引用本文

周铭文, 王和伟, 叶继丹. 斜带石斑鱼生长性能、体成分和组织游离氨基酸含量对饲料中牛磺酸含量的响应[J]. 动物营养学报, 2015, 27(3): 785-794.

ZHOU Mingwen, WANG Hewei, YE Jidan. Responses of Growth Performance, Body Composition and Tissue Free Amino Acid Contents of Grouper (
Epinephelus coioides) to Dietary Taurine Content[J]. Chinese Journal of Animal Nutrition, 2015, 27(3): 785-794.

斜带石斑鱼生长性能、体成分和组织游离氨基酸含量对饲料中牛磺酸含量的响应
1. 集美大学水产学院, 厦门市饲料检测与安全评价重点实验室, 厦门 361021;
2. 农业部东海海水健康养殖重点实验室, 厦门 361021
收稿日期:2014-10-21
基金项目:国家自然科学基金(31372546)
作者简介:周铭文(1988—),男,福建三明人,硕士研究生,从事水产动物营养与饲料研究。E-mail:381115404@qq.com
通讯作者:叶继丹,研究员,硕士生导师,E-mail:yjdwk@sina.com
摘要:本试验旨在探讨饲料中牛磺酸含量对斜带石斑鱼生长性能、体成分及组织游离氨基酸含量的影响。以酪蛋白和明胶为蛋白质源配制低牛磺酸含量的基础饲料,在基础饲料中分别添加0(对照)、0.4%、0.8%、1.2%、1.6%的牛磺酸,配制成5种试验饲料。将300尾平均体重为(19.14±0.06) g斜带石斑鱼幼鱼随机分为5组(每组3个重复,每个重复20尾),每组投喂1种试验饲料,饲养周期为56 d。结果表明:与对照组相比,各牛磺酸添加组试验鱼的增重率、摄食率和饲料效率均显著增加(P<0.05),内脏指数显著降低(P<0.05),肝脏指数、肥满度无显著变化(P>0.05)。与对照组相比,各牛磺酸添加组试验鱼全鱼粗蛋白质含量显著升高(P<0.05),全鱼粗脂肪含量显著降低(P<0.05),全鱼水分和粗灰分含量则无显著变化(P>0.05)。各牛磺酸添加组试验鱼肝脏和肌肉中总非必需氨基酸、总氨基酸、游离丝氨酸含量以及肝脏游离蛋氨酸含量均显著低于对照组(P<0.05)。试验鱼血清、肝脏、肌肉和全鱼中牛磺酸含量随饲料中牛磺酸 含量的增加而呈上升趋势,且与饲料中牛磺酸含量存在显著正相关关系(r>0.907,P<0.05)。随饲料中牛磺酸含量的增加,肝脏牛磺酸保留率呈上升趋势,而肌肉中牛磺酸保留率则呈先上升后下降的趋势;此外,各组试验鱼肌肉牛磺酸保留率均高于肝脏。由此得出,饲料中一定含量的牛磺酸能促进斜带石斑鱼生长和摄食,降低全鱼脂肪沉积,增加全鱼蛋白质沉积,降低组织中游离氨基酸含量。以增重率为指标,通过回归分析得出斜带石斑鱼饲料中牛磺酸的适宜含量为1.04%。
关键词:
斜带石斑鱼
牛磺酸
生长性能
体成分
游离氨基酸
Responses of Growth Performance, Body Composition and Tissue Free Amino Acid Contents of Grouper (Epinephelus coioides) to Dietary Taurine Content
ZHOU Mingwen
1
, WANG Hewei
1, YE Jidan
1,2

1. Xiamen Key Laboratory for Feed Quality Testing and Safety Evaluation, Fisheries College of Jimei University, Xiamen 361021, China;
2. Key Laboratory of Healthy Mariculture for the East China Sea, Ministry of Agriculture, Xiamen 361021, China
Abstract: This experiment was conducted to study the effects of dietary taurine content on growth performance, body composition and tissue free amino acid contents of grouper (Epinephelus coioides). Casein and gelatin were used as protein sources, a low taurine content basal diet was formulated. The taurine was supplemented to the basal diet at 0 (control), 0.4%, 0.8%, 1.2% and 1.6%, respectively, to formulate 5 experimental diets. Three hundred juvenile groupers with the average body weight of (19.14±0.06) g were randomly divided into 5 groups with 3 replicates per group and 20 fish per replicate. Five groups of fish were allocated one of five experimental diets. The feeding trial lasted for 56 d. The results showed as follows: compared with control group, the weight gain rate, feed intake rate and feed efficiency were significantly increased and viscerasomatic index was significantly decreased in taurine supplementation groups (P<0.05), but no significant differences were found in hepatosomatic index and condition factor among all groups (P>0.05). Compared with control group, the whole-body crude protein content was significantly increased (P<0.05) and whole-body crude lipid content was significantly decreased in taurine supplementation groups (P<0.05), but no significant differences were found in whole-body moisture and ash contents among all groups (P>0.05). The contents of total non-essential amino acids, total amino acids, free serine in liver and muscle, and free methionine content in liver in taurine supplementation groups were significantly lower than those in control group (P<0.05). Taurine content in serum, liver, muscle and whole-body of grouper showed an increasing tendency with the dietary taurine content increasing, and there were significant positive correlations between them and dietary taurine content (r>0.907, P<0.05). With the dietary taurine content increasing, the taurine retention in liver was increased and that in muscle was firstly incresed and then decreased; moreover, the taurine retention in muscle was higher than that in liver in all groups. In conclusion, a certain content of taurine in diet can improve the growth and feeding, decrease whole-body lipid deposition, increase whole-body protein deposition, and decrease tissue free amino acid contents of grouper. Regression analysis based on weight gain rate indicates that the optimal dietary taurine content of grouper is 1.04%.
Key words:
grouper (Epinephelus coioides)
taurine
growth performance
body composition
free amino acids
牛磺酸是一种β含硫氨基酸,虽不参与蛋白质的合成,但在动物的渗透压调节、神经递质释放、抗氧化等生理代谢中起着重要的作用[1, 2]。由于鱼类牛磺酸合成的关键酶的活性较弱[3],特别是仔稚鱼期自身几乎不能合成牛磺酸[4],因此,牛磺酸被认为是鱼类的一种条件性必需氨基酸,为仔稚鱼生长发育所必需的营养物质[5]。饲料中添加牛磺酸可以明显促进 鱼(Seriola quinqueradiata)[6]、牙鲆(Paralichthys olivaceus)[7]和真鲷(Pagrus major)[8]的生长, 但当饲料中缺乏牛磺酸时真鲷出现绿肝综合征[9], 鱼发生贫血[10]。不同鱼类合成牛磺酸的能力也存在差异[4],即使同一种鱼类其不同生长阶段牛磺酸合成能力亦不同[3]。研究表明,在动物体内牛磺酸是以游离氨基酸的形式存在,饲料中牛磺酸含量的高低直接影响大西洋鲑(Salmo salar)[11]和真鲷[12]组织中游离氨基酸的含量。迄今为止,饲料中牛磺酸含量与斜带石斑鱼(Epinephelus coioides)生长和组织游离氨基酸含量关系的研究仍未见报道,为此,本试验研究了饲料中牛磺酸含量对斜带石斑鱼生长性能、体成分和组织游离氨基酸含量的影响,旨在探讨饲料中牛磺酸含量与斜带石斑鱼不同组织牛磺酸沉积和游离氨基酸含量之间的关系,确定斜带石斑鱼对饲料中牛磺酸的适宜需要量,为斜带石斑鱼饲料的配制提供理论依据。
1 材料与方法
1.1 饲料配制
以酪蛋白和明胶为蛋白质源,设计1个低牛 磺酸含量的斜带石斑鱼饲料配方,配制成基础饲料,在基础饲料中分别添加0、0.4%、0.8%、1.2%、1.6%的牛磺酸,配制成5种试验饲料,并分别记为D1(对照)、D2、D3、D4和D5组。试验饲料组成及营养水平见表1。饲料原料粉碎过80目筛网,将所有原料加水混匀后制成粒径为2.5 mm的颗粒饲料,经自然风干后于-20 ℃冰箱保存备用。
表1
Table 1
表1(Table 1)
 表1 试验饲料组成及营养水平(风干基础)
Table 1 Composition and nutrient levels of experimental diets (air-dry basis)
| % |
项目
Items |
组别 Groups
| |
D1 | D2 | D3 | D4 | D5
| |
原料 Ingredients | |
酪蛋白 Casein | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | |
面粉 Flour | 19.00 | 19.00 | 19.00 | 19.00 | 19.00 | |
明胶 Gelatin | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | |
鱼油 Fish oil | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | |
大豆卵磷脂 Soybean lecithin | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | |
氯化胆碱 Choline chloride | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | |
微晶纤维素 Microcrystalline cellulose | 2.40 | 2.00 | 1.60 | 1.20 | 0.80 | |
维生素预混物 Vitamin premix1) | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
矿物质预混物 Mineral premix2) | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | |
南极磷虾膏 Antarctic krill cream | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | |
牛磺酸 Taurine | 0.00 | 0.40 | 0.80 | 1.20 | 1.60 | |
合计 Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
营养水平 Nutrient levels | | |
干物质 Dry matter | 89.83 | 89.64 | 89.39 | 89.61 | 89.31 | |
粗蛋白质 Crude protein | 50.06 | 50.20 | 50.55 | 50.35 | 50.54 | |
粗脂肪 Crude lipid | 7.78 | 7.81 | 7.78 | 7.80 | 7.71 | |
粗灰分 Ash | 5.40 | 5.53 | 5.56 | 5.50 | 5.40 | |
牛磺酸 Taurine/(10-2 mg/g) | 103.00 | 439.00 | 788.00 | 1 112.00 | 1 453.00 |
1)维生素为每千克饲料提供Vitamin premix supplied the following per kg of diets:VA 10 mg,VD 10 mg,VC 1 000 mg,VK 40 mg,VE 500 mg,VB1 60 mg,VB2 70 mg,VB6 80 mg,VB12 0.4 mg,烟酸 nicotinic acid 200 mg,泛酸钙 calcium pantothenate 200 mg,生物素 biotin 2 mg,肌醇 inositol 500 mg,叶酸 folic acid 8 mg,对氨基苯甲酸钠 para-aminobenzoic acid sodium salt 90 mg,纤维素 cellulose 17 229.6 mg。
2)矿物质预混料为每千克饲料提供Mineral premix supplied the following per kg of diets:Ca(H2PO4)2·H2O 20 000 mg,NaH2PO4·2H2O 5 000 mg,KH2PO4 8 000 mg,乳酸钙 calcium lactate 5 000 mg,KCl 1 000 mg,NaCl 1 000 mg,MgSO4·7H2O 6 000 mg,柠檬酸铁 ferric citrate 800 mg,CuSO4·5H2O 24 mg,ZnSO4·7H2O 190 mg,MnSO4·4H2O 100 mg,CoSO4·7H2O 50 mg,KI 8 mg,Na2SeO3 2 mg,Al2(SO4)3·18H2O 25 mg,纤维素 cellulose 12 801 mg。 |
| 表1 试验饲料组成及营养水平(风干基础)
Table 1 Composition and nutrient levels of experimental diets (air-dry basis)
|
试验用斜带石斑鱼幼鱼购自厦门当地一家水产养殖场,运至集美大学养殖基地后放入3个1 000 L的循环桶驯养15 d,以适应试验饲料和养殖条件。驯养结束后,选取规格一致、平均体重(19.14±0.06) g的斜带石斑鱼,随机放入15个有循环水的水族箱(有效体积120 L)中,每箱放养20尾鱼,每3个水族箱随机作为1个饲料组,投喂1种试验饲料。饲养试验进行56 d,期间,每天饱食投喂2次(08:30和18:30),每日记录投喂量,投喂0.5 h后吸出残饵,烘干称重,计算摄食量,同时排污,排出的水量用沙滤的海水补足。每天观察试验鱼活动情况,养殖试验期间水体温度为(28±2) ℃,溶解氧浓度(5.50±0.85) mg/L,硝酸盐氮浓度(0.24±0.12) mg/L。
1.3 样品采集
在试验鱼分组前,随机选取10尾鱼作为初始样品用于全鱼体成分分析。饲养试验结束后,称取每箱鱼总重。从每箱中随机取3尾鱼作为全鱼样品,储存于-20 ℃冰箱中待测。再从每箱中随机取5尾鱼,丁香酚麻醉,逐尾采血,采集的血液样品放置在4 ℃冰箱中过夜,第2天取出,用离心机在10 000 r/min下离心10 min,收集血清。采血后将鱼解剖,摘取内脏,并记录每尾鱼的体长、体重、内脏重和肝脏重。血清和肝脏放入冻存管置于-80 ℃冰箱中待测。
1.4 样品测定
1.4.1 常规营养成分测定
水分含量的测定采用105 ℃恒温干燥法。粗蛋白质含量的测定采用凯氏定氮法,用Kjeltec-8400型自动凯氏定氮仪进行测定。粗脂肪含量的测定采用索氏提取法。粗灰分含量的测定采用马弗炉(550 ℃)灼烧法。
1.4.2 牛磺酸含量的测定
测定血清牛磺酸含量时,先在每毫升血清中加入4%磺基水杨酸1 mL,混匀后15 000 r/min离心15 min,取上清液用氨基酸自动分析仪(日立L-8900型)测定。牛磺酸测定条件:缓冲液流速为0.4 mL/min,衍生试剂流速为0.35 mL/min;通道1检测波长为570 nm,通道2检测波长为440 nm;分离柱填料为3 μm磺酸型阳离子树脂,分离柱温度为57.0 ℃,反应柱温度为(135.0±0.1) ℃。
测定组织和饲料中牛磺酸含量时,先准确称取1~2 g(精确至0.000 2 g)样品放入10 mL离心管中,加入4%磺基水杨酸溶液约4 mL,超声波仪超声匀质5 min后,于15 000 r/min离心15 min,将上清液全部转移至25 mL容量瓶中,沉淀物再用上述方法提取1次,合并上清液,混匀,用0.02 mol/L盐酸溶液定容。溶液经0.45 μm滤膜过滤,滤液上机测定牛磺酸含量。
1.4.3 游离氨基酸含量的测定
测定组织中的游离氨基酸含量时,对样品的处理方法与测定牛磺酸含量时的样品处理方法相同。在使用氨基酸自动分析仪测定时,用混合氨基酸标准溶液调整仪器操作参数和洗脱用缓冲液的转换时间,使各氨基酸达到最佳分离。混合氨基酸标准溶液和待测样品放入进样盘中,按照日立L-8900型氨基酸自动分析仪的操作程序进行分析测定。
1.5 计算公式
增重率(WGR,%)=100×(末重-初重)/初重;
摄食率(FIR,%)=100×总摄食量/[(初重+末重)/2]/饲喂天数;
饲料效率(FE)=(末重-初重)/总摄食量;
肝体指数(HSI,%)=100×肝脏重/体重;
脏体指数(VSI,%)=100×内脏重/体重;
肥满度(CF)=100×体重/体长3;
牛磺酸保留率(%)=100×(试验鱼组织终末牛磺酸含量-试验鱼组织初始牛磺酸含量)/试验饲料牛磺酸含量。
1.6 数据处理与分析
试验数据用SPSS 13.0分析软件进行单因素方差分析(one-way ANOVA),结果以平均值±标准差(mean±SD)表示,若存在显著差异时,则采用Student-Newman-Keuls法进行多重比较,显著性差异水平为P<0.05。将各指标数据值与饲料牛磺酸含量进行拟合,建立各指标与饲料牛磺酸含量的相关关系。
2 结 果
2.1 饲料中牛磺酸含量对斜带石斑鱼生长性能的影响
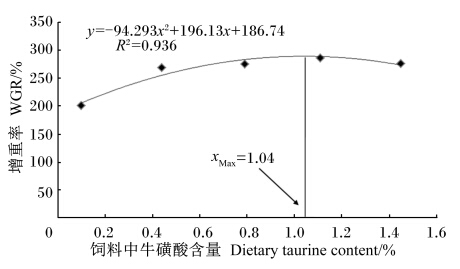
由表2可知,与对照组相比,各牛磺酸添加组试验鱼的增重率、摄食率和饲料效率均显著增加(P<0.05),但各牛磺酸添加组间差异不显著(P>0.05)。各牛磺酸添加组试验鱼的脏体指数与对照组相比均显著降低(P<0.05)。各组间试验鱼的肝体指数和肥满度无显著差异(P>0.05)。如图1所示,以饲料中牛磺酸含量为自变量(x),以增重率为因变量(y),对二者进行二次回归分析,得出斜带石斑鱼饲料中牛磺酸的适宜含量为1.04%。
表2
Table 2
表2(Table 2)
 表2 饲料中牛磺酸含量对斜带石斑鱼生长性能的影响
Table 2 Effects of dietary taurine content on growth performance of grouper (Epinephelus coioides)
|
组别 Groups | 初重
IBW/g | 末重
FBW/g | 增重率
WGR/% | 饲料效率
FE | 摄食率
FIR/% | 肝体指数
HSI/% | 脏体指数
VSI/% | 肥满度
CF | |
D1 | 19.19±0.02 | 57.55±2.31a | 199.87±11.77a | 0.46±0.03a | 1.22±0.02a | 3.27±0.11 | 8.57±0.19a | 2.64±0.09 | |
D2 | 19.13±0.07 | 70.52±1.47b | 268.58±7.85b | 0.54±0.02b | 1.33±0.03b | 3.20±0.11 | 8.10±0.05b | 2.84±0.18 | |
D3 | 19.11±0.03 | 71.53±3.12b | 274.37±15.71b | 0.55±0.02b | 1.33±0.02b | 2.96±0.18 | 7.80±0.07c | 2.58±0.18 | |
D4 | 19.16±0.07 | 73.88±1.39b | 285.68±8.61b | 0.55±0.01b | 1.36±0.01b | 2.95±0.22 | 7.70±0.14c | 2.68±0.13 | |
D5 | 19.12±0.05 | 71.81±1.52b | 275.65±7.41b | 0.56±0.03b | 1.33±0.03b | 2.97±0.25 | 7.86±0.03c | 2.60±0.04
| |
集合标准误Pooled SE | 0.01 | 1.63 | 8.60 | 0.01 | 0.02 | 0.05 | 0.16 | 0.04 | |
P值 P-value | 0.325 | <0.001 | <0.001 | 0.002 | <0.001 | 0.090 | <0.001 | 0.215 |
同列数据肩标不同小写字母表示差异显著(P<0.05)。表3同。
Values in the same column with different small letter superscripts mean significant difference (P<0.05). The same as Table 3. |
| 表2 饲料中牛磺酸含量对斜带石斑鱼生长性能的影响
Table 2 Effects of dietary taurine content on growth performance of grouper (Epinephelus coioides)
|
由表3可知,与对照组相比,各牛磺酸添加组 试验鱼的全鱼粗蛋白质含量显著增加(P<0.05),粗脂肪含量显著降低(P<0.05),但各牛磺酸添加组间差异不显著(P>0.05)。各组间试验鱼的全鱼粗灰分和水分含量均无显著差异(P>0.05)。
表3
Table 3
表3(Table 3)
 表3 饲料中牛磺酸含量对斜带石斑鱼体成分的影响(湿重基础)
Table 3 Effects of dietary taurine content on body composition of grouper (Epinephelus coioides) (wet weight basis)
| % |
|
组别 Groups | 水分
Moisture | 粗蛋白质
Crude protein | 粗脂肪
Crude lipid | 粗灰分
Ash
| |
D1 | 71.11±0.19 | 16.55±0.15a | 5.86±0.10b | 4.75±0.11 | |
D2 | 71.14±0.20 | 17.13±0.09b | 5.56±0.11a | 4.49±0.08 | |
D3 | 71.19±0.21 | 17.26±0.21b | 5.46±0.15a | 4.44±0.11 | |
D4 | 71.19±0.20 | 17.34±0.10b | 5.43±0.10a | 4.48±0.10 | |
D5 | 71.21±0.34 | 17.21±0.17b | 5.40±0.16a | 4.49±0.18
| |
集合标准误Pooled SE | 0.05 | 0.08 | 0.05 | 0.04 | |
P值 P-value | 0.982 | <0.001 | 0.007 | 0.064 | | 表3 饲料中牛磺酸含量对斜带石斑鱼体成分的影响(湿重基础)
Table 3 Effects of dietary taurine content on body composition of grouper (Epinephelus coioides) (wet weight basis)
|
由表4、表5可知,各牛磺酸添加组试验鱼肝脏和肌肉中总非必需氨基酸(TNEAA)和总氨基酸(TAA)含量均显著低于对照组(P<0.05)。各牛磺酸添加组试验鱼肝脏和肌肉中游离丝氨酸含量均显著低于对照组(P<0.05)。各牛磺酸添加组试验鱼肝脏和肌肉中游离蛋氨酸含量显著低于对照组(P<0.05),但各牛磺酸添加组间差异不显著(P>0.05)。
表4
Table 4
表4(Table 4)
 表4 饲料中牛磺酸含量对斜带石斑鱼肝脏游离氨基酸含量的影响(湿重基础)
Table 4 Effects of dietary taurine content on liver free amino acid contents of grouper (Epinephelus coioides)(wet weight basis)
| 10-2 mg/g |
氨基酸
Amino acids | 组别 Groups
| 集合标准误
Pooled SE | P值
P-value | |
D1 | D2 | D3 | D4 | D5 | |
总必需氨基酸TEAA | 46.15±2.23b | 37.07±2.01a | 44.73±2.49b | 38.31±1.91a | 34.41±1.50a | 1.29 | <0.001 | |
精氨酸 Arg | 4.39±0.53c | 2.19±0.16ab | 2.94±0.65b | 2.21±0.36ab | 1.62±0.35a | 0.27 | <0.001 | |
赖氨酸 Lys | 3.27±0.75a | 4.19±0.34ab | 5.16±0.53b | 4.95±0.51b | 3.63±0.43a | 0.23 | 0.006 | |
组氨酸 His | 3.06±0.25 | 2.79±0.27 | 3.20±0.22 | 2.82±0.24 | 2.65±0.08 | 0.07 | 0.077 | |
苯丙氨酸 Phe | 1.95±0.85ab | 1.47±0.07a | 2.43±0.34b | 2.30±0.45b | 1.23±0.30a | 0.16 | 0.049 | |
酪氨酸 Tyr | 3.50±0.65 | 3.18±0.19 | 3.66±0.46 | 3.16±0.45 | 2.92±0.09 | 0.12 | 0.279 | |
亮氨酸 Leu | 6.03±0.06a | 4.98±0.44b | 6.96±0.63c | 5.39±0.24b | 4.09±0.73b | 0.28 | <0.001 | |
异亮氨酸 Ile | 2.54±0.28b | 2.01±0.18a | 3.01±0.21c | 2.08±0.21a | 1.78±0.21a | 0.13 | <0.001 | |
蛋氨酸 Met | 2.38±0.22b | 1.68±0.19a | 1.61±0.17a | 1.31±0.13a | 1.28±0.22a | 0.11 | <0.001 | |
缬氨酸 Val | 6.20±1.02b | 4.01±0.25a | 5.36±0.11b | 3.96±0.26a | 3.20±0.25a | 0.31 | <0.001 | |
苏氨酸 Thr | 12.84±0.50c | 10.63±0.37a | 10.38±0.70a | 10.13±0.18a | 12.00±0.36b | 0.30 | <0.001 |
总非必需氨基酸
TNEAA | 427.39±4.89d | 361.69±4.40c | 365.41±3.18c | 308.94±2.50a | 340.96±4.27b | 10.41 | <0.001 | |
牛磺酸 Tau | 102.30±11.0a | 259.70±4.70b | 342.50±2.20c | 364.00±4.40d | 396.70±6.20e | 28.25 | <0.001 | |
丙氨酸 Ala | 67.96±6.12 | 57.09±6.14 | 66.92±3.06 | 56.48±2.77 | 66.32±1.18 | 1.65 | 0.160 | |
甘氨酸 Gly | 73.91±7.36 | 69.01±3.75 | 64.33±6.85 | 64.60±1.25 | 75.18±2.45 | 1.42 | 0.065 | |
谷氨酸 Glu | 253.53±7.23d | 211.35±12.03c | 206.54±1.30c | 158.75±2.01a | 177.79±3.13b | 8.75 | <0.001 | |
丝氨酸 Ser | 5.59±0.48c | 4.31±0.61ab | 4.51±0.31b | 3.90±0.24ab | 3.50±0.25a | 0.21 | 0.001 | |
天冬氨酸 Asp | 4.48±0.40b | 2.30±0.50a | 3.93±0.49b | 2.39±0.51a | 2.16±0.25a | 0.27 | <0.001 | |
脯氨酸 Pro | 21.91±2.15 | 17.62±1.72 | 19.19±1.39 | 22.81±2.20 | 16.02±1.35 | 0.79 | 0.050 | |
总氨基酸 TAA | 473.54±3.11e | 398.76±3.24c | 410.13±5.13d | 347.24±3.02a | 375.37±4.20b | 11.30 | <0.001 |
同行数据肩标不同小写字母表示差异显著(P<0.05)。下表同。
Values in the same row with different small letter superscripts mean significant differences (P<0.05). The same as below. |
| 表4 饲料中牛磺酸含量对斜带石斑鱼肝脏游离氨基酸含量的影响(湿重基础)
Table 4 Effects of dietary taurine content on liver free amino acid contents of grouper (Epinephelus coioides)(wet weight basis)
|
表5
Table 5
表5(Table 5)
 表5 饲料中牛磺酸含量对斜带石斑鱼肌肉游离氨基酸含量的影响(湿重基础)
Table 5 Effects of dietary taurine level on muscle free amino acid contents of grouper (Epinephelus coioides) (wet weight basis)
| 10-2 mg/g |
氨基酸
Amino acids | 组别 Groups
| 集合标准误
Pooled SE | P值
P-value | |
D1 | D2 | D3 | D4 | D5 |
总必需氨基酸
TEAA | 30.04±2.97b | 30.49±1.62b | 22.98±0.87a | 23.62±1.57a | 24.81±2.25a | 0.96 | 0.020 | |
精氨酸 Arg | 0.84±0.15b | 0.70±0.06b | 0.74±0.01b | 0.44±0.09a | 0.45±0.05a | 0.05 | 0.001 | |
赖氨酸 Lys | 7.45±0.71a | 9.08±1.48b | 5.61±0.62a | 6.52±1.01a | 7.00±0.59a | 0.44 | 0.041 | |
组氨酸 His | 1.29±0.18b | 1.19±0.31b | 0.78±0.24a | 0.78±0.03a | 0.85±0.09a | 0.07 | 0.025 | |
苯丙氨酸 Phe | 1.25±0.05 | 0.86±0.28 | 1.02±0.02 | 0.71±0.26 | 0.84±0.25 | 0.07 | 0.071 | |
酪氨酸 Tyr | 4.16±0.46 | 3.54±0.38 | 3.68±0.23 | 3.73±0.62 | 3.81±0.18 | 0.10 | 0.474 | |
亮氨酸 Leu | 1.45±0.07ab | 1.30±0.03a | 1.17±0.16a | 1.30±0.19a | 1.64±0.06b | 0.05 | 0.006 | |
异亮氨酸 Ile | 1.03±0.10bc | 0.98±0.16bc | 0.70±0.01a | 0.88±0.16ab | 1.11±0.08c | 0.05 | 0.013 | |
蛋氨酸 Met | 0.65±0.02b | 0.41±0.05a | 0.41±0.15a | 0.34±0.03a | 0.42±0.08a | 0.03 | 0.006 | |
缬氨酸 Val | 4.48±0.08b | 4.18±0.53b | 2.56±0.25a | 2.70±0.85a | 2.13±0.85a | 0.28 | 0.002 | |
苏氨酸 Thr | 7.44±0.27 | 7.24±0.48 | 6.30±0.65 | 6.22±0.29 | 6.47±0.98 | 0.19 | 0.089 |
总非必需氨基酸
TNEAA | 694.63±14.41e | 530.48±7.39d | 465.15±2.53c | 426.19±6.59b | 353.97±1.32a | 30.90 | <0.001 | |
牛磺酸 Tau | 117.10±4.44a | 323.30±4.70b | 453.60±4.30c | 500.20±8.00d | 539.30±10.70e | 40.97 | <0.001 | |
丙氨酸 Ala | 23.44±4.35 | 23.00±1.19 | 21.13±0.69 | 24.36±2.77 | 26.60±2.23 | 0.74 | 0.207 | |
甘氨酸 Gly | 233.12±4.70c | 139.47±7.87b | 132.08±2.59b | 116.93±8.58a | 109.09±3.31a | 12.04 | <0.001 | |
谷氨酸 Glu | 29.16±2.74b | 28.11±1.73b | 26.48±0.45b | 26.05±1.38b | 20.68±1.59a | 0.96 | 0.019 | |
丝氨酸 Ser | 12.63±1.87b | 5.04±0.39a | 5.64±0.49a | 4.57±0.52a | 4.37±0.17a | 0.86 | <0.001 | |
天冬氨酸 Asp | 18.44±1.61d | 15.52±0.82c | 10.83±1.14b | 12.11±1.64b | 6.97±0.78a | 1.09 | <0.001 | |
脯氨酸 Pro | 377.84±8.70e | 319.34±1.92d | 268.98±3.10c | 242.17±14.03b | 186.26±4.60a | 17.59 | <0.001 | |
总氨基酸 TAA | 724.68±17.31e | 560.97±5.78d | 488.13±2.48c | 449.82±5.69b | 378.78±1.92a | 31.56 | <0.001 |
| 表5 饲料中牛磺酸含量对斜带石斑鱼肌肉游离氨基酸含量的影响(湿重基础)
Table 5 Effects of dietary taurine level on muscle free amino acid contents of grouper (Epinephelus coioides) (wet weight basis)
|
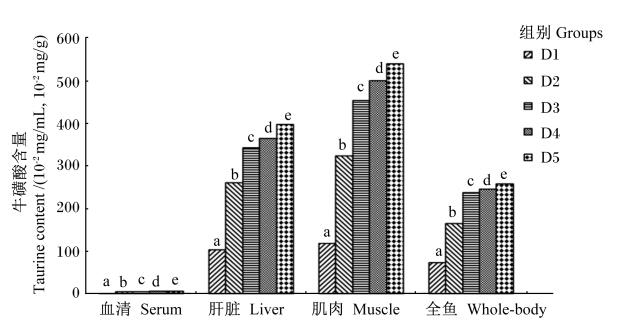
试验前试验鱼每100 g肌肉、肝脏和全鱼中牛磺酸含量分别为294.70、252.95和123.78 mg。图2显示,试验结束时,试验鱼血清、肝脏、肌肉和全鱼牛磺酸含量均随饲料中牛磺酸含量的增加而呈上升趋势,各牛磺酸添加组试验鱼血清、肝脏、肌肉和全鱼牛磺酸含量均显著高于对照组(P<0.05)。由表6可知,试验鱼血清、肝脏、肌肉和全鱼牛磺酸含量与饲料中牛磺酸含量呈显著正相关(r>0.907,P<0.05)。从试验鱼肝脏和肌肉对饲料中牛磺酸的保留来看,肝脏牛磺酸保留率随饲料中牛磺酸含量的增加呈上升趋势,而肌肉中牛磺酸保留率随饲料中牛磺酸含量的增加呈先上升后下降的趋势,以D3组最高;此外,各组试验鱼肌肉牛磺酸保留率均高于肝脏(表7)。
表6
Table 6
表6(Table 6)
 表6 斜带石斑鱼血清、肝脏、肌肉和全鱼牛磺酸含量(y)与饲料中牛磺酸含量(x)的关系
Table 6 Relationships between taurine content in serum,liver,muscle and whole body (y) of grouper (Epinephelus coioides) and dietary taurine content (x)
组织
Tissues | 线性回归方程
Linear regressive equation | 相关系数
Correlation coefficient (r) | P值 P-value
| |
血清 Serum | y=3.120x+1.624 | 0.916 | 0.029 | |
肝脏 Liver | y=206.4x+132.4 | 0.932 | 0.021 | |
肌肉 Muscle | y=303.9x+150.2 | 0.946 | 0.015 | |
全鱼 Whole-body | y=133.8x+90.99 | 0.920 | 0.027 |
| 表6 斜带石斑鱼血清、肝脏、肌肉和全鱼牛磺酸含量(y)与饲料中牛磺酸含量(x)的关系
Table 6 Relationships between taurine content in serum,liver,muscle and whole body (y) of grouper (Epinephelus coioides) and dietary taurine content (x)
|
本试验中,各牛磺酸添加组均提高了斜带石斑鱼的末重和增重率,这与牙鲆[13]、鲈鱼(Lateolabrax japonicus)[14]、草鱼(Ctenopharyngodon idellus)[15]的研究结果一致。牛磺酸是氨基酸衍生物,可以作为诱食剂作用于鱼类的嗅觉器官,增加对食物的摄取[16]。本试验中,各牛磺酸添加组斜带石斑鱼的摄食率与对照组相比均显著升高,这说明牛磺酸可以促进斜带石斑鱼摄食。各牛磺酸添加组斜带石斑鱼摄食率和饲料效率均比对照组高,这与其增重率的变化一致,据此推测牛磺酸可能是通过提高斜带石斑鱼的摄食率和饲料效率来促进生长的。经二次曲线回归分析,斜带石斑鱼饲料中牛磺酸的适宜含量为1.04%。这一结果高于鲈鱼(0.2%)[14]和草鱼(0.6%)[15],但低于牙鲆(1.5%~2.0%)[13]。鱼类对牛磺酸需要量的不同可能与鱼的种类有关。
表7
Table 7
表7(Table 7)
 表7 饲料中牛磺酸含量对斜带石斑鱼肌肉和肝脏牛磺酸保留率的影响(湿重基础)
Table 7 Effects of dietary taurine content on taurine retention in muscle and liver of grouper (Epinephelus coioides) (wet weight basis)
| % |
组织
Tissues | 组别 Groups
| 集合标准误
Pooled SE | P值
P-value | |
D1 | D2 | D3 | D4 | D5 | |
肝脏 Liver | -186.21±10.62a | -7.98±1.06b | 6.06±0.28c | 6.18±0.39c | 7.02±0.43c | 20.29 | <0.001 | |
肌肉 Muscle | -131.43±4.30a | 16.01±1.07b | 25.46±0.54d | 22.03±0.71cd | 19.70±0.74bc | 16.30 | <0.001 | | 表7 饲料中牛磺酸含量对斜带石斑鱼肌肉和肝脏牛磺酸保留率的影响(湿重基础)
Table 7 Effects of dietary taurine content on taurine retention in muscle and liver of grouper (Epinephelus coioides) (wet weight basis) |
牛磺酸能够通过调节与蛋白质合成代谢相关激素的分泌来影响全鱼的蛋白质含量,饲料中添加牛磺酸能够显著地提高鲤鱼(Cyprinus carpio L.)血清甲状腺激素含量,从而控制鱼类生长激素的基因表达和合成,达到促进鱼类体蛋白质合成的目的[17]。在本试验中,各牛磺酸添加组斜带石斑鱼全鱼粗蛋白质含量与对照组相比均显著升高,这与在草鱼[15]、虹鳟(Oncorhynchus mykiss)[18]和牙鲷[19]上的研究结果一致。牛磺酸在鱼类体内能够参与胆汁酸的合成,而胆汁酸能够促进脂类合成代谢[20],进而增加全鱼的粗脂肪含量。但在本试验中,各牛磺酸添加组斜带石斑鱼的全鱼粗脂肪含量却显著下降,该结果与在大西洋鲑[11]上的研究结果一致,可能是由于牛磺酸在鱼体内参与了胆汁酸合成以外的其他代谢合成途径[21]。各牛磺酸添加组斜带石斑鱼体内蛋白质沉积的增加以其体内脂肪沉积的降低为代价,说明牛磺酸具有营养物质再分配的作用,饲料中过低的牛磺酸含量不利于鱼体内蛋白质的沉积,而鱼体内蛋白质沉积的增加也反映在鱼体生长速度上。
3.3 饲料中牛磺酸含量对斜带石斑鱼组织中游离氨基酸含量的影响
动物体内游离氨基酸的含量明显受饲料组成的影响[22, 23]。在本试验中,对照组斜带石斑鱼肝脏和肌肉中大部分游离氨基酸含量均显著高于各牛磺酸添加组,表明饲料中牛磺酸含量同样对斜带石斑鱼肝脏和肌肉中游离氨基酸含量产生了影响,这一结果与在真鲷[12]上得出的结果一致。丝氨酸和蛋氨酸是鱼类牛磺酸合成过程中的关键氨基酸[24],本试验中,对照组斜带石斑鱼肝脏和肌肉中游离丝氨酸和蛋氨酸含量均显著高于各牛磺酸添加组,说明斜带石斑鱼摄入牛磺酸不足时,机体会调动更多的游离丝氨酸和蛋氨酸用于牛磺酸的合成。在本试验中,牛磺酸不仅对肝脏和肌肉中游离蛋氨酸和丝氨酸含量产生了显著的影响,对肝脏和肌肉中大部分游离氨基酸含量也同样产生了显著的影响。各牛磺酸添加组斜带石斑鱼肝脏和肌肉中游离氨基酸含量均低于对照组,其具体的原因不明,还需要对斜带石斑鱼体内含硫氨基酸的代谢机制做更进一步的研究。
3.4 饲料中牛磺酸含量对斜带石斑鱼组织中牛磺酸分布的影响
本试验发现,石斑鱼肌肉、肝脏、血清和全鱼牛磺酸含量与饲料中牛磺酸含量呈良好的线性关系,这表明斜带石斑鱼具有将外源摄入的牛磺酸直接蓄积到鱼体组织中的能力。牛磺酸在鱼体内的分布与牛磺酸合成过程中的关键酶——半胱次磺酸脱羧酶(CSAD)[4]在这些组织中的活性有关[25, 26]。牛磺酸在斜带石斑鱼不同组织中的含量差异较大,其中以肌肉中的含量最高,这个结果与Qi等[3]对大菱鲆(Scophthalmus maximus L.)的研究结果一致。肝脏牛磺酸保留率随着饲料中牛磺酸含量的增加呈逐渐升高趋势,而肌肉牛磺酸保留率则随着饲料中牛磺酸含量的增加呈现先升高后降低趋势,反映出斜带石斑鱼肝脏和肌肉组织对外源牛磺酸的代谢与利用有所不同。无论牛磺酸添加量多与少,肌肉牛磺酸保留率始终高于肝脏,说明肌肉对牛磺酸的蓄积能力比肝脏强,而牛磺酸在眼睛、脑等一些特定组织中的含量尤其高[27],说明这些特定组织代谢需要更多的牛磺酸参与,以利于这些组织生理功能的发挥,这也表明了鱼类不同的组织对牛磺酸的蓄积能力不同[11, 28]。
4 结 论
① 饲料中牛磺酸含量影响斜带石斑鱼的生长性能和体成分,一定含量(0.4%~1.2%)的牛磺酸能促进斜带石斑鱼生长和摄食,降低全鱼脂肪沉积,增加全鱼蛋白质沉积。
② 饲料中适量添加牛磺酸可降低斜带石斑鱼组织中游离氨基酸含量,增加体内牛磺酸的累积。
③ 以增重率为衡量指标,斜带石斑鱼饲料中牛磺酸的适宜含量为1.04%。
参考文献
|
[1] | HUXTABLE R J.Physiological actions of taurine[J]. Physiological Reviews,1992,72(1):101-163. ( 1) 1)
|
|
[2] | GVRER H,ÖZGVNES H,SAYGIN E,et al.Antioxidant effect of taurine against lead-induced oxidative stress[J]. Archives of Environmental Contamination and Toxicology,2001,41(4):397-402. ( 1) 1)
|
|
[3] | QI G S,AI Q H,MAI K S,et al.Effects of dietary taurine supplementation to a casein-based diet on growth performance and taurine distribution in two sizes of juvenile turbot (Scophthalmus maximus L.)[J]. Aquaculture,2012,358-359:122-128. ( 3) 3)
|
|
[4] | YOKOYAMA M,TAKEUCHI T,PARK G S,et al.Hepatic cysteine sulfinate decarboxylase activity in fish[J]. Aquaculture Research,2001,32(1):216-220. ( 3) 3)
|
|
[5] | TAKEUCHI T,PARK G S,SEIKAI T,et al.Taurine content in Japanese flounder Paralichthys olivaceus T. & S. and red sea bream Pagrus major T. & S. during the period of seed production[J]. Aquaculture Research,2001,32(S):244-248. ( 1) 1)
|
|
[6] | MATSUNARI H,TAKEUCHI T,TAKAHASHI M,et al.Effect of dietary taurine supplementation on growth performance of yellowtail juveniles Seriola quinqueradiata[J]. Fisheries Science,2005,71(5):1131-1135. ( 1) 1)
|
|
[7] | KIM S K,MATSUNARI H,TAKEUCHI T,et al.Effect of different dietary taurine levels on the conjugated bile acid composition and growth performance of juvenile and fingerling Japanese flounder Paralichthys olivaceus[J]. Aquaculture,2007,273(4):595-601. ( 1) 1)
|
|
[8] | MATSUNARI H,FURUITA H,YAMAMOTO T,et al.Effect of dietary taurine and cystine on growth performance of juvenile red sea bream Pagrus major[J]. Aquaculture,2008,274(1):142-147. ( 1) 1)
|
|
[9] | TAKAGI S,MURATA H, GOTO T,et al.Efficacy of taurine supplementation for preventing green liver syndrome and improving growth performance in yearling red sea bream Pagrus major fed low-fishmeal diet[J]. Fisheries Science,2006,72(6):1191-1199. ( 1) 1)
|
|
[10] | TAKAGI S,MURATA H,GOTO T,et al.Taurine is an essential nutrient for yellowtail Seriola quinqueradiata fed non-fish meal diets based on soy protein concentrate[J]. Aquaculture,2008,280(1/2/3/4):198-205. ( 1) 1)
|
|
[11] | ESPE M,RUOHONEN K,EL-MOWAFI A.Effect of taurine supplementation on the metabolism and body lipid-to-protein ratio in juvenile Atlantic salmon (Salmo salar)[J]. Aquaculture Research,2012,43(3):349-360. ( 3) 3)
|
|
[12] | MATSUNARI H,YAMAMOTO T,KIM S K,et al.Optimum dietary taurine level in casein-based diet for juvenile red sea bream Pagrus major[J]. Fisheries Science,2008,74(2):347-353. ( 2) 2)
|
|
[13] | PARK G S,TAKEUCHI T,YOKOYAMA M,et al.Optimal dietary taurine level for growth of juvenile Japanese flounder Paralichthys olivaceus[J]. Fisheries Science,2002,68(4):824-829. ( 2) 2)
|
|
[14] | MARTINEZ J B,CHATZIFOTIS S,DIVANACH P,et al.Effect of dietary taurine supplementation on growth performance and feed selection of sea bass Dicentrarchus labrax fry fed with demand-feeders[J]. Fisheries Science,2004,70(1):74-79. ( 2) 2)
|
|
[15] | 罗莉,王琳, 龙勇, 等. 牛磺酸对草鱼生长效应研究 [J]. 饲料工业,2005(12):25-27. ( 3) 3)
|
|
[16] | DØVING K B,SELSET R,THOMMESEN G.Olfactory sensitivity to bile acids in salmonid fishes[J]. Acta Physiologica Scandinavica,1980,108(2):123-131. ( 1) 1)
|
|
[17] | 邱小琮,赵红雪.牛磺酸对鲤生长及血清T3、T4含量的影响[J]. 淡水渔业,2006,36(1):22-24. ( 1) 1)
|
|
[18] | GAYLORD G T,TEAGUE A M,BARROWS F T.Taurine supplementation of all-plant protein diets for rainbow trout (Oncorhynchus mykiss)[J]. Journal of the World Aquaculture Society,2006,37(4):509-517. ( 1) 1)
|
|
[19] | CHATZIFOTIS S,POLEMITOU I,DIVANACH P,et al.Effect of dietary taurine supplementation on growth performance and bile salt activated lipase activity of common dentex,Dentex dentex,fed a fish meal/soy protein concentrate-based diet[J]. Aquaculture,2008,275(1/2/3/4):201-208. ( 1) 1)
|
|
[20] | RUSSELL D W.The enzymes,regulation,and genetics of bile acid synthesis[J]. Annual Review of Biochemistry,2003,72(1):137-174. ( 1) 1)
|
|
[21] | GOTO T,TAKAGI S,ICHIKI T,et al.Studies on the green liver in cultured red sea bream fed low level and non-fish meal diets:relationship between hepatic taurine and biliverdin levels[J]. Fisheries Science,2001,67(1):58-63. ( 1) 1)
|
|
[22] | GUNASEKERA R M,SHIM K F,LAM T J.Influence of dietary protein content on the distribution of amino acids in oocytes,serum and muscle of Nile tilapia,Oreochromis niloticus (L.)[J]. Aquaculture,1997,152(1/2/3/4):205-221. ( 1) 1)
|
|
[23] | GÓMEZ-REQUENI P,MINGARRO M,CALDUCH-GINER J A,et al.Protein growth performance,amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata)[J]. Aquaculture,2004,232(1/2/3/4):493-510. ( 1) 1)
|
|
[24] | FINKELSTEIN J D,MARTIN J J.Methionine metabolism in mammals.Adaptation to methionine excess[J]. The Journal of Biological Chemistry,1986,261(4):1582-1587. ( 1) 1)
|
|
[25] | KIM S K,TAKEUCHI T,YOKOYAMA M,et al.Effect of dietary supplementation with taurine,β-alanine and GABA on the growth of juvenile and fingerling Japanese flounder Paralichthys olivaceus[J]. Fisheries Science,2003,69(2):242-248. ( 1) 1)
|
|
[26] | KIM S K,TAKEUCHI T,AKIMOTO A,et al.Effect of taurine supplemented practical diet on growth performance and taurine contents in whole body and tissues of juvenile Japanese flounder Paralichthys olivaceus[J]. Fisheries Science,2005,71(3):627-632. ( 1) 1)
|
|
[27] | PINTO W,RØNNESTAD I,JORDAL A E O,et al.Cloning,tissue and ontogenetic expression of the taurine transporter in the flatfish Senegalese sole (Solea senegalensis)[J]. Amino Acids,2012,42(4):1317-1327. ( 1) 1)
|
|
[28] | PINTO W,FIGUEIRA L,RIBEIRO L,et al.Dietary taurine supplementation enhances metamorphosis and growth potential of Solea senegalensis larvae[J]. Aquaculture,2010,309(1/2/3/4):159-164. ( 1) 1)
|
1
本文献在全文中的定位:
... 但在动物的渗透压调节、神经递质释放、抗氧化等生理代谢中起着重要的作用
[1, 2] ...
1
本文献在全文中的定位:
... 但在动物的渗透压调节、神经递质释放、抗氧化等生理代谢中起着重要的作用
[1, 2] ...
3
本文献在全文中的定位:
... 由于鱼类牛磺酸合成的关键酶的活性较弱
[3] ...
... 即使同一种鱼类其不同生长阶段牛磺酸合成能力亦不同
[3] ...
... 这个结果与Qi等
[3]对大菱鲆(
Scophthalmus maximus L.)的研究结果一致 ...
3
本文献在全文中的定位:
... 特别是仔稚鱼期自身几乎不能合成牛磺酸
[4] ...
... 不同鱼类合成牛磺酸的能力也存在差异
[4] ...
... 牛磺酸在鱼体内的分布与牛磺酸合成过程中的关键酶——半胱次磺酸脱羧酶(CSAD)
[4]在这些组织中的活性有关
[25, 26] ...
1
本文献在全文中的定位:
... 为仔稚鱼生长发育所必需的营养物质
[5] ...
1
本文献在全文中的定位:
... 饲料中添加牛磺酸可以明显促进 鱼(
Seriola quinqueradiata)
[6]、牙鲆(
Paralichthys olivaceus)
[7]和真鲷(
Pagrus major)
[8]的生长 ...
1
本文献在全文中的定位:
... 饲料中添加牛磺酸可以明显促进 鱼(
Seriola quinqueradiata)
[6]、牙鲆(
Paralichthys olivaceus)
[7]和真鲷(
Pagrus major)
[8]的生长 ...
1
本文献在全文中的定位:
... 饲料中添加牛磺酸可以明显促进 鱼(
Seriola quinqueradiata)
[6]、牙鲆(
Paralichthys olivaceus)
[7]和真鲷(
Pagrus major)
[8]的生长 ...
1
本文献在全文中的定位:
... 但当饲料中缺乏牛磺酸时真鲷出现绿肝综合征
[9] ...
3
本文献在全文中的定位:
... 饲料中牛磺酸含量的高低直接影响大西洋鲑(
Salmo salar)
[11]和真鲷
[12]组织中游离氨基酸的含量 ...
... 该结果与在大西洋鲑
[11]上的研究结果一致 ...
... 这也表明了鱼类不同的组织对牛磺酸的蓄积能力不同
[11, 28] ...
2
本文献在全文中的定位:
... 饲料中牛磺酸含量的高低直接影响大西洋鲑(
Salmo salar)
[11]和真鲷
[12]组织中游离氨基酸的含量 ...
... 这一结果与在真鲷
[12]上得出的结果一致 ...
2
本文献在全文中的定位:
... 这与牙鲆
[13]、鲈鱼(
Lateolabrax japonicus)
[14]、草鱼(
Ctenopharyngodon idellus)
[15]的研究结果一致 ...
... 但低于牙鲆(1.5%~2.0%)
[13] ...
2
本文献在全文中的定位:
... 这与牙鲆
[13]、鲈鱼(
Lateolabrax japonicus)
[14]、草鱼(
Ctenopharyngodon idellus)
[15]的研究结果一致 ...
... 这一结果高于鲈鱼(0.2%)
[14]和草鱼(0.6%)
[15] ...
3
本文献在全文中的定位:
... 这与牙鲆
[13]、鲈鱼(
Lateolabrax japonicus)
[14]、草鱼(
Ctenopharyngodon idellus)
[15]的研究结果一致 ...
... 这一结果高于鲈鱼(0.2%)
[14]和草鱼(0.6%)
[15] ...
... 这与在草鱼
[15]、虹鳟(
Oncorhynchus mykiss)
[18]和牙鲷
[19]上的研究结果一致 ...
1
本文献在全文中的定位:
... 达到促进鱼类体蛋白质合成的目的
[17] ...
1
本文献在全文中的定位:
... 这与在草鱼
[15]、虹鳟(
Oncorhynchus mykiss)
[18]和牙鲷
[19]上的研究结果一致 ...
1
本文献在全文中的定位:
... 这与在草鱼
[15]、虹鳟(
Oncorhynchus mykiss)
[18]和牙鲷
[19]上的研究结果一致 ...
1
本文献在全文中的定位:
... 而胆汁酸能够促进脂类合成代谢
[20] ...
1
本文献在全文中的定位:
... 可能是由于牛磺酸在鱼体内参与了胆汁酸合成以外的其他代谢合成途径
[21] ...
1
本文献在全文中的定位:
... 饲料中牛磺酸含量对斜带石斑鱼组织中游离氨基酸含量的影响
动物体内游离氨基酸的含量明显受饲料组成的影响[22, 23] ...
1
本文献在全文中的定位:
... 饲料中牛磺酸含量对斜带石斑鱼组织中游离氨基酸含量的影响
动物体内游离氨基酸的含量明显受饲料组成的影响[22, 23] ...
1
本文献在全文中的定位:
... 丝氨酸和蛋氨酸是鱼类牛磺酸合成过程中的关键氨基酸
[24] ...
1
本文献在全文中的定位:
... 牛磺酸在鱼体内的分布与牛磺酸合成过程中的关键酶——半胱次磺酸脱羧酶(CSAD)
[4]在这些组织中的活性有关
[25, 26] ...
1
本文献在全文中的定位:
... 牛磺酸在鱼体内的分布与牛磺酸合成过程中的关键酶——半胱次磺酸脱羧酶(CSAD)
[4]在这些组织中的活性有关
[25, 26] ...
1
本文献在全文中的定位:
... 而牛磺酸在眼睛、脑等一些特定组织中的含量尤其高
[27] ...
1
本文献在全文中的定位:
... 这也表明了鱼类不同的组织对牛磺酸的蓄积能力不同
[11, 28] ...
 1)
1)
 1)
1)
 3)
3)
 3)
3)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 3)
3)
 2)
2)
 2)
2)
 2)
2)
 3)
3)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)
 1)
1)




