrus onset delayed by protein restriction |
氨基酸对动物情期启动的影响可能通过肝脏内分泌胰岛素样生长因子-1介导。肝脏是动物机体的首要代谢器官,是感知动物能量平衡和代谢状态的第1道“门”。胰岛素样生长因子-1是肝脏在机体处于合成代谢时分泌的代谢激素,血液循环中的胰岛素样生长因子-1有70%来自肝脏。胰岛素样生长因子-1广泛参与动物的繁殖活动,对卵泡发育、胚胎存活均表现出积极效果。有关胰岛素样生长因子-1对后备母猪情期启动的研究较少,已有的研究结果表明高浓度的胰岛素样生长因子-1与雌性动物的早熟有关[60]。Roongsitthichai等[61]对80头后备母猪按照初情日龄进行划分,发现初情日龄早于200 d的后备母猪血液中胰岛素样生长因子-1浓度高于初情日龄晚于200 d的后备母猪[(30.2±1.2) nmol/L vs. (25.4±1.1) nmol/L,P=0.002]。在牛上的研究表明,初情日龄与18月龄时的血清胰岛素样生长因子-1浓度呈显著负相关关系[62]。Fortes等[63]应用单核苷酸多态性(single nucleotide polymorphism,SNP)技术,发现胰岛素样生长因子-1受体的SNP与动物初情日龄显著相关。
下丘脑胰岛素样生长因子-1信号途径直接参与雌激素正负反馈途径对Kisspeptin神经元的影响。通过中枢和外周灌注胰岛素样生长因子-1,6 h后小鼠AVPV区域的Kiss-1基因的表达量显著提高。当处理胰岛素样生长因子-1受体拮抗剂JB-1之后,胰岛素样生长因子-1对Kisspeptin神经元的激活作用消失,雌激素的正负反馈调节效应中断[64]。胰岛素样生长因子-1的作用主要通过其受体介导的信号途径发挥作用,Todd等[65]和Sun等[66]通过中枢灌注胰岛素样生长因子-1及其受体拮抗剂JB-1,证实了胰岛素样生长因子-1受体是雌激素正反馈作用和GnRH神经元激活的必需组成部分,下丘脑胰岛素样生长因子-1信号途径减弱,则动物的情期循环受到干扰。
2.3 维生素D3实际生产中,光照影响后备母猪的情期启动。光照影响体内维生素D3的代谢,因此维生素D3可能参与调控动物的情期启动。下丘脑-垂体-性腺轴均表达维生素D3受体,已有研究表明维生素D3缺乏动物繁殖能力受损,但是机理不详[67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88]。最新研究证实,维生素D3缺乏导致雌性小鼠初情日龄推迟6 d,此外,维生素D3缺乏导致雌性成年小鼠无法维持正常的情期循环[69]。维生素D3广泛参与下丘脑-垂体-性腺轴的细胞增殖和分化、激素分泌等活动,为了进一步探索维生素D3影响小鼠情期启动的作用靶点,对维生素D3缺乏或Cyp27b1基因缺失小鼠进行外源促性腺激素处理,发现小鼠的垂体及卵巢功能并未受损,证实了下丘脑GnRH分泌抑制是维生素D3影响小鼠情期启动的关键靶点[69]。骨骼代谢与维生素D3密切相关,因此维生素D3可能通过影响骨骼代谢影响动物情期启动。Patterson等[70]观察了431头 后备母猪初情启动与骨骼发育的关系,发现317头180日龄之前启动初情期的后备母猪无腿病发生;180日龄后发情的母猪中,高达16%的后备母猪因发生腿病而被淘汰。骨骼分泌蛋白骨钙素(osteocalcin)已被证明可以调控动物的能量代谢,同时可作用于雄性动物繁殖组织而影响睾丸细胞的功能[39],因此也可能参与雌性动物情期启动的调节,但此领域研究甚少,有待进一步证实。
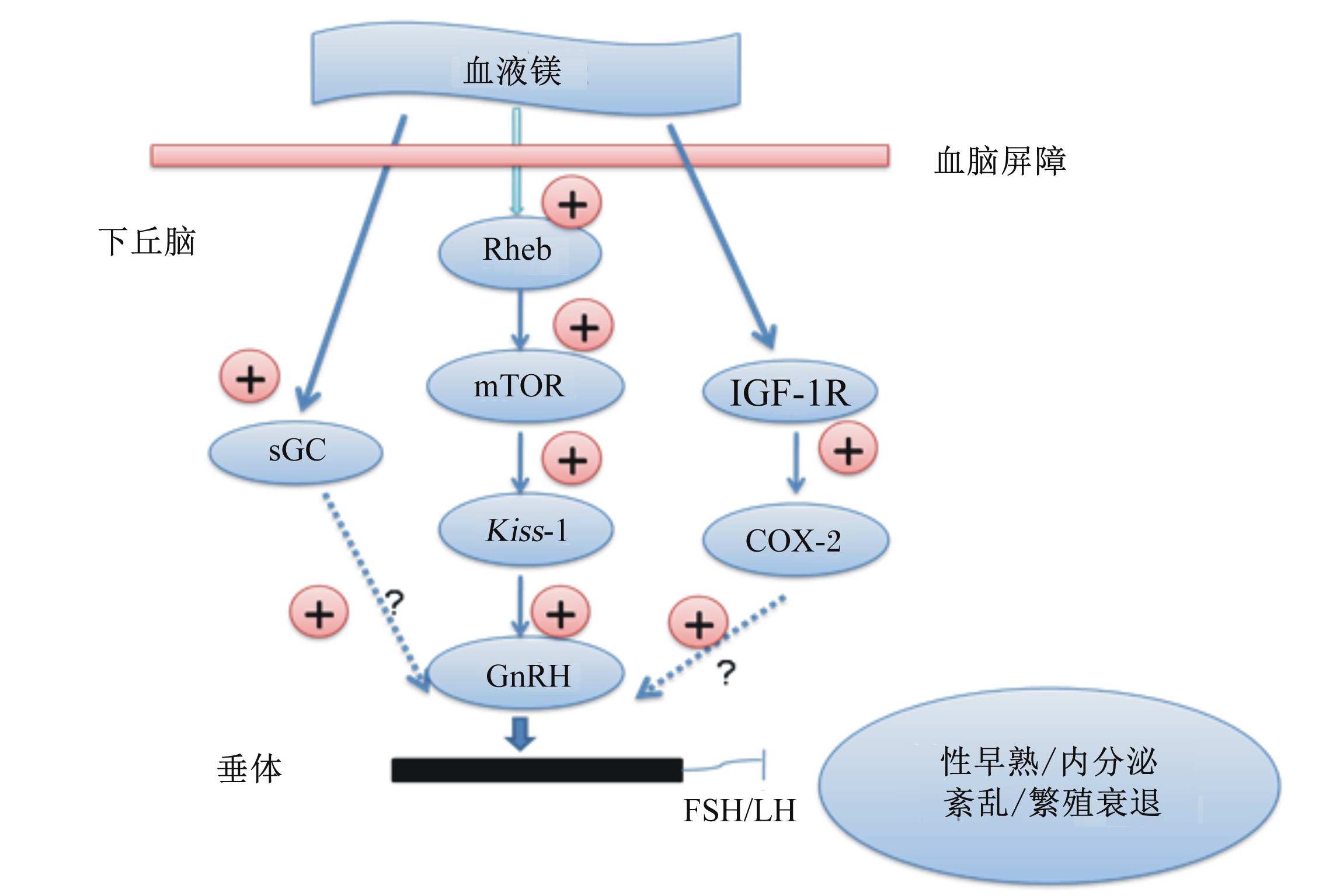 | sGC:鸟苷酸环化酶soluble guanylyl cyclase;Rheb:脑内Ras同系物ras homolog enriched in brain;mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;IGF-1R:胰岛素样生长因子-1受体insulin-like growth factor-1;IGF-1R:胰岛素样生长因子-1受体insulin-like growth factor-1 receptor;COX-2:环氧化酶-2 cyclooxygenase-2;GnRH:促性腺激素释放激素 gonadotropin-releasing hormone;LH:促黄体素 luteinizing hormone;FSH:促卵泡素 follicle-stimulating hormone。 图7 血液镁干扰下丘脑GnRH分泌及性早熟机制 Fig. 7 Mechanism of GnRH secretion and precocious puberty interfered by blood manganese |
矿物元素广泛参与调控细胞的增殖和分化、组织器官的发育。众多微量元素如铁、锌、硒等被发现广泛参与动物的繁殖活动,但是否调控动物的情期启动及卵泡发育,相关研究较少。镁是动物骨代谢和骨胶原生长和发育的必需矿物元素,其缺乏会导致生长和发育停滞。血液中镁在生长发育期跨过血脑屏障的效率是成年期的4倍,更有趣的是,镁十分容易在下丘脑中沉积。Pine等[71]在雌性生长大鼠第3脑室中灌注氯化镁,发现可显著刺激下丘脑促黄体素释放激素(luteinizing hormone-releasing hormone,LHRH)基因表达量及血液LH浓度;此外,生长大鼠饲粮中添加10 mg/kg氯化镁,血液中LH、FSH、雌二醇等繁殖激素的浓度显著提高,且初情日龄显著提前。通过体外研究[72]及体内研究[73]进一步证实了氯化镁通过下丘脑鸟苷酸环化酶、胰岛素样生长因子受体、环氧合酶-2刺激下丘脑分泌LHRH及垂体分泌LH。Srivastava等[74]进一步证实,在饲粮中添加10 mg/kg的氯化镁,大鼠血液GnRH浓度显著提高,并且引起下丘脑情期启动相关基因Kiss-1表达显著上调,并诱发大鼠早熟(图8)。在母猪养殖过程中,妊娠母猪便秘是困扰母猪健康的一大难题,在母猪饲粮中通常添加2~3 kg/t硫酸镁以防止便秘。鉴于镁对神经内分泌及繁殖系统的干扰效应,因此在母猪饲粮配制时需足够谨慎。
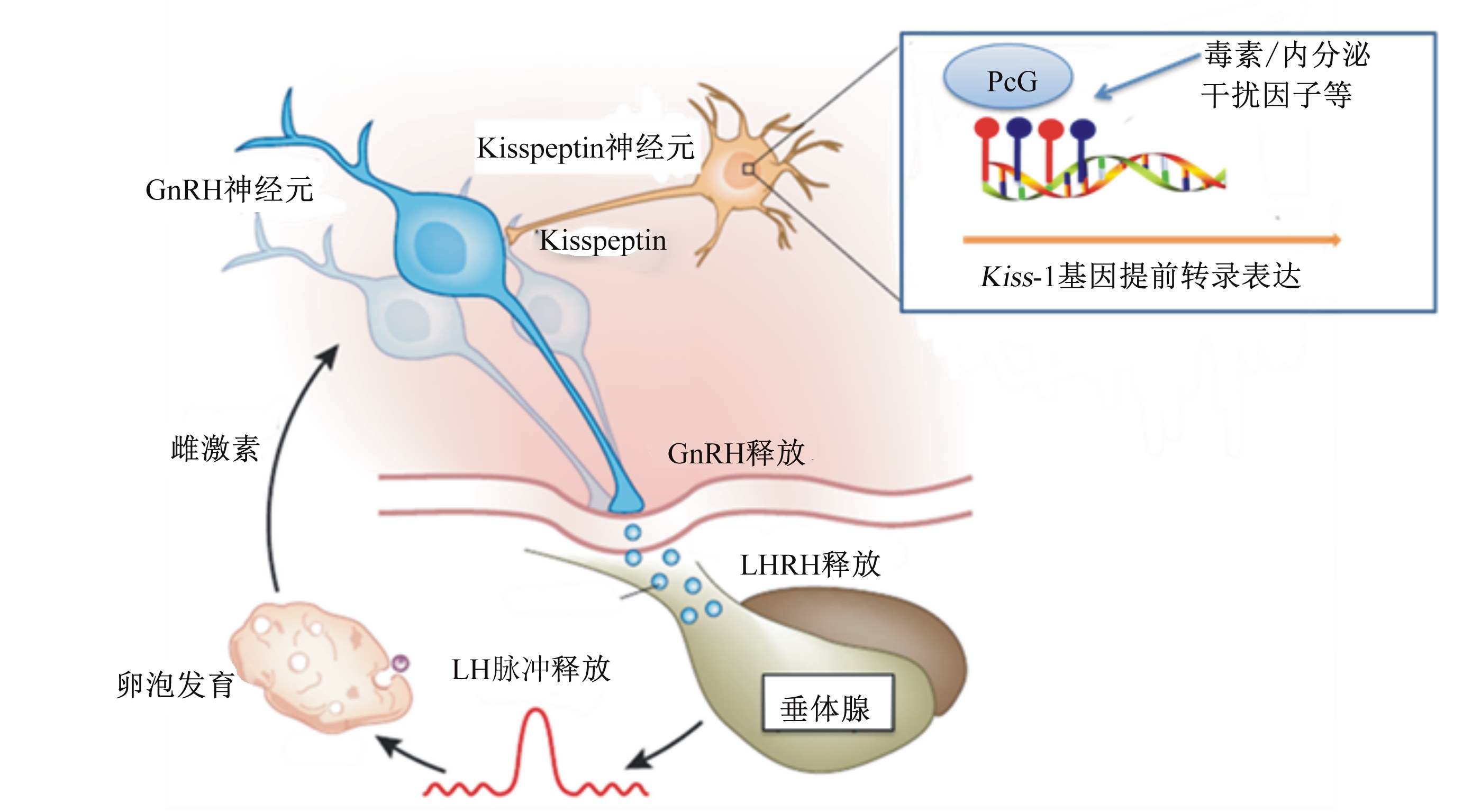 | GnRH:促性腺激素释放激素 gonadotropin-releasing hormone;LH:促黄体素 luteinizing hormone;LHRH:促黄体素释放激素luteinizing hormone-releasing hormone。 正常发育情况下,PcG处于低甲基化,抑制Kiss-1基因转录,使体成熟与性成熟保持同步。毒素与内分泌干扰因子打破这种表观遗传修饰对Kiss-1基因的转录调控,诱发机体GnRH、LHRH等内分泌紊乱,引起雌性动物早熟或者繁殖障碍。 During normal development, polycomb group (PcG) was hypomethylated to repress the Kiss-1 gene transcription to ensure the synchronous development of peripheral body tissues and reproductive tissue. Toxins and endocrine disruptors can interrupt the repression of Kiss-1 genes through epigenetic modification of PcG, which induces the precocious stimulation of GnRH and LHRH secretion and contribute to precocious puberty and reproductive disturbance. 图8 毒素与内分泌干扰因子干扰繁殖功能的理论假设 Fig. 8 Theoryhypothesis of reproduction function interfered by toxins and endocrine disruptors |
雌性动物由生长向繁殖转换过程中,表观遗传修饰发挥着决定性作用。Tomikawaa等[75]分别观察了雌激素对下丘脑AVPV和ARC区域Kiss-1基因的表观遗传修饰状态,发现雌激素对AVPV神经元的正反馈调控与Kiss-1基因的表观遗传修饰有关[75]。不同区域Kiss-1基因表达模式的改变反映了雌激素受体诱导的正负反馈调控敏感性的改变,是触发情期启动的关键生理过程[34]。Lomniczi等[76]对小鼠注射甲基化抑制剂,小鼠初情期推迟甚至消失。进一步研究证实,小鼠正常情期启动依赖于PcG(polycomb group)蛋白对Kiss-1基因启动区域的一系列表观遗传修饰,导致Kiss-1基因表达量上调,促进性腺发育及初情期来临。
值得注意的是,基因的表观遗传修饰对外界环境十分敏感,尤其是具有内分泌干扰效应的化学物质。多酚A是广泛存在于食品、饲料中的一种化学物质,可诱导基因启动子富含双核苷酸“CG”的区域(即CpG岛)低甲基化。研究表明[77],在小鼠饲粮中添加低剂量多酚A(50 μg/kg),可通过影响Kiss-1基因表达及GnRH神经元数量诱发性早熟。苯甲雌二醇和甲氧氯具有明显的类雌激素结构,可显著干扰动物的内分泌状态并诱发早熟[76]。研究表明,当动物在发育早期被苯甲雌二醇和甲氧氯干扰时,下丘脑Kiss-1的基因表达及雌激素受体α(estrogen receptor α,ERα)基因启动区域的甲基化模式改变,导致情期循环紊乱,繁殖系统提前衰退(图8),繁殖功能提前终止[78]。因此,母猪饲粮中应特别关注重金属、霉菌毒素、内分泌干扰因子对母猪情期启动及繁殖系统的损害。
4 小 结情期启动是提高母猪终身繁殖成绩的关键环节。体组织的生长和发育是营养积累的结果,各器官组织只有在营养储备达到一定标准后才能激活下丘脑Kisspeptin神经元并触发下丘脑-垂体-性腺轴活性,推动母猪由生长转向繁殖,或由泌乳转向发情。雌激素正负反馈效应参与了营养调控情期启动,但机理不详,待进一步研究。
| [1] | TUMMARUK P,TANTASUPARUK W,TECHAKUMPHU M,et al.Age,body weight and backfat thickness at first observed oestrus in crossbred Landrace×Yorkshire gilts,seasonal variations and their influence on subsequence reproductive performance[J]. Animal Reproduction Science,2007,99(1/2):167-181. ( 1) 1)
|
| [2] | LE COZLER Y,DAGORN J,LINDBERG J E,et al.Effect of age at first farrowing and herd management on long-term productivity of sows[J]. Livestock Production Science,1998,53(2):135-142. ( 1) 1)
|
| [3] | TUMMARUK P,LUNDEHEIM N,EINARSSON S,et al.Effect of birth litter size,birth parity number,growth rate,backfat thickness and age at first mating of gilts on their reproductive performance as sows[J]. Animal Reproduction Science,2001,66(3/4):225-237. ( 1) 1)
|
| [4] | ROONGSITTHICHAI A,CHEUCHUCHART P,CHATWIJITKUL S,et al.Influence of age at first estrus,bodyweight,and average daily gain of replacement gilts on their subsequent reproductive performance as sows[J]. Livestock Science,2013,151(2/3):238-245. ( 1) 1)
|
| [5] | PINILLA L,AGUILAR E,DIEGUEZ C,et al.Kisspeptins and reproduction:physiological roles and regulatory mechanisms[J]. Physiological Reviews,2012,92(3):1235-1316. ( 1) 1)
|
| [6] | GOTTSCH M L,CLIFTON D K,STEINER R A.Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis[J]. Molecular and Cellular Endocrinology,2006,254/255:91-96. ( 1) 1)
|
| [7] | PLANT T M,BARKER-GIBB M L.Neurobiological mechanisms of puberty in higher primates[J]. Human Reproduction Update,2004,10(1):67-77. ( 1) 1)
|
| [8] | TENA-SEMPERE M.GPR54 and kisspeptin in reproduction[J]. Human Reproduction Update,2006,12(5):631-639. ( 1) 1)
|
| [9] | OHTAKI T,SHINTANI Y,HONDA S,et al.Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor[J]. Nature,2001,411(6837):613-617. ( 2) 2)
|
| [10] | LEE J H,MIELE M E,HICKS D J,et al.KiSS-1,a novel human malignant melanoma metastasis-suppressor gene[J]. Journal of the National Cancer Institute,1996,88(23):1731-1737. ( 1) 1)
|
| [11] | KOTANI M,DETHEUX M,VANDENBOGAERDE A,et al.The metastasis suppressor gene KiSS-1 encodes kisspeptins,the natural ligands of the orphan G protein-coupled receptor GPR54[J]. The Journal of Biological Chemistry,2001,276:34631-34636. ( 1) 1)
|
| [12] | SEMINARA S B,MESSAGER S,CHATZIDAKI E E,et al.The GPR54 gene as a regulator of puberty[J]. The New England Journal of Medicine,2003,349:1614-1627. ( 1) 1)
|
| [13] | DE ROUX N,GENIN E,CAREL J C,et al.Hypogonadotropichypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54[J]. The Proceedings of the National Academy of Sciences of the United States of America,2003,100(19):10972-10976. ( 1) 1)
|
| [14] | KINOSHITA M,TSUKAMURA H,ADACHI S,et al.Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats[J]. Endocrinology,2005,146(10):4431-4436. ( 1) 1)
|
| [15] | DHILLO W S,CHAUDHRI O B,PATTERSON M,et al.Kisspeptin-54 stimulates the hypothalamic-pituitarygonadal axis in human males[J]. The Journal of Clinical Endocrinology and Metabolism,2005,90(12):6609-6615. ( 1) 1)
|
| [16] | NAVARRO V M,CASTELLANO J M,FERNNDEZ-FERNÁNDEZ R,et al.Effects of KiSS-1 peptide,the natural ligand of GPR54,on follicle-stimulating hormone secretion in the rat[J]. Endocrinology,2005,146(4):1689-1697. ( 1) 1)
|
| [17] | NAVARRO V M,CASTELLANO J M,FERNÁNDEZ-FERNÁNDEZ R,et al.Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide,the natural ligand of GPR54[J]. Endocrinology,2005,146 (1):156-163. ( 1) 1)
|
| [18] | SHAHAB M,MASTRONARDI C,SEMINARA S B,et al.Increased hypothalamic GPR54 signaling:a potential mechanism for initiation of puberty in primates[J]. The Proceedings of the National Academy of Sciences of the United States of America,2005,102(6):2129-2134. ( 1) 1)
|
| [19] | GREIVES T J,MASON A O,SCOTTI M A L,et al.Environmental control of kisspeptin:implications for seasonal reproduction[J]. Endocrinology,2007,148(3):1158-1166. ( 1) 1)
|
| [20] | KAUFFMAN A S,PARK J H,MCPHIE-LALMANSINGH A A,et al.The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior[J]. The Journal of Neuroscience:the Official Journal of the Society for Neuroscience,2007,27(33):8826-8835. ( 1) 1)
|
| [21] | XU S Y,LINHER-MELVILLE K,YANG B B,et al.Micro-RNA378 (miR-378) regulates ovarian estradiol production by targeting aromatase[J]. Endocrinology,2011,152(10):3941-3951. ( 1) 1)
|
| [22] | IRWIG M S,FRALEY G S,SMITH J T,et al.Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat[J]. Neuroendocrinology,2004,80(4):264-272. ( 1) 1)
|
| [23] | HAN S K,GOTTSCH M L,LEE K J,et al.Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty[J]. The Journal of Neuroscience,2005,25(49):11349-11356. ( 1) 1)
|
| [24] | PIELECHA-FORTUNNA J,CHU Z G,MOENTER S M.Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol[J]. Endocrinology,2008,149(4):1979-1986. ( 1) 1)
|
| [25] | CLARKSON J,HERBISON A E.Postnatal development of kisspeptin neurons in mouse hypothalamus;sexual dimorphism and projections to gonadotropin-releasing hormone neurons[J]. Endocrinology,2006,147(12):5817-5825. ( 1) 1)
|
| [26] | DECOURT C,TILLET Y,CARATY A,et al.Kisspeptinimmunoreactive neurons in the equine hypothalamus interactions with GnRH neuronal system[J]. Journal of Chemical Neuroanatomy,2008,36(3/4):131-137. ( 1) 1)
|
| [27] | RAMASWAMY S,GUERRIERO K A,GIBBS R B,et al.Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy[J]. Endocrinology,2008,149(9):4387-4395. ( 1) 1)
|
| [28] | NAVARROV M,TENA-SEMPERE M.Neuroendocrine control by kisspeptins:role in metabolic regulation of fertility[J]. Nature Reviews Endocrinology,2012,8(1):40-53. ( 1) 1)
|
| [29] | LENTS C A,HEIDORN N L,BARB C R,et al.Central andperipheral administration of kisspeptin activates gonadotropinbut not somatotropin secretion in prepubertal gilts[J]. Reproduction,2008,135:879-887. ( 1) 1)
|
| [30] | ADACHI S,YAMADA S,TAKATSU Y,et al.Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormonerelease in female rats[J]. The Journal of Reproduction and Development,2007,53(2):367-378. ( 1) 1)
|
| [31] | TOMIKAWA J,HOMMA T,TAJIMA S,et al.Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig[J]. Biology of Reproduction,2010,82(2):313-319. ( 1) 1)
|
| [32] | IEDA N,UENOYAMA Y,TAJIMA Y,et al.KISS1 gene expression in the developing brain of female pigs in pre- and peripubertal periods[J]. The Jounal of Reproduction and Development,2014,60(4):312-316. ( 1) 1)
|
| [33] | SMITH J T,CUNNINGHAM M J,RISSMAN E F,et al.Regulation of Kiss1 gene expression in the brain of the female mouse[J]. Endocrinology,2005,146(9):3686-3692. ( 1) 1)
|
| [34] | FRISH R E.Body fat,menarche,fitness and fertility[J]. Human Reproduction,1987,2(6):521-533. ( 2) 2)
|
| [35] | ARMSTRONG J D,BRITT J H.Nutritionally-induced anestrus in gilts:metabolic and endocrine changes associated with cessation and resumption of estrous cycles[J]. Journal of Animal Science,1987,65(2):508-523. ( 1) 1)
|
| [36] | ROZEBOOM D W,MOSER R L,CORNELIUS S G,et al.Body composition of postpubertal gilts at nutritionally induced anestrus[J]. Journal of Animal Science,1993,71(2):426-435. ( 1) 1)
|
| [37] | LENTS C A,REMPEL L A,KLINDT J,et al.The relationship of plasma urea nitrogen with growth traits and age at first estrus in gilts[J]. Journal of Animal Science,2013,91(7):3137-3142. ( 1) 1)
|
| [38] | OURY F,SUMARA G,SUMARA O,et al.Endocrine regulationof male fertility by the skeleton[J]. Cell,2011,144(5):796-809. ( 1) 1)
|
| [39] | MAYER C,ACOSTA-MARTINEZ M,DUBOIS S L,et al.Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons[J]. The Proceedings of the National Academy of Sciences of the United States of America,2010,107(52):22693-22698. ( 2) 2)
|
| [40] | ZHOU D,ZHUO Y,CHE L.Nutrient restriction induces failure of reproductive functionand molecular changes in hypothalamus-pituitary-gonadal axisin postpubertal gilts[J]. Molecular Biology Reprots,2014,41(7):4733-4742. ( 1) 1)
|
| [41] | CASTELLANO J M,NAVARRO V M R,FERNANDEZ-FERNANDEZ R,et al.Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition[J]. Endocrinology,2005,146(9):3917-3925. ( 1) 1)
|
| [42] | OWEN B M,BOOKOUT A L,DING X S,et al.FGF21 contributes to neuroendocrine control of female reproduction[J]. Nature Medicine,2013,19(9):1153-1156. ( 1) 1)
|
| [43] | POTTHOFF M J,INAGAKI T,SATAPATI S,et al.FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response[J]. The Proceedings of the National Academy of Sciences of the United States of America,2009,106(26):10853-10858. ( 1) 1)
|
| [44] | SONIGO C,BOUILLY J,CARRN,et al.Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration[J]. The Journal of Clinical Investigation,2012,122(10):3791-3795. ( 1) 1)
|
| [45] | XU J,KIRIGITI M A,GROVE K L,et al.Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation:role of insulin and leptin[J]. Endocrinology,2009,150(9):4231-4240. ( 1) 1)
|
| [46] | TRUE C,KIRIGITI M A,KIEVIT P,et al.Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance[J]. Journal of Neuroendocrinology,2011,23(11):1099-1112. ( 1) 1)
|
| [47] | ROLAND A V,MOENTER S M.Glucosensing by GnRH neurons:inhibition by androgens and involvement of AMP-activated protein kinase[J]. Molecular Endocrinology,2011,25(5):847-858. ( 1) 1)
|
| [48] | ZHANG C G,BOSCH M A,LEVINE J E,et al.Gonadotropin-releasing hormone neurons express KATP channels that are regulatedby estrogen and responsive to glucose and metabolic inhibition[J]. The Journal of Neuroscience,2007,27(38):10153-10164. ( 1) 1)
|
| [49] | ROA J,GARCIA-GALIANO D,VARELA L,et al.The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system[J]. Endocrinology,2009,150(11):5016-5026. ( 1) 1)
|
| [50] | ALTAREJOS J Y,GOEBEL N,CONKRIGHT M D,et al.The Creb1 coactivator Crtc1 is required for energybalance and fertility[J]. Nature Medicine,2008,14(10):1112-1117. ( 2) 2)
|
| [51] | ZHUO Y,ZHOU D S,CHE L Q,et al.Feeding prepubescent gilts a high-fat diet induces molecularchanges in the hypothalamus-pituitary-gonadal axis and predictsearly timing of puberty[J]. Nutrition,2014,30(7/8):890-896. ( 1) 1)
|
| [52] | BARB C R,HAUSMAN G J,LENTS C A.Energy metabolism and leptin:effects on neuroendocrine regulation of reproduction in the gilt and sow[J]. Reproduction in Domestic Animals,2008,43(Suppl.2):324-330. ( 1) 1)
|
| [53] | 李方方.日粮能量来源对大鼠初情启动的影响及调控机理研究[D]. 博士学位论文.雅安:四川农业大学,2012. ( 1) 1)
|
| [54] | SMITH J T,ACOHIDO B V,CLIFTON D K,et al.KiSS-1 neurones are direct targets for leptin in the ob/ob mouse[J]. Journal of Neuroendocrinology,2006,18(4):298-303. ( 1) 1)
|
| [55] | QUENNELL J H,HOWELL C S,ROA J,et al.Leptindeficiency and diet-induced obesity reduce hypothalamic kisspeptinexpression in mice[J]. Endocrinology,2011,152(4):1541-1550. ( 1) 1)
|
| [56] | CUNNINGHAM P J,NABER C H,ZIMMERMAN D R,et al.Influence of nutritional regime on age at puberty in gilts[J]. Journal of Animal Science,1974,39(1):63-67. ( 1) 1)
|
| [57] | PATTERSON J L,BALL R O,WILLIS H J,et al.The effect of lean growth rate on puberty attainment in gilts[J]. Journal of Animal Science,2002,80(5):1299-1310. ( 1) 1)
|
| [58] | TORRE S D,RANDO G,MEDA C,et al.Amino acid-dependent activation of liver estrogen receptor alpha integrates metabolic and reproductive functions via IGF-1[J]. Cell Metabolism,2011,13(2):205-214. ( 1) 1)
|
| [59] | NARITA K,NAGAO K,BANNAI M,et al.Dietary deficiency of essential amino acids rapidly induces cessation of the rat estrous cycle[J]. PLoS One,2011,6(11):e28136. ( 1) 1)
|
| [60] | ROLDAN M B,WHITE C,WITCHEL S F.Association of the GAA1013→GAG polymorphism of the insulin-like growth factor-1 receptor (IGF1R) gene with premature pubarche[J]. Fertility and Sterility,2007,88(2):410-417. ( 1) 1)
|
| [61] | ROONGSITTHICHAI A,KOONJAENAK S,TUMMARUK P.Association among serum insulin-like growth factor-Ⅰ,backfat thickness,and age at first observed estrus in gilts[J]. Thai Journal of Veterinary Medicine,2013,43(1):41-48. ( 1) 1)
|
| [62] | JOHNSTON D J,BARWICK S A,CORBET N J,et al.Genetics of heifer puberty in two tropical beef genotypes in northern Australia and associations with heifer- and steer-production traits[J]. Animal Production Science,2009,49(6):399-412. ( 1) 1)
|
| [63] | FORTES M R S,LI Y T,COLLIS E,et al.The IGF1 pathway genes and their association with age of puberty in cattle[J]. Animal Genetics,2013,44(1):91-95. ( 1) 1)
|
| [64] | HINEY J K,SRIVASTAVA V K,PINE M D,et al.Insulin-like growth factor-Ⅰactivates KiSS-1 gene expression in the brain of the prepubertal female rat[J]. Endocrinology,2009,150(1):376-384. ( 1) 1)
|
| [65] | TODD B J,MERHI Z O,SHU J,et al.Hypothalamic insulin-like growth factor-Ⅰ receptors are necessary for hormone-dependent luteinizing hormone surges:implications for female reproductive aging[J]. Endocrinology,2010,151(3):1356-1366. ( 1) 1)
|
| [66] | SUN Y,TODD B J,THORNTON K,et al.Differential effects of hypothalamic IGF-Ⅰ on gonadotropin releasing hormone neuronal activation during steroid-induced LH surges in young and middle-aged female rats[J]. Endocrinology,2011,152(11):4276-4287. ( 1) 1)
|
| [67] | OZKAN S,JINDAL S,GREENSEID K,et al.Replete vitamin D stores predict reproductive success following in vitro fertilization[J]. Fertility and Sterility,2009,94(5):1314-1319. ( 1) 1)
|
| [68] | SUN W,XIE H,JI J,et al.Defective female reproductive function in 1, 25(OH)2D-deficient mice results from indirect effect mediated by extracellular calcium and/or phosphorus[J]. American Journal of Physiology:Endocrinology and Metabolism,2011,299(6):E928-E935. ( 1) 1)
|
| [69] | DICKEN C L,ISRAEL D D,DAVIS J B,et al.Peripubertalvitamin D3 deficiency delays puberty and disrupts the estrous cycle in adult female mice[J]. Biology of Reproduction,2012,87(2):51. ( 3) 3)
|
| [70] | PATTERSON J L,BELTRANENA E,FOXCROFT G R.The effect of gilt age at first estrus and breeding on third estruson sow body weight changes and long-term reproductive performance[J]. Journal of Animal Science,2010,88(7):2500-2513. ( 2) 2)
|
| [71] | PINE M,LEE B,DEARTH R,et al.Manganese acts centrally to stimulate luteinizing hormonesecretion:a potential influence on female pubertaldevelopment[J]. Toxicological Science,2005,85(2):880-885. ( 2) 2)
|
| [72] | LEE B,HINEY J K,PINE M D,et al.Manganese stimulates luteinizing hormone releasing hormone secretion in prepubertal female rats:hypothalamic site and mechanism of action[J]. Journal of Physiology,2007,578:765-772. ( 2) 2)
|
| [73] | HINEY J K,SRIVASTAVA V K,DEES W L.Manganese induces IGF-1 and cyclooxygenase-2 gene expressionsin the basal hypothalamus during prepubertalfemale development[J]. Toxicological Science,2011,121(2):389-396. ( 2) 2)
|
| [74] | SRIVASTAVA V K,HINEY J K,DEES W L.Early life manganese exposure upregulatestumor-associated genes in the hypothalamus of female rats:relationship to manganese-induced precocious puberty[J]. Toxicological Science,2013,136(2):373-381. ( 2) 2)
|
| [75] | TOMIKAWAA J,YOSHIHISA U,OZAWAA M,et al.Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain[J]. The Proceedings of the National Academy of Sciences of the United States of America,2012,109(20):E1294-E1301. ( 2) 2)
|
| [76] | LOMNICZI A,LOCHE A,CASTELLANO J M,et al.Epigenetic control of female puberty[J]. Nature Neuroscience,2013,16(3):281-289. ( 3) 3)
|
| [77] | LOSA-WARD S M,TODD K L,MCCAFFREY K A,et al.Disrupted organization of Rfami depathways in the hypothalamus is associated with advanced puberty in female rats neonatallyexposed to bisphenol A[J]. Biology of Reproduction,2012,87(2):28. ( 2) 2)
|
| [78] | GORE A C,WALKER D M,ZAMA A M,et al.Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging[J]. Molecular Endocrinology,2011,25(12):2157-2168. ( 2) 2)
|



