水不仅仅是鱼类必需的生存环境,也是其他病原菌的良好生存介质和有毒物质的良好溶剂。因此,随着集约化高密度养殖模式的推广,鱼类常常面临着生存环境拥挤、中毒和感染等各种应激,导致鱼类免疫力降低,甚至爆发感染而引起大面积死亡[1]。然而,抗生素和化学制剂等传统鱼类疾病预防和治疗措施存在严重的环境污染和食品安全问题[2]。近年来,通过营养措施提高鱼类自身疾病抵抗力和抗应激能力预防疾病爆发因其低环境污染、保证食品安全而成为研究热点[3]。目前,关于营养物质对鱼类疾病抵抗力及其免疫调控作用已开展了一些研究,本文将对部分氨基酸[蛋氨酸(methionine,Met)[4]和异亮氨酸(isoleucine,Ile)][5]、脂溶性维生素[维生素A(vitamin A,VA)][6]、水溶性维生素[维生素B1(vitamin B1,VB1)[7]、吡哆醇[8]、泛酸(pantothenic acid,PA)[9]、胆碱[10]和肌醇(myo-inositol,MI)][11]及营养性添加物[蛋氨酸羟基类似物(methionine hydroxyl analogue,MHA)][12]等营养物质与鱼类疾病抵抗力、非特异和特异性免疫防御、免疫器官生长发育、结构完整性和抗氧化防御、细胞因子及其相关信号途径之间关系的研究进展作一综述。
1 营养物质与鱼类疾病抵抗力和免疫防御的 关系疾病抵抗力是衡量营养物质对机体免疫作用的综合指标,而细菌攻毒后的存活率能敏感反映鱼类疾病抵抗力[13]。近来研究表明,饲料中适宜水平的Met[4]、Ile[5]、VA[6]、VB1[7]、吡哆醇[8]、PA[9]、胆碱[10]和MHA[12]可显著提高幼建鲤细菌攻毒后的存活率(P<0.05)(表1),说明这些营养物质能够增强鱼类疾病抵抗力。鱼类疾病抵抗力主要依赖于免疫防御系统,其主要包括非特异和特异性免疫防御[14]。因此,营养物质增强鱼类疾病抵抗力可能与其提高了鱼类非特异和特异性免疫防御有关。
非特异性免疫防御在鱼类抵抗病原菌入侵过程中非常重要,主要包括细胞和体液免疫2部分[15]。吞噬细胞是鱼类非特异性细胞免疫防御系统的关键组成之一,能有效吞噬和杀灭病原菌[16]。白细胞吞噬活性(leucocytes phagocytic activity,LPA)可敏感反映白细胞吞噬能力,是鱼类非特异性细胞免疫的重要标识[17]。研究表明,饲料中适宜水平的Ile[5]、VB1[7]、吡哆醇[8]、PA[9]、胆碱[10]、MI[11]以及MHA[12]能够显著提高攻毒后幼建鲤的LPA(P<0.05)(表1),增强白细胞吞噬能力。鱼类白细胞发挥吞噬作用需要吞噬细胞经过对病原菌的识别、黏附及溶菌等一系列过程,从而杀灭病原菌[18]。凝集素广泛存在于脊椎动物体液和细胞表面,在细胞免疫识别中起重要作用[19]。补体3(C3)和补体4(C4)与鱼类补体介导的调理作用有关,通过与病原菌共价结合后与吞噬细胞表面补体受体作用,进而有利于吞噬细胞对病原菌的识别和吞噬[20]。溶菌酶和酸性磷酸酶(acid phosphatase,ACP)是重要的非氧依赖免疫防御分子,是吞噬细胞消化摄入病原菌能力的重要标识[14]。有限的研究表明,饲料中适宜水平的Ile[5]、胆碱[10]和MHA[12]可显著提高攻毒后幼建鲤的血清凝集素效价、C3和C4含量以及溶菌酶和ACP活力(P<0.05);饲料中适宜水平的Met[4]可显著提高攻毒后幼建鲤的血清凝集素效价、C3和C4含量以及溶菌酶活力(P<0.05);饲料中适宜水平的VB1[7]、吡哆醇[8]和PA[9]对血清凝集素效价以及溶菌酶和ACP活力的影响与胆碱一致,而MI[11]仅显著提高了攻毒后幼建鲤血清凝集素效价和溶菌酶活力(P<0.05),对血清ACP活力没有显著影响(P>0.05)(表1)。这些结果说明,营养物质提高鱼类白细胞吞噬能力与其增强了吞噬细胞对病原菌的识别、黏附和溶菌能力有关。此外,抑制病原菌生长也是机体非特异性免疫防疫的主要作用之一[21]。铁是微生物生长所必需的营养元素之一,转铁蛋白有很强的铁结合能力,可以抑制微生物生长和繁殖[22],也可以直接作用于病原菌膜蛋白,增强鱼类巨噬细胞对病原菌的杀伤能力[23]。常用总铁结合力(total iron-binding capacity,TIBC)来反映血清中转铁蛋白含量[24]。相关研究表明,饲料中适宜水平的Met[4]、VB1[7]、吡哆醇[8]、PA[9]以及MHA[12]能够显著提高攻毒后幼建鲤的血清TIBC(P<0.05)(表1),从而增强鱼类的抑菌作用和吞噬细胞杀菌能力。然而,饲料中胆碱缺乏显著提高了攻毒后幼建鲤的血清TIBC(P<0.05)(表1),这可能与胆碱缺乏破坏了细胞结构完整有关[10]。此外,MI对攻毒后幼建鲤的血清TIBC没有显著影响(P>0.05)[11](表1)。
| 表1 营养物质对嗜水气单孢菌攻毒后幼建鲤存活率、非特异和特异性免疫指标的影响 Table 1 Effects of nutrients on survival rate and parameters of non-specific and specific immunity in juvenile Jian carp after challenged with Aeromonas hydrophila |
尽管鱼类特异性免疫应答相对哺乳动物不完善,但其仍然是鱼类免疫防疫体系的重要组成部分[14]。非特异性免疫防御系统未处理的胞外抗原或通过抗原呈递细胞识别并捕获后的胞内抗原可激活体内特异性免疫系统,产生特异性抗体来专一性的清除抗原[25]。研究表明,饲料中适宜水平的Met[4]、Ile[5]、VB1[7]、吡哆醇[8]、PA[9]、胆碱[10]、MI[11]以及MHA[12]可显著提高攻毒后幼建鲤的血清抗体含量(P<0.05)(表1),说明这些营养物质能够提高鱼类的特异性免疫能力,进而增强其疾病抵抗力。然而,VA对幼建鲤血清免疫球蛋白M(IgM)含量没有显著影响(P>0.05)[6](表1)。
2 营养物质与鱼类血液红细胞(red blood cell,RBC)、白细胞(white blood cell,WBC)数量及免疫器官生长发育的关系营养物质提高鱼类非特异和特异性免疫力可能与其提高RBC、WBC数量有关。研究报道,鱼类非特异和特异性免疫力与其血液中RBC和WBC数量呈正相关[26]。近来研究表明,饲料中适宜水平的Ile[5]、VA[6]、VB1[7]、吡哆醇[8]、PA[9]、胆碱[10]、MI[11]以及MHA[12]可显著提高幼建鲤的血液RBC和WBC数量(P<0.05)(表2),说明这些营养物质能够通过增加鱼类血液中RBC和WBC数量来提高鱼类的非特异和特异性免疫力。Ellis[27]报道,头肾和脾脏是硬骨鱼类重要的造血和免疫器官。因此,营养物质增加鱼类RBC和WBC数量可能与其促进了鱼类免疫器官生长发育有关。已有研究表明,饲料中适宜水平的VA[6]、VB1[7]、吡哆醇[8]、PA[9]、胆碱[10]、MI[11]以及MHA[12]能够显著提高幼建鲤脾体指数(spleen index,SI)(P<0.05)(表2),促进鱼类脾脏生长发育。然而,除Ile[5]和MHA[12]对幼建鲤头肾生长发育的影响与脾脏一致外,VB1[7]、吡哆醇[8]、PA[9]和MI[11]缺乏导致幼建鲤头肾指数(head kidney index,HKI)显著提高(P<0.05)(表2),这可能由于当这些维生素缺乏时鱼类优先保证重要器官的生长发育和功能正常,但具体作用机制需要进一步研究。此外,VA[6]和胆碱[10]对幼建鲤HKI没有显著影响(P>0.05)(表2)。综上所述,营养物质能够促进幼建鲤免疫器官生长发育,且不同营养物质和不同免疫器官间存在差异。鱼类组织器官生长发育通常依赖于其细胞结构完整性和抗氧化状态[28],营养物质可能通过调控免疫器官抗氧化防御保证细胞结构的完整性。
| 表2 营养物质对幼建鲤红细胞、白细胞数量以及脾脏和头肾指数的影响 Table 2 Effects of nutrients on the counts of red blood cells and white blood cells,and the indexes of spleen and head kidney in juvenile Jian carp |
大量研究表明,硬骨鱼类脾脏和头肾含有大量淋巴细胞等免疫细胞[29],这些免疫细胞的正常代谢及免疫过程将产生大量活性氧簇(reactive oxygen species,ROS)[30]。然而,过量ROS将引起氧化应激,导致鱼脾脏[31]和头肾[32]的氧化损伤。此外,鱼类细胞膜中含有大量多不饱和脂肪酸,非常容易遭受ROS攻击[27, 33]。因此,保护鱼类免疫器官免受氧化损伤,保证其结构完整性在鱼类免疫防疫中非常重要。然而,目前关于营养物质对鱼类免疫器官氧化损伤的影响仅见少量研究报道。丙二醛(malondialdehyde,MDA)和蛋白质羰基(protein carbonyl,PC)能够分别敏感反映鱼类组织器官脂质过氧化和蛋白质氧化损伤程度[34]。饲料中适宜水平的胆碱和MHA可显著降低幼建鲤脾脏中MDA和PC含量(P<0.05)(表3)。Ile和胆碱对幼建鲤头肾中MDA和PC含量的影响与脾脏类似,而MHA仅显著降低了MDA含量(P<0.05)(表3)。这些结果说明Ile[5]、胆碱[35]和MHA[12]能够降低鱼类免疫器官的氧化损伤,保证鱼类免 疫器官结构完整和功能正常。
| 表3 营养物质对幼建鲤免疫器官抗氧化相关参数的影响 Table 3 Effects of nutrients on antioxidant-related parameters in immune organs of juvenile Jian carp |
超氧阴离子和羟自由基是导致机体氧化损伤常见的2种氧自由基[36]。抗超氧阴离子(anti-superoxide anion,ASA)和抗羟自由基(anti-hydroxy radical,AHR)活力能够分别敏感反映细胞对这2种自由基的清除能力[37]。饲料中适宜水平的胆碱[35]和MHA[12]可显著降低幼建鲤脾脏ASA活力(P<0.05);饲料中适宜水平的胆碱对幼建鲤脾脏中AHR活力的影响与其对ASA的影响一致[35],而饲料中适宜水平的MHA则提高了脾脏AHR活力(P<0.05)[12](表3)。这些结果说明:1)胆碱可能作为细胞膜结构组成直接保护鱼类脾脏细胞结构的完整性,而不通过提高ASA和AHR活力来发挥作用[35]。Saito等[38]报道,胆碱含有的羟胺基团能够分解氢过氧化物,降低沙丁鱼油混合物的过氧化值。2)胆碱提高鱼类脾脏ROS水平可能与增强氧依赖方式杀菌能力有关[35]。3)MHA通过降低鱼类脾脏中ASA活力增强免疫细胞氧依赖方式杀菌能力,同时也通过提高AHR活力提高抗氧化损伤能力[12]。在鱼类头肾上的研究表明,饲料中适宜水平的Ile可显著提高幼建鲤头肾中ASA和AHR活力(P<0.05)[5],而MHA对头肾中ASA和AHR活力的影响与Ile的趋势相反[12];胆碱仅显著降低幼建鲤头肾中AHR活力(P<0.05)[35](表3)。
鱼类的ROS清除能力通常与非酶性和酶性抗氧化系统有关[39]。谷胱甘肽(glutathione,GSH)是脊椎动物主要的非蛋白质低分子抗氧化物质之一[40]。饲料中胆碱缺乏可显著提高幼建鲤脾脏GSH含量(P<0.05),该结果与ASA和AHR活力的变化趋势相一致[35](表3)。胆碱缺乏导致幼建鲤脾脏GSH含量增加可能与GSH生物合成途径有关[35]。Zhang等[41]报道,胆碱缺乏诱导小鼠肝脏GSH生物合成限速酶谷氨酸-半胱氨酸连接酶催化亚基(glutamate-cysteine ligase catalytic subunit,GCLC)的mRNA表达,进而增加小鼠肝脏GSH含量。然而,MHA对幼建鲤脾脏GSH含量没有显著影响(P>0.05)[12]。在鱼类头肾上的研究显示,Ile和胆碱对幼建鲤头肾GSH含量的影响与胆碱对脾脏GSH含量的影响具有相似变化趋势,但MHA与Ile的作用趋势相反(表3)。这些结果说明,Ile[5]、胆碱[35]和MHA[12]能够影响鱼类免疫器官中GSH含量,且在不同免疫器官间存在差异,具体作用方式有待进一步研究。
抗氧化酶在鱼类免疫器官抵抗氧化应激免受ROS导致的氧化损伤过程中发挥着重要的作用。超氧化物歧化酶(superoxide dismutase,SOD)、过氧化氢酶(catalase,CAT)、谷胱甘肽过氧化物酶(glutathione peroxidase,GPx)、谷胱甘肽硫转移酶(glutathione-S-transferase,GST)和谷胱甘肽还原酶(glutathione peroxidase,GR)是构成鱼类酶性抗氧化系统的重要抗氧化酶类[39]。研究表明,Ile[5]、胆碱[35]和MHA[12]对鱼类免疫器官抗氧化酶活力有显著影响(表3)。饲料中胆碱缺乏导致幼建鲤脾脏中SOD、CAT、GPx、GST和GR活力显著增加[35](P<0.05),该结果与脾脏ASA和AHR活力变化趋势一致;MHA对脾脏SOD、CAT和GPx活力的影响与胆碱一致,但饲料适宜水平的MHA显 著提高了脾脏GST和GR活力(P<0.05)[12](表 3)。在鱼类头肾上的研究显示,饲料中适宜水平的Ile显著提高了幼建鲤头肾SOD、CAT、GPx、GST和GR活力(P<0.05)[5];胆碱和MHA对头肾CAT活力以及MHA对头肾GR活力的影响与Ile的趋势一致,而胆碱[35]和MHA[12]对头肾GPx以及MHA[12]对SOD活力的影响与Ile的趋势相反;胆碱[35]和MHA[12]对头肾GST活力以及胆碱[35]对头肾SOD和GR活力没有显著影响(P>0.05)(表3)。这些结果说明,Ile[5]、胆碱[35]和MHA[12]能够部分通过影响脾脏和头肾的抗氧化酶活力来影响免疫器官的ASA和AHR活力,调节免疫器官的ROS水平,使其在发挥免疫功能的同时保证鱼类免疫器官结构的完整性。
3.2 营养物质与鱼类免疫器官抗氧化酶基因表达的关系及调控途径
在大鼠上的研究表明,抗氧化酶活力与其基因表达有关[42]。而关于营养物质对鱼类免疫器官抗氧化酶基因表达的影响仅见胆碱上有零星报道。饲料中胆碱缺乏显著上调幼建鲤脾脏和头肾铜锌超氧化物歧化酶(copper-zinc superoxide dismutase,CuZnSOD)、锰超氧化物歧化酶(manganese superoxide dismutase,MnSOD)、CAT、谷胱甘肽过氧化物酶1a(glutathione peroxidase 1a,GPx1a)、谷胱甘肽过氧化物酶1b(glutathione peroxidase 1b,GPx1b)和GR mRNA的表达(P<0.05)[10, 35](图1),说明胆碱可以通过影响鱼类脾脏和头肾抗氧化酶基因的表达来调控抗氧化酶活力。胆碱对抗氧化酶基因表达的影响在大鼠上有相似报道,报道认为胆碱-蛋氨酸缺乏上调了大鼠NAD(P)H:苯醌氧化还原酶-1[NAD(P)H:quinone oxidoreductase 1,NQO1]等抗氧化反应相关酶类基因的表达[43]。动物细胞抗氧化酶基因的表达受到多种关键信号分子的调控[44]。核因子E2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)是调控斑马鱼抗氧化酶基因转录的关键信号分子之一[45]。Kelch样ECH相关蛋白1(Kelch-like ECH-associated protein 1,Keap1)与斑马鱼Nrf2的稳定性调控有关[46]。鲤鱼上已成功克隆了鲤鱼Nrf2和Kelch样ECH相关蛋白1a(Kelch-like ECH-associated protein 1a,Keap1a)cDNA(NCBI登录号分别为JX462955和JX470752)[47]。关于营养物质对鱼类免疫器官Nrf2及Keap1表达的影响仅见胆碱上有1篇研究报道。Wu等[35]报道,饲料中胆碱缺乏显著上调幼建鲤头肾Nrf2以及脾脏和头肾Keap1a mRNA的表达(P<0.05)(图1),该结果与抗氧化酶基因的表达结果一致,说明胆碱可通过影响Nrf2和Keap1a的表达调控鱼类免疫器官抗氧化酶基因的表达,具体作用机制有待进一步研究。
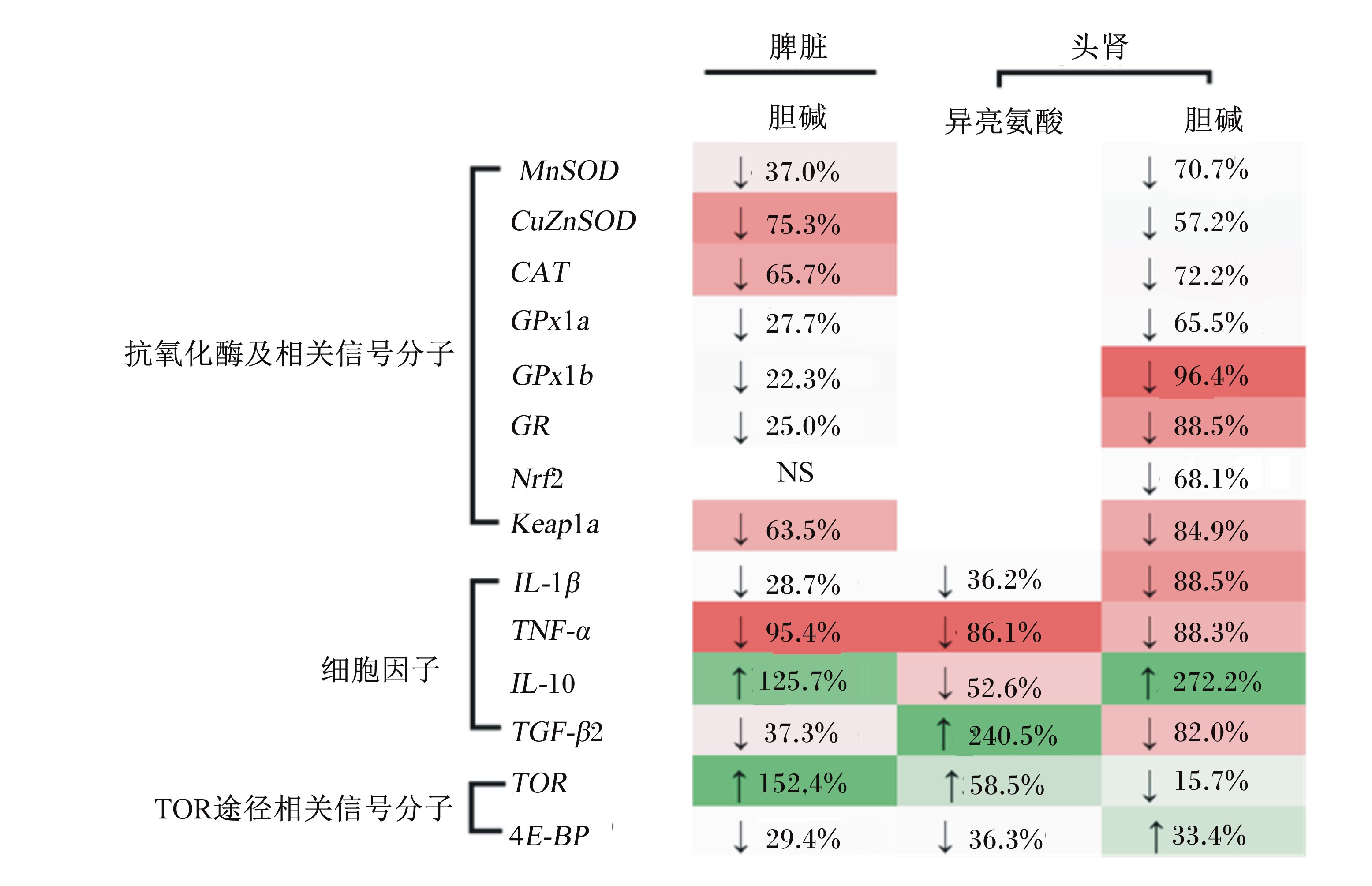 | MnSOD:锰超氧化物歧化酶 manganese superoxide dismutase;CuZnSOD:铜锌超氧化物歧化酶 copper-zinc superoxide dismutase;CAT:过氧化氢酶 catalase;GPx1a:谷胱甘肽过氧化物酶1a glutathione peroxidase 1a;GPx1b:谷胱甘肽过氧化物酶1b glutathione peroxidase 1b;GR:谷胱甘肽还原酶 glutathione reductase;Nrf2:核因子E2相关因子2 nuclear factor erythroid 2-related factor 2;Keap1a:Kelch样ECH相关蛋白1 Kelch-like ECH-associated protein 1a;IL-1β:白细胞介素-1β interleukin-1β;TNF-α:肿瘤坏死因子-α tumor necrosis factor α;IL-10:白细胞介素-10 interleukin-10;TGF-β2:转化生长因子-β2 transforming growth factor-β2;TOR:雷帕霉素靶蛋白 target of papamycin;4E-BP:真核翻译起始因子4E结合蛋白 eukaryotic translation initiation factor 4E binding protein。 ↑:提高increase;↓:降低decrease。 图1 营养物质对嗜水气单孢菌攻毒后幼建鲤免疫器官基因表达的影响 Fig. 1 Effects of nutrients on gene expression in immune organs of juvenile Jian carp after being challenged with Aeromonas hydrophila[5, 10, 35] |
前面提到Ile[5]和胆碱[35]等营养物质缺乏会导致鱼类脾脏和头肾发生氧化应激,破坏其结构完整性。在小鼠上研究表明,氧化应激与细胞内促炎症细胞因子白细胞介素-1(interleukin-1,IL-1)和肿瘤坏死因子-α(tumor necrosis factor α,TNF-α)的产生有关[48]。白细胞介素-10(interleukin-10,IL-10)和转化生长因子-β2(transforming growth factor-β2,TGF-β2)等抗炎细胞因子能够有效抑制脊椎动物白细胞介素-1β(interleukin-1β,IL-1β)和TNF-α的产生[49]。目前,关于营养物质对鱼类脾脏和头肾细中胞因子的研究仅见Ile和胆碱上有报道。研究表明,饲料中适宜水平的Ile[5]和胆碱[10]下调了攻毒后幼建鲤头肾中促炎症细胞因子IL-1β和TNF-α mRNA的表达,同时Ile上调抗炎症细胞因子IL-10 mRNA表达,而下调了TGF-β2 mRNA表达[5](P<0.05),但胆碱对头肾IL-10和TGF-β2 mRNA表达的影响与Ile趋势相反;同时,胆碱对脾脏中细胞因子的影响与头肾相似[10](图1)。这些结果说明,Ile[5]和胆碱[10]可以分别通过上调TGF-β2和IL-10的表达来降低鱼类免疫器官促炎症细胞因子IL-1β和TNF-α的产生。在陆生动物上的研究表明,细胞因子同样也受到多种胞内信号途径的调控[50]。近年来研究表明,哺乳动物雷帕霉素靶蛋白(mammalian target of papamycin,mTOR)在调控人类免疫细胞细胞因子的产生过程中发挥重要作用,能够通过抑制转录因子核因子-κB(nuclear factor-κB,NF-κB)来降低促炎症细胞因子的产生,同时还能够增强转录因子信号转导与转录激活因子3(signal transducer and activator of transcription 3,STAT3)的活性,促进抗炎症细胞因子IL-10的产生[51]。在鲤鱼上已成功克隆到雷帕霉素靶蛋白(target of papamycin,TOR)[52]及其下游信号分子真核翻译起始因子4E结合蛋白(eukaryotic translation initiation factor 4E binding protein,4E-BP)(NCBI登录号分别为FJ899680和HQ010440)基因的cDNA序列。研究表明,饲料中适宜水平的胆碱上调攻毒后幼建鲤脾脏TOR mRNA表达而下调4E-BP mRNA的表达,且头肾TOR和4E-BP mRNA的表达与脾脏相反[10];Ile对幼建鲤头肾TOR和4E-BP mRNA表达的影响同胆碱对脾脏TOR和4E-BP mRNA表达的影响趋势一致[5](图1),表明Ile[5]和胆碱[10]可通过TOR信号途径调控鱼类免疫器官细胞因子的产生,且调控作用存在差异,具体作用机制有待进一步研究。
5 小 结营养物质能够通过提高非特异和特异性免疫力增强鱼类疾病抵抗力。非特异和特异性免疫力的提高与营养物质促进了鱼类免疫器官的生长发育有关。鱼类免疫器官的生长发育部分依赖于其结构完整性和抗氧化能力。营养物质主要通过作为细胞组成成分(如胆碱等)以及影响细胞非酶性和酶性抗氧化系统2个方面来提高鱼类免疫器官的抗氧化能力。营养物质对酶性抗氧化系统的影响与其通过抗氧化关键信号分子Nrf2调控鱼类免疫器官抗氧化酶mRNA的表达有关。此外,营养物质还能够通过影响鱼类免疫器官TOR信号途径上调抗炎症细胞因子mRNA的表达,下调促炎症细胞因子mRNA的表达,进而减少促炎症细胞因子诱导的氧化应激。然而,不同营养物质对鱼类不同免疫器官的生长发育、抗氧化防御及细胞因子的调控存在差异。这种差异提示我们虽然各种营养物质在最适添加水平时不约而同地表现出提高免疫力进而增强鱼类疾病抵抗力的终端效应,但由于各种营养物质自身物理化学特点,鱼类对不同营养物质消化吸收、代谢以及器官需求和利用的差异,可能导致不同营养物质对鱼类免疫调控的特异性。目前,关于营养物质对鱼类免疫功能调控的研究仅处于起步阶段,更深入的调控机制有待进一步研究。
| [1] | EL-BOSHY M E,EL-ASHRAM A M,ABDELHAMID F M,et al.Immunomodulatory effect of dietary Saccharomyces cerevisiae,β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila[J]. Fish & Shellfish Immunology,2010,28(5/6):802-808. ( 1) 1)
|
| [2] | KIRON V.Fish immune system and its nutritional modulation for preventive health care[J]. Animal Feed Science and Technology,2012,173(1/2):111-133. ( 1) 1)
|
| [3] | ZUO R T,AI Q H,MAI K S,et al.Effects of dietary n-3 highly unsaturated fatty acids on growth,nonspecific immunity,expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans)[J]. Fish & Shellfish Immunology,2012,32(2):249-258. ( 1) 1)
|
| [4] | TANG L,WANG G X,JIANG J,et al.Effect of methionine on intestinal enzymes activities,microflora and humoral immune of juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Aquaculture Nutrition,2009,15(5):477-483. ( 6) 6)
|
| [5] | ZHAO J,LIU Y,JIANG J,et al.Effects of dietary isoleucine on the immune response,antioxidant status and gene expression in the head kidney of juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Fish & Shellfish Immunology,2013,35(2):572-580. ( 21) 21)
|
| [6] | YANG Q H,ZHOU X Q,JIANG J,et al.Effect of dietary vitamin A deficiency on growth performance,feed utilization and immune responses of juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Aquaculture Research,2008,39(8):902-906. ( 8) 8)
|
| [7] | FENG L,HUANG H H,LIU Y,et al.Effect of dietary thiamin supplement on immune responses and intestinal microflora in juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Aquaculture Nutrition,2011,17(5):557-569. ( 11) 11)
|
| [8] | FENG L,HE W,JIANG J,et al.Effects of dietary pyridoxine on disease resistance,immune responses and intestinal microflora in juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Aquaculture Nutrition,2010,16(3):254-261. ( 11) 11)
|
| [9] | WEN Z P,FENG L,JIAN G J,et al.Immune response,disease resistance and intestinal microflora of juvenile Jian carp (Cyprinus carpio var.Jian) fed graded levels of pantothenic acid[J]. Aquaculture Nutrition,2010,16(4):430-436. ( 11) 11)
|
| [10] | WU P,JIANG J,LIU Y,et al.Dietary choline modulates immune responses,and gene expressions of TOR and eIF4E-binding protein 2 in immune organs of juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Fish & Shellfish Immunology,2013,35(3):697-706. ( 17) 17)
|
| [11] | JIANG W D,FENG L,LIU Y,et al.Effects of graded levels of dietary myo-inositol on non-specific immune and specific immune parameters in juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Aquaculture Research,2010,41(10):1413-1420. ( 10) 10)
|
| [12] | KUANG S Y,XIAO W W,FENG L,et al.Effects of graded levels of dietary methionine hydroxy analogue on immune response and antioxidant status of immune organs in juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Fish & Shellfish Immunology,2012,32(5):629-636. ( 22) 22)
|
| [13] | ZHOU Q,WANG L G,WANG H L,et al.Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum)[J]. Fish & Shellfish Immunology,2012,32(6):969-975. ( 1) 1)
|
| [14] | SONG S K,BECK B R,KIM D,et al.Prebiotics as immunostimulants in aquaculture:a review[J]. Fish & Shellfish Immunology,2014,40(1):40-48. ( 3) 3)
|
| [15] | AOKI T,TAKANO T,SANTOS M D,et al.Molecular innate immunity in teleost fish:review and future perspectives[C]//TSUKAMOTO K,KAWAMURA T,TAKEUCHI T,et al.Fisheries for global welfare and environment.Tokyo:TERRAPUB,2008:263-276. ( 1) 1)
|
| [16] | SALINAS I,CUESTA A,ESTEBAN M ,et al.Dietary administration of Lactobacillus delbrueckii and Bacillus subtilis,single or combined,on gilthead seabream cellular innate immune responses[J]. Fish & Shellfish Immunology,2005,19(1):67-77. ( 1) 1)
|
| [17] | CEREZUELA R,GUARDIOLA F A,MESEGUER J,et al.Increases in immune parameters by inulin and Bacillus subtilis dietary administration to gilthead seabream (Sparus aurata L.) did not correlate with disease resistance to Photobacterium damselae[J]. Fish & Shellfish Immunology,2012,32(6):1032-1040. ( 1) 1)
|
| [18] | WHYTE S K.The innate immune response of finfish:a review of current knowledge[J]. Fish & Shellfish Immunology,2007,23(6):1127-1151. ( 1) 1)
|
| [19] | RAUTA P R,NAYAK B,DA S S.Immune system and immune responses in fish and their role in comparative immunity study:a model for higher organisms[J]. Immunology Letters,2012,148(1):23-33. ( 1) 1)
|
| [20] | L VOLL M,DALMO R A,B GWALD J.Extrahepatic synthesis of complement components in the rainbow trout (Oncorhynchus mykiss)[J]. Fish & Shellfish Immunology,2007,23(4):721-731. ( 1) 1)
|
| [21] | TURNER R J.Immunology:a comparative approach[M]. New York:John Wiley & Sons Ltd.,1994. ( 1) 1)
|
| [22] | DIETRICH M A,Z . MIJEWSKI D K,AROL H,et al.Isolation and characterization of transferrin from common carp (Cyprinus carpio L.) seminal plasma[J]. Fish & Shellfish Immunology,2010,29(1):66-74. ( 1) 1)
|
| [23] | STAFFORD J L,BELOSEVIC M.Transferrin and the innate immune response of fish:identification of a novel mechanism of macrophage activation[J]. Developmental & Comparative Immunology,2003,27(6):539-554. ( 1) 1)
|
| [24] | ESLAMLOO K,FALAHATKAR B,YOKOYAMA S.Effects of dietary bovine lactoferrin on growth,physiological performance,iron metabolism and non-specific immune responses of Siberian sturgeon Acipenser baeri[J]. Fish & Shellfish Immunology,2012,32(6):976-985. ( 1) 1)
|
| [25] | PARKIN J,COHEN B.An overview of the immune system[J]. The Lancet,2001,357(9270):1777-1789. ( 1) 1)
|
| [26] | HARIKRISHNAN R,BALASUNDARAM C,HEO M S.Effect of Inonotus obliquus enriched diet on hematology,immune response,and disease protection in kelp grouper,Epinephelus bruneus against Vibrio harveyi[J]. Aquaculture,2012,344-349:48-53. ( 1) 1)
|
| [27] | ELLIS A E.Fish immune system[M]//ROITT I M.Encyclopedia of immunology.London:Academic Press,1998:920-926. ( 2) 2)
|
| [28] | ZAPATA A,DIEZ B,CEJALVO T,et al.Ontogeny of the immune system of fish[J]. Fish & Shellfish Immunology,2006,20(2):126-136. ( 1) 1)
|
| [29] | PRESS C M,EVENSEN Ø.The morphology of the immune system in teleost fishes[J]. Fish & Shellfish Immunology,1999,9(4):309-318. ( 1) 1)
|
| [30] | CHENG Z,GATLIN III D M,BUENTELLO A.Dietary supplementation of arginine and/or glutamine influences growth performance,immune responses and intestinal morphology of hybrid striped bass (Morone chrysops × Morone saxatilis)[J]. Aquaculture,2012,362/363:39-43. ( 1) 1)
|
| [31] | YONAR M E.The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss,W.)[J]. Fish Shellfish Immunol,2012,32(6):994-1001. ( 1) 1)
|
| [32] | FATIMA M,AHMAD I,SAYEED I,et al.Pollutant-induced over-activation of phagocytes is concomitantly associated with peroxidative damage in fish tissues[J]. Aquatic Toxicology,2000,49(4):243-250. ( 1) 1)
|
| [33] | WAAGBÓ R,HEMRE G I,HOLM J C,et al.Tissue fatty acid composition,haematology and immunity in adult cod,Gadus morhua L.,fed three dietary lipid sources[J]. Journal of Fish Diseases,1995,18(6):615-622. ( 1) 1)
|
| [34] | TKACHENKO H,KURHALUK N,GRUDNIEWSKA J,et al.Tissue-specific responses of oxidative stress biomarkers and antioxidant defenses in rainbow trout Oncorhynchus mykiss during a vaccination against furunculosis[J]. Fish Physiology and Biochemistry,2014,40(4):1289-1300 ( 1) 1)
|
| [35] | WU P,JIANG W D,LIU Y,et al.Effect of choline on antioxidant defenses and gene expressions of Nrf2 signaling molecule in the spleen and head kidney of juvenile Jian carp (Cyprinus carpio var.Jian)[J]. Fish & Shellfish Immunology,2014,38(2):374-382. ( 14) 14)
|
| [36] | HALLIWELL B.Free radicals and antioxidants-quo vadis?[J]. Trends in Pharmacological Sciences,2011,32(3):125-130. ( 1) 1)
|
| [37] | JIANG J,ZHENG T,ZHOU X Q,et al.Influence of glutamine and vitamin E on growth and antioxidant capacity of fish enterocytes[J]. Aquaculture Nutrition,2009,15:409-414. ( 1) 1)
|
| [38] | SAITO H,ISHIHARA K.Antioxidant activity and active sites of phospholipids as antioxidants[J]. Journal of the American Oil Chemists’ Society,1997,74(12):1531-1536. ( 1) 1)
|
| [39] | MARTÍNEZ-ÁLVAREZ R M,MORALES A E,SANZ A.Antioxidant defenses in fish:biotic and abiotic factors[J]. Reviews in Fish Biology and Fisheries,2005,15(1/2):75-88. ( 2) 2)
|
| [40] | DE LA ROSA L C,GARCÍA-RUIZ C,FERNÁNDEZ-CHECA J C.Glutathione in mammalian biology[M]//Systems biology of free radicals and antioxidants.Berlin:Springer-Verlag,2014:617-644. ( 1) 1)
|
| [41] | ZHANG Y K J,YEAGER R L,TANAKA Y,et al.Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet[J]. Toxicology and Applied Pharmacology,2010,245(3):326-334. ( 1) 1)
|
| [42] | ATTIA A A,ELMAZOUDY R H,EL-SHENAWY N S.Antioxidant role of propolis extract against oxidative damage of testicular tissue induced by insecticide chlorpyrifos in rats[J]. Pesticide Biochemistry and Physiology,2012,103(2):87-93. ( 1) 1)
|
| [43] | LICKTEIG A J,FISHER C D,AUGUSTINE L M,et al.Genes of the antioxidant response undergo upregulation in a rodent model of nonalcoholic steatohepatitis[J]. Journal of Biochemical and Molecular Toxicology,2007,21(4):216-220. ( 1) 1)
|
| [44] | BRYAN H K,OLAYANJU A,GOLDRING C E,et al.The Nrf2 cell defence pathway:Keap1-dependent and -independent mechanisms of regulation[J]. Biochemical Pharmacology,2013,85(6):705-717. ( 1) 1)
|
| [45] | KOBAYASHI M,LI L,IWAMOTO N,et al.The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds[J]. Molecular and Cellular Biology,2009,29(2):493-502. ( 1) 1)
|
| [46] | KOBAYASHI M,ITOH K,SUZUKI T,et al.Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system[J]. Genes to Cells,2002,7(8):807-820. ( 1) 1)
|
| [47] | JIANG W D,LIU Y,HU K,et al.Copper exposure induces oxidative injury,disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain:protective effects of myo-inositol[J]. Aquatic Toxicology,2014,155:303-313. ( 1) 1)
|
| [48] | TYAGI E,AGRAWAL R,NATH C,et al.Influence of LPS-induced neuroinflammation on acetylcholinesterase activity in rat brain[J]. Journal of Neuroimmunology,2008,205(1/2):51-56. ( 1) 1)
|
| [49] | REYES-CERPA S,MAISEY K,REYES-LÓPEZ F,et al.Fish cytokines and immune response[M]//TVRKER H.New advances and contributions to fish biology.Croatia:InTech,2013:3-58. ( 1) 1)
|
| [50] | WEICHHART T,SAEMANN M D.The multiple facets of mTOR in immunity[J]. Trends in Immunology,2009,30(5):218-226. ( 1) 1)
|
| [51] | WEICHHART T,COSTANTINO G,POGLITSCH M,et al.The TSC-mTOR signaling pathway regulates the innate inflammatory response[J]. Immunity,2008,29(4):565-577. ( 1) 1)
|
| [52] | JIANG J,FENG L,LIU Y,et al.Mechanistic target of rapamycin in common carp:cDNA cloning,characterization,and tissue expression[J]. Gene,2013,512(2):566-572. ( 1) 1)
|



