2. 江西农业大学动物科学技术学院, 南昌 330045
2. School of Animal Science and Technology, Jiangxi Agricultural University, Nanchang 330045, China
水产养殖业可持续发展与鱼油资源有限性的矛盾日益激化,寻找合适的替代脂肪源势在必行。植物油因具备供应稳定、相对低的二 英和其他有机污染物含量、较大可获得性和较高含量不饱和脂肪酸等优点,已成为极具潜力的鱼油替代者。相对于其他植物油,菜籽油具有适宜的C18 ∶ 2n-6[亚油酸(LA)],低含量的C18 ∶ 3n-3[α-亚麻酸(ALA)]和高含量的C18 ∶ 1n-9,被认为是能量代谢偏好的底物[1]。有研究表明,菜籽油替代少量鱼油不会显著影响鱼体生长和饲料利用[2, 3, 4, 5, 6, 7, 8, 9],但菜籽油全部替代鱼油会对鱼体生长和品质产生显著影响,如生长速度下降[10]、腹腔或肝脏脂肪过多沉积[11]、肝脏组织发生病变[12]、肌肉中n-3长链多不饱和脂肪酸(n-3 LC-PUFA)含量显著下降[2, 3, 4, 5, 8, 9]等。鱼肉对人类营养的最大价值在于其是n-3 LC-PUFA等活性物质的来源。摄食高含量n-3 LC-PUFA鱼肉后会对人体健康产生积极的影响,如抑制心脏病的发生和降低炎性反应的发生[13]。因此,对植物油替代效果的衡量不仅需关注鱼体的生长和饲料利用,还需关注鱼体健康和鱼肉n-3 LC-PUFA含量。
大菱鲆(Scophthalmus maximus L.)是我国北方重要的经济养殖种类,因其美味的肉质、快速生长的特性及较高的经济价值,在亚洲和欧洲被广泛养殖。目前,已有的关于大菱鲆饲料中植物油替代鱼油的研究主要涉及豆油、亚麻籽油、橄榄油和红花油[14, 15, 16],有关菜籽油在大菱鲆上的替代效果研究还未见报道。本试验以菜籽油分别替代饲料中0、33.3%、66.7%和100.0%的鱼油,探究其对大菱鲆生长、脂肪酸组成及脂肪沉积的影响,为大菱鲆饲料中植物油替代鱼油提供理论依据,以缓解鱼油短缺难题,更好地促进水产养殖业的可持续发展。
1 材料与方法 1.1 试验饲料配方和制备以白鱼粉、豆粕、谷朊粉和酪蛋白作为主要的蛋白质源,以菜籽油分别替代0、33.3%、66.7%和100.0%的鱼油,配制粗蛋白质含量为50%(干物质基础)、粗脂肪含量为12%(干物质基础)的4种等氮等脂饲料(表1)。鱼油和菜籽油分别购于福山大茂饲料有限公司和宁波奉化大堰阿拉榨油坊,其脂肪酸组成见表2。因鱼粉中含有脂肪,鱼油中LC-PUFA含量占总脂肪酸的18%~25%(参照李庆民等[17]、Turchini等[18]的方法计算)。以鱼油总脂肪酸中含有18%的LC-PUFA计,可推测出全鱼油组、33.3%菜籽油组、66.7%菜籽油组和全菜籽油组饲料中n-3 LC-PUFA含量分别约为1.70%、1.25%、0.80%和0.35%。
| 表1 试验饲料组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of experimental diets (DM basis) |
| 表2 脂肪源和饲料脂肪酸组成(占总脂肪酸的百分比) Table 2 Fatty acid composition of lipid sources and diets (percentage of total fatty acids) |
饲料制备之前,鱼粉和豆粕等饲料原料先经粉碎,过320 μm筛网,再按配比从小到大逐级定量均匀混合;较大的混合物进入V型搅拌机充分混合25 min。将大豆卵磷脂、菜籽油和鱼油混合均匀,随后将其与已混好的干粉充分混匀,再加入适量的水揉匀,全自动渔用饵料机(F-26Ⅱ,华南理工大学研制)将其加工制成直径为3.0 mm的硬颗粒饲料,45~55 ℃烘干至饲料水分含量为10%左右,待饲料颗粒干燥冷却后,双层塑料袋包装并封口,所制饲料置于-20 ℃冰箱保存备用,以防止饲料脂肪被氧化。 1.2 饲养管理和样品采集
试验用统一规格的同批次健康大菱鲆幼鱼购于青岛胶州养殖场。在正式试验之前,所有试验鱼先用大菱鲆0#料(七好生物科技有限公司提供)暂养1周适应养殖系统,再用自制硬颗粒饲料(混合的本试验配制的4种饲料)暂养1周使其适应自制饲料。在饥饿24 h后,初重为(5.89±0.02) g的大菱鲆幼鱼被随机分到12个桶(300 L,35尾/桶)。每种饲料随机饲喂3桶试验鱼。每天投喂2次(08:00和18:00),饱食投喂,养殖周期为92 d。大菱鲆在饲料下沉过程时摄食,摄食后,桶内多余的饲料和粪便立即用吸管吸去。记录摄食量,如有死鱼记录数量并称重。海水持续经由水泵泵到泡沫分离器,经由生物塔砂滤到高位池后最终进入循环系统,控制每桶水流速2 L/min。每次摄食完后,循环水系统排污,在试验第1个月,每桶更换1/2的水,之后更换70%桶体积水。循环水系统使用气石持续充氧。在养殖过程中,水温17.5~19.0 ℃;盐度28.0%~31.0%;溶氧浓度在7 mg/L左右;氨态氮(NH4-N)、硝态氮(NO3-N)和亚硝酸盐氮(NO2-N)浓度均低于100.0 μg/L。从试验第6周开始,投喂5 h后收集桶内成形的粪便,放入5 mL离心管,2 000×g离心10 min后保存于-20 ℃,待后期用于表观消化率的测定。
在试验开始之前,随机挑出15尾鱼做全鱼常规营养成分分析。养殖试验结束时,所有鱼都饥饿处理24 h。每桶各取6尾鱼称重,取背部肌肉、肝脏,称重。将肝脏和肌肉放入5 mL离心管,液氮速冻,随后保存在-80 ℃,待后期用于脂肪酸组成以及水分、粗脂肪含量的测定。此外,每桶取5尾鱼放入样品袋,-20 ℃保存,待后期用于全鱼常规营养成分分析。
1.3 指标测定 1.3.1 常规营养成分分析饲料原料、饲料和试验鱼均测定水分、粗蛋白质、粗脂肪和粗灰分含量。样品在105 ℃烘干到恒重后求得干物质含量,然后进行生化组分分析。粗蛋白质含量的测定采用凯氏定氮法;粗脂肪含量的测定采用索氏抽提法;粗灰分含量的测定采用马弗炉灼烧法。每份样品均重复测定2次,若相对偏差大于2%,则增加重复次数,采用相对偏差在2%以下的2个测定值的平均值为测定结果。
肝脏和肌肉脂肪含量分析参照Floch等[19]方法略作修改,详见彭墨等[20]的方法。
脂肪酸组成的测定参照Metcalfe等[21]的方法略作修改,详见彭墨等[20]的方法。
1.3.3 钇含量测定将粪便和饲料样品冷冻干燥20 h,研磨成粉,再次冷冻干燥6 h。称取100 mg粪便(或饲料)于干净烧瓶中,加入10 mL高氯酸,电炉上灼烧,直到烧瓶中液体呈透明状。将透明液体转移至100 mL容量瓶中,用双蒸水定容,0.22 μm滤膜过滤后即可借助电感耦合等离子体原子发射光谱仪(ICP-OES,瓦里安公司,美国)测定钇的含量。
1.4 计算公式存活率(survival rate,SR,%)=100×终末鱼数量/初始鱼数量;
特定生长率(special growth rate,SGR,%/d)=100×(ln终末体重-ln初始体重)/试验天数;
饲料效率(feed conversion rate,FCR)=(终末体重-初始体重)/饲料干物质消耗量;
摄食率(feed intake ratio,FIR,%/d)=100×饲料干物质消耗量/[(初始体重+终末体重)/2]/试验天数;
表观净蛋白质利用率(apparent net protein utilization,ANPU,%)=100×(末期鱼体蛋白质含量-初始鱼体蛋白质含量)/摄食饲料蛋白质的量;
肝体比(hepatosomatic index,HSI,%)=100×肝脏重/体重;
脂肪表观消化率[apparent digestibility coefficient (ADC) of lipid,%]=100×[1-(饲料中Y2O3含量/饲料脂肪含量)×(粪便脂肪含量/粪便中Y2O3含量)]。
1.5 统计分析试验数据采用SPSS 16.0 软件进行统计分析,在单因素方差分析(one-way ANOVA)达到显著水平时(P<0.05),采用Turkey’s检验进行多重比较,数据表示为平均值±标准误的形式。
2 结 果 2.1 菜籽油替代鱼油对大菱鲆幼鱼存活、生长和形体指标的影响由表3可知,全菜籽油组大菱鲆幼鱼SR较其他组显著下降(P<0.05),与其他各组间差异不显著(P>0.05)。各组大菱鲆幼鱼的FIR在1.51%/d~1.63%/d范围内,但菜籽油替代水平并未显著影响大菱鲆幼鱼的FIR(P>0.05)。大菱鲆幼鱼的终末体重和SGR均随菜籽油替代水平的升高而下降,全菜籽油组的终末体重和SGR显著低于全鱼油组(P<0.05),其他组间差异不显著(P>0.05)。菜籽油替代水平并未显著影响大菱鲆幼鱼的FCR和ANPU(P>0.05)。大菱鲆幼鱼的HSI随菜籽油替代水平的升高而升高(P<0.05),且全菜籽油组的HSI显著高于全鱼油组(P<0.05),与其他组间差异不显著(P>0.05)。
| 表3 菜籽油替代鱼油对大菱鲆幼鱼存活、生长和形体指数的影响 Table 3 Effects of fish oil replacement by rapeseed oil on growth,survival and body parameters of juvenile turbot (Scophthalmus maximus L.)(n=3) |
由表4可知,菜籽油替代水平并未显著影响大菱鲆幼鱼的体成分和肌肉脂肪含量以及饲料脂肪表观消化率(P>0.05)。大菱鲆幼鱼的肝脏脂肪含量随着菜籽油替代水平的升高而升高,当菜籽油替代66.7%和100.0%的鱼油时,该组肝脏脂肪含量显著高于全鱼油组(P<0.05),且全菜籽油组肝脏脂肪含量还显著高于33.3%菜籽油组(P<0.05),但与66.7%菜籽油组间差异不显著(P>0.05)。
| 表4 菜籽油替代鱼油对大菱鲆幼鱼体成分、肝脏和肌肉脂肪含量以及饲料脂肪表观消化率的影响
Table 4 Effects of fish oil replacement by rapeseed oil on body composition,lipid content in muscle and liver of juvenile turbot (Scophthalmus maximus L.) and dietary lipid apparent digestibility (n=3)
|
随着菜籽油替代水平的升高,大菱鲆幼鱼肝脏中C18 ∶ 1及肝脏和肌肉中LA和ALA含量随之升高,肝脏和肌肉中C14 ∶ 0、C18 ∶ 0、C20 ∶ 4n-6[花生四烯酸(ARA)]、C20 ∶ 5n-3(EPA)和C22 ∶ 6n-3(DHA)含量随之下降(表5)。相对于全鱼油组,66.7%菜籽油组和全菜籽油组肌肉和肝脏中LA和ALA含量显著升高(P<0.05),而EPA和DHA含量则显著下降(P<0.05)。饲料C18 ∶ 1含量与大菱鲆幼鱼肝脏和肌肉中C18 ∶ 1、LA、ALA、EPA和DHA含量存在正相关关系(图1)。
| 表5 菜籽油替代鱼油对大菱鲆幼鱼肝脏和肌肉脂肪酸组成的影响(占总脂肪酸的百分比) Table 5 Effects of fish oil replacement by rapeseed oil on fatty acid composition in liver and muscle of turbot (Scophthalmus maximus L.) (percentage of total fatty acids) |
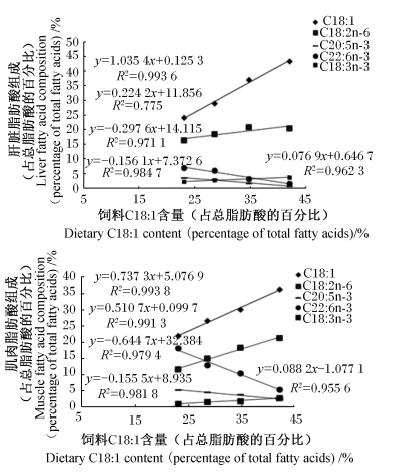 | 图1 饲料C18 ∶ 1含量与大菱鲆幼鱼肝脏和肌肉中C18 ∶ 1、C18 ∶ 2n-6、C18 ∶ 3n-3、C20 ∶ 5n-3和C22 ∶ 6n-3含量的关系 Fig. 1 Relationship between dietary C18 ∶ 1 content and C18 ∶ 1n,C18 ∶ 2n-6,C18 ∶ 3n-3,C20 ∶ 5n-3 and C22 ∶ 6n-3 contents in liver and muscle of turbot (Scophthalmus maximus L.) |
本试验研究发现,在养殖周期为92 d时,饲料中菜籽油替代66.7%的鱼油并未显著影响大菱鲆幼鱼的生长和饲料利用。该结果与一些海水鱼如欧洲鲈鱼(Dicentrarchus labrax L.)[6]和金头鲷(Sparus aurata)[7]的研究结果一致。但当鱼油全部被菜籽油替代时,则会显著影响大菱鲆的生长,这与布鲁克嘉鱼(Salvelinus fontinalis)[2]、大西洋鲑鱼(Salmo salar L.)[3, 4, 5]、虹鳟(Oncorhynchus mykiss)[8]和青鱼(Mylopharyngodon piceus)[9]上的研究不一致,上述这些研究表明菜籽油全部替代鱼油并未显著鱼体的生长。Turchini等[18]指出,当植物油替代全部鱼油时,鱼体出现生长下降的原因大多与饲料中的n-3 LC-PUFA不足有关。本试验中,大菱鲆幼鱼的FE、FIR及饲料的脂肪表观消化率均未受到菜籽油替代水平的显著影响,因此全菜籽油组鱼体生长下降的原因可能是饲料中n-3 LC-PUFA的含量不能满足鱼体需求。在本试验中,全鱼油组、33.3%菜籽油组、66.7%菜籽油组和全菜籽油组n-3 LC-PUFA的含量分别约为1.70%、1.25%、0.80%和0.35%。Léger等[22]研究指出,大菱鲆必需脂肪酸需求可能低于0.6%;Gatesoupe等[23]指出,大菱鲆必需脂肪酸需求可能在0.8%。因此,全菜籽油组饲料中0.35%的n-3 LC-PUFA含量不能满足鱼体对n-3 LC-PUFA的需求,故可能影响大菱鲆对饲料其他营养素的利用。
本试验中,菜籽油替代水平并未显著影响大菱鲆幼鱼的体成分。而另有研究表明饲料中菜籽油含量过高时显著影响青鱼的鱼体水分含量[9],结果的不同可能与养殖鱼类和养殖时间有关。本研究发现,大菱鲆幼鱼的HSI随菜籽油替代水平的升高而升高,且全菜籽油组的HSI显著高于全鱼油组。这可能与肝脏脂肪含量升高有关。大菱鲆幼鱼的肝脏脂肪含量随菜籽油替代水平的升高而升高,其结果与在青鱼[9]和金头鲷[11]上所得结果一致。脂肪过多沉积于肝脏可能与肝脏n-3/n-6 PUFA的不平衡密切有关[24]。本试验中全鱼油组、33.3%菜籽油组、66.7%菜籽油组和全菜籽油组饲料中n-3/n-6 PUFA分别为1.20%、0.88%、0.61%和0.42%,相对应的4种饲料中n-3 LC-PUFA的含量分别约为1.70%、1.25%、0.80%和0.35%。n-3 LC-PUFA一方面能激活过氧化物酶体增殖物激活受体α(peroxisome proliferators-activated receptor α,PPARα),进而促进脂肪分解[25];另一方面能抑制固醇调节元件结合蛋白-1c(sterol regulatory element-binding protein 1c,SREBP-1c)的表达,进而抑制脂肪合成[26]。因此,菜籽油替代鱼油后n-3/n-6 PUFA的不平衡和较低n3 LC-PUFA含量可能造成肝脏脂肪过多沉积。
鱼类脂肪酸组成与人类健康息息相关[27]。本试验研究发现,随菜籽油替代水平的升高,肝脏ARA、EPA和DHA含量以及n-3/n-6 PUFA均随之下降,而肝脏C18 ∶ 1、LA和ALA含量则随之升高,且与饲料C18 ∶ 1之间存在显著的相关性。虽然饲料中LA和ALA含量随菜籽油替代水平的升高而升高,但肝脏和肌肉中ARA、EPA和DHA含量则随之下降。这可能与大菱鲆没有或只具有较低转化LA为ARA或ALA为EPA和DHA的能力有关。尽管大菱鲆△6脂肪酸去饱和酶(Fad)活力较高,但其△5Fad和延长酶(从C18到C20)活力相对较低[28, 29]。此外,肝脏和肌肉中ARA含量降低可能还与ARA转化为类二十烷酸产物,如前列腺素2(PGE2)等炎性物质有关,最终影响鱼体的免疫能力。本试验研究发现,全菜籽油组存活率低于其他3组。因此,菜籽油高水平替代鱼油可能会影响鱼体的免疫力,其机理还未知,需进一步深入研究。
4 结 论菜籽油替代66.7%的鱼油虽未显著影响大菱鲆幼鱼生长和饲料利用,但显著影响大菱鲆肝脏脂肪含量和肌肉n-3 LC-PUFA含量,故从营养品质的角度,在大菱鲆幼鱼饲料中菜籽油替代鱼油的水平应低于66.7%。
致谢:感谢国家鲆鲽类产业技术体系(CARS 50-G08)经费的资助,感谢同实验室左然涛博士在试验方案设计以及孟玉琼、王震、韩丽蓉、路凯和阳钢同学在饲料制备、养殖试验及样品采集时给予的帮助。
| [1] | HENDERSON R J.Fatty acid metabolism in freshwater fish with particular reference to polyunsaturated fatty acids[J]. Archiv fûr Tierernährung,1996,49(1):5-22. ( 1) 1)
|
| [2] | GUILLOU A,SOUCY P,KHALIL M,et al.Effects of dietary vegetable and marine lipid on growth,muscle fatty acid composition and organoleptic quality of flesh of brook charr (Salvelinus fontinalis)[J]. Aquaculture,1995,136(3/4):351-362. ( 3) 3)
|
| [3] | TOCHER D R,BELL J G,DICK J R,et al.Polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr-smolt transformation and the effects of dietary linseed and rapeseed oils[J]. Fish Physiology and Biochemistry,2000,23(1):59-73. ( 3) 3)
|
| [4] | BELL J G,TOCHER D R,HENDERSON R J,et al.Altered fatty acid compositions in Atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet[J]. The Journal of Nutrition,2003,133(9):2793-2801. ( 3) 3)
|
| [5] | TORSTENSEN B E,FRØYLAND L,LIE Ø.Replacing dietary fish oil with increasing levels of rapeseed oil and olive oil—effects on Atlantic salmon (Salmo salar L.) tissue and lipoprotein lipid composition and lipogenic enzyme activities[J]. Aquaculture Nutrition,2004,10(3):175-192. ( 3) 3)
|
| [6] | MOURENTE G,GOOD J E,BELL J G.Partial substitution of fish oil with rapeseed,linseed and olive oils in diets for European sea bass (Dicentrarchus labrax L.):effects on flesh fatty acid composition,plasma prostaglandins E2 and F2α,immune function and effectiveness of a fish oil finishing diet[J]. Aquaculture Nutrition,2005,11(1):25-40. ( 2) 2)
|
| [7] | IZQUIERDO M S,MONTERO D,ROBAINA L,et al.Alterations in fillet fatty acid profile and flesh quality in gilthead seabream (Sparus aurata) fed vegetable oils for a long term period.Recovery of fatty acid profiles by fish oil feeding[J]. Aquaculture,2005,250(1/2):431-444. ( 2) 2)
|
| [8] | PETTERSSON A,JOHNSSON L,BRÄNNÄS E,et al.Effects of rapeseed oil replacement in fish feed on lipid composition and self-selection by rainbow trout (Oncorhynchus mykiss)[J]. Aquaculture Nutrition,2009,15(6):577-586. ( 3) 3)
|
| [9] | SUN S,YE J,CHEN J,et al.Effect of dietary fish oil replacement by rapeseed oil on the growth,fatty acid composition and serum non-specific immunity response of fingerling black carp,Mylopharyngodon piceus[J]. Aquaculture Nutrition,2011,17(4):441-450. ( 5) 5)
|
| [10] | 王骥腾,韩涛,田丽霞,等.3种植物油源对军曹鱼生长、体组成和脂肪酸组成的影响[J]. 浙江海洋学院学报:自然科学版,2007,26(3):237-246. ( 1) 1)
|
| [11] | FOUNTOULAKI E,VASILAKI A,HURTADO R,et al.Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.):effects on growth performance,flesh quality and fillet fatty acid profile:recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures[J]. Aquaculture,2009,289(3/4):317-326. ( 2) 2)
|
| [12] | CABALLERO M J,OBACH A,ROSENLUND G,et al.Impact of different dietary lipid sources on growth,lipid digestibility,tissue fatty acid composition and histology of rainbow trout,Oncorhynchus mykiss[J]. Aquaculture,2002,214(1/2/3/4):253-271. ( 1) 1)
|
| [13] | SIMOPOULOS A P.Essential fatty acids in health and chronic disease[J]. The American Journal of Clinical Nutrition,1999,70(3):560s-569s. ( 1) 1)
|
| [14] | BELL J G,TOCHER D R,MACDONALD F M,et al.Effects of diets rich in linoleic (18:2n-6) and α-linolenic (18:3n-3) acids on the growth,lipid class and fatty acid compositions and eicosanoid production in juvenile turbot (Scophthalmus maximus L.)[J]. Fish Physiology and Biochemistry,1994,13(2):105-118. ( 1) 1)
|
| [15] | BEll J G,TOCHER D R,FARNDALE B M,et al.Effects of essential fatty acid-deficient diets on growth,mortality,tissue histopathology and fatty acid compositions in juvenile turbot (Scophthalmus maximus)[J]. Fish Physiology and Biochemistry,1999,20(3):263-277. ( 1) 1)
|
| [16] | ALTUNDAG M S,TIRIL S U,OZDEMIR A.Effects of safflower oil supplementation in diet on growth performance and body fatty acid composition of turbot (Psetta maxima)[J]. Aquaculture International,2014,22(2):597-605. ( 1) 1)
|
| [17] | 李庆民,陈桂范,高实,等.气相色谱外标法测定鱼油中EPA和DHA的含量[J]. 沈阳药科大学学报,1996,13(4):259-261. ( 1) 1)
|
| [18] | TURCHINI G M,TORSTENSEN B E,NG W K.Fish oil replacement in finfish nutrition[J]. Reviews in Aquaculture,2009,1(1):10-57. ( 2) 2)
|
| [19] | FOLCH J,LEES M,SLOANE STANLEY G H.A simple method for the isolation and purification of total lipides from animal tissues[J]. The Journal of Biological Chemistry,1957,226(1):497-509. ( 1) 1)
|
| [20] | 彭墨,徐玮,麦康森,等.亚麻籽油替代鱼油对大菱鲆幼鱼生长、脂肪酸组成及脂肪沉积的影响[J]. 水产学报,2014,38(8):1131-1139. ( 2) 2)
|
| [21] | METCALFE L D,SCHMITZ A A,PELKA J R.Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis[J]. Analytical Chemistry,1966,38(3):514-515. ( 1) 1)
|
| [22] | LÉGER C,GATESOUPE FJ,METAILLER R,et al.Effect of dietary fatty acids differing by chain lengths and ω series on the growth and lipid composition of turbot Scophthalmus maximus L.[J]. Comparative Biochemistry and Physiology Part B:Comparative Biochemistry,1979,64(4):345-350. ( 1) 1)
|
| [23] | GATESOUPE F J,LEGER C,BOUDON M,et al.Lipid feeding of turbot (Scophthalmus maximus L.):influence on growth of supplementation with methyl esters of linolenic acid and fatty acid acids of w-9 series[J]. Annales d'Hydrobiologie,1977,8:247-254. ( 1) 1)
|
| [24] | TAKEUCHI T,WATANABE T.Effect of excess amounts of essential fatty acids on growth of rainbow trout[J]. Bulletin of the Japanese Society of Scientific Fisheries,1979,45:1517-1519. ( 1) 1)
|
| [25] | VARGA T,CZIMMERER Z,NAGY L.PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation[J]. Biochimica et Biophysica Acta:Molecular Basis of Disease,2011,1812(8):1007-1022. ( 1) 1)
|
| [26] | YOSHIKAWA T,SHIMANO H,YAHAGI N,et al.Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements[J]. The Journal of Biological Chemistry,2002,277(3):1705-1711. ( 1) 1)
|
| [27] | VALDIMARSSON G,JAMES D.World fisheries-utilisation of catches[J]. Ocean & Coastal Management,2001,44(9/10):619-633. ( 1) 1)
|
| [28] | TOCHER D R,CARR J,SARGENT J R.Polyunsaturated fatty acid metabolism in fish cells:differential metabolism of (n-3) and (n-6) series acids by cultured cells orginating from a freshwater teleost fish and from a marine teleost fish[J]. Comparative Biochemistry and Physiology Part B:Comparative Biochemistry,1989,94(2):367-374. ( 1) 1)
|
| [29] | GHIONI C,TOCHER D R,BELL M V,et al.Low C18 to C20 fatty acid elongase activity and limited conversion of stearidonic acid,18:4(n-3),to eicosapentaenoic acid,20:5(n-3),in a cell line from the turbot,Scophthalmus maximus[J]. Biochimica et Biophysica Acta:Molecular and Cell Biology of Lipids,1999,1437(2):170-181. ( 1) 1)
|




