氧化应激可影响机体健康,降低仔猪生长性能[1, 2]。养猪生产中,许多因素使机体自由基产生过量或细胞内抗氧化防御系统受损,从而诱导氧化应激[3]。精氨酸(Arg)是合成生物学重要分子如一氧化氮(NO)、多胺、肌酸的重要前体物,可促进胰岛素和生长激素分泌、调节养分代谢等[4]。在应激或疾病状态下,Arg被认为是猪的必需氨基酸[5]。近年来的研究发现,补充Arg可以缓解代谢综合征[6, 7],促进动物生长[8],提高胚胎存活率[9, 10],增强机体抗氧化功能[11],降低氧化应激的危害。本实验室前期的研究发现,补充Arg可以提高氧化应激仔猪的采食量,缓解氧化应激仔猪的失重[12],那么Arg抗氧化应激的作用途径是什么呢?目前还缺乏相关研究报道。本试验通过Diquat诱导仔猪的氧化应激[13, 14, 15, 16, 17],研究Arg对氧化应激仔猪内分泌及生长相关因子基因表达的影响,探寻Arg抗氧化应激的作用机制,为生产上合理应用Arg提供理论支持。 1 材料与方法 1.1 试验材料
Diquat(货号:PS365),购于美国Sigma公司。 1.2 试验设计
试验选择24头21日龄健康PIC断奶仔公猪,经过1周的预饲,按体重相近的原则随机分为4组,每组6个重复,每个重复1头猪,分别饲喂基础饲粮+注射生理盐水(对照组)、基础饲粮+注射Diquat(氧化应激组)、基础饲粮+0.8% Arg+注射Diquat(0.8% Arg组)、基础饲粮+1.6% Arg+注射Diquat(1.6% Arg组)。正式试验第8天08:00,各试验组仔猪空腹称重采血后腹腔注射用灭菌生理盐水配成的10 mg/mL的Diquat溶液(10 mg/kg BW)[12]诱导氧化应激,对照组仔猪注射等量的灭菌生理盐水。正式试验期共11 d。 1.3 试验饲粮
试验用基础饲粮为玉米-豆粕型饲粮,饲粮配制参照NRC(1998)5~10 kg仔猪营养需要以及猪场PIC仔猪饲料营养水平配制[18],基础饲粮组成及营养成分见表1。0.8%和1.6% Arg组分别以0.8%和1.6%的Arg等量替代基础饲粮中的玉米。
| 表1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
试验在四川农业大学动物营养研究所试验猪场进行,猪舍温度控制在(25±1) ℃,每天饲喂4次(08:00、12:00、16:00、20:00),每次以采食后料槽略有少量余料为度,所有仔猪自由采食和饮水,按常规程序进行消毒。 1.5 样品采集与处理
分别于应激前(0 h),应激后24、48和96 h,全部仔猪空腹用含肝素的真空采血管于前腔静脉采血10 mL,3 000 r/min离心10 min,收集血浆,-20 ℃保存待测。于试验第11天,空腹称重后采血,然后进行麻醉屠宰,迅速取肝脏、背最长肌样品,用液氮速冻后转入-80 ℃保存备用。 1.6 测定指标及方法 1.6.1 血浆胰岛素、胰岛素样生长因子-Ⅰ(IGF-Ⅰ)的浓度
血浆中胰岛素、IGF-Ⅰ的浓度采用猪胰岛素和IGF-Ⅰ酶联免疫吸附试验(ELISA)试剂盒(R&D,美国)进行测定。具体操作按试剂盒说明书进行。 1.6.2 血浆和肝脏一氧化氮合成酶(NOS)的活性
采用试剂盒测定血浆和肝脏中总一氧化氮合成酶(tNOS)和诱导型一氧化氮合成酶(iNOS)的活性,详细方法参见南京建成生物工程研究所试剂盒操作说明。 1.6.3 肝脏和肌肉生长相关因子基因表达
所测肝脏和肌肉生长相关因子基因包括IGF-Ⅰ、胰岛素样生长因子-Ⅰ受体(IGF-ⅠR)、胰岛素样生长因子结合蛋白3(IGFBP3)。肝脏和肌肉组织总RNA提取按照Trizol试剂(TaKaRa,大连)说明书进行,提取的总RNA溶解于无RNA酶水溶液(TaKaRa,大连)中。总RNA提取后,进行琼脂糖凝胶电泳检测RNA的完整性;核酸蛋白仪(Beckman Coulter DU800,CA,美国)于260 nm波长处检测RNA浓度。cDNA合成采用逆转录试剂盒(TaKaRa,大连),反转录反应条件为:在PCR仪(Bio-Rad,美国)中,37 ℃下孵化15 min(反转录反应),85 ℃下静置5 s(反转录酶的失活反应),反应产物即cDNA,-20 ℃保存备用。
实时荧光定量PCR用Option Monitor 3 Real-Time PCR检测系统(Bio-Rad,美国),采用TaKaRa SYBR Green Supermix Ⅱ试剂盒,引物序列及参数见表2。反应程序:95 ℃预变性30 s;95 ℃变性10 s;退火温度下退火20 s;40个循环。反应结束后作熔解曲线,产生单峰以证实产物的单一性。结果用Pfaffl[19]的方法进行计算。
| 表2 实时荧光定量PCR引物序列及参数 Table 2 Primer sequences and parameters for real-time PCR |
所有数据采用SPSS 17.0统计软件进行单因素方差分析,差异显著者进行Duncan氏多重比较。结果以平均值±标准误表示,以P<0.05为差异显著标准。 2 结果与分析 2.1 饲粮补充Arg对氧化应激仔猪血浆胰岛素、IGF-Ⅰ浓度的影响
由表3可知,注射Diquat前,各组仔猪血浆胰岛素浓度无显著差异(P>0.05),Arg对血浆胰岛素浓度无显著影响(P>0.05)。氧化应激显著降低仔猪应激后24 h血浆胰岛素浓度(P<0.05),对应激后48和96 h的血浆胰岛素浓度影响不显著(P>0.05)。与氧化应激组相比,补充1.6% Arg使仔猪应激后24、96 h的血浆胰岛素浓度分别升高了113%(P>0.05)、121%(P>0.05);与对照组相比,补充1.6% Arg可显著升高仔猪应激后96 h的血浆胰岛素浓度(P<0.05)。
注射Diquat前,补充1.6% Arg显著升高了血浆IGF-Ⅰ的浓度(P<0.05)。与对照组相比,氧化应激可显著降低仔猪应激后24 h的血浆IGF-Ⅰ浓度(P<0.05),对应激后48 h的血浆IGF-Ⅰ浓度影响不显著(P>0.05),但显著升高了应激后96 h的血浆IGF-Ⅰ浓度(P<0.05)。与氧化应激组相比,补充0.8% Arg可显著增加仔猪应激后48 h的血浆IGF-Ⅰ浓度(P<0.05),补充1.6% Arg使仔猪应激后24、48、96 h的血浆IGF-Ⅰ浓度分别升高了13%(P>0.05)、44%(P<0.05)、15%(P<0.05)。
| 表3 饲粮补充Arg对氧化应激仔猪血浆胰岛素和IGF-Ⅰ浓度的影响 Table 3 Effects of arginine supplementation on plasma concentrations of insulin and IGF-Ⅰ of piglets under oxidative stress |
由表4可见,氧化应激和补充Arg对仔猪血浆tNOS、iNOS活性的影响均不显著(P>0.05)。
| 表4 饲粮补充Arg对氧化应激仔猪血浆一氧化氮合成酶活性的影响 Table 4 Effects of arginine supplementation on plasma activities of NOS of piglets under oxidative stress |
由表5可见,氧化应激显著降低仔猪肝脏tNOS、iNOS的活性(P<0.05)。补充0.8% Arg能显著抑制因氧化应激导致的tNOS活性的降低(P<0.05),补充1.6% Arg能显著升高氧化应激下仔猪肝脏tNOS、iNOS的活性(P<0.05)。 2.4 饲粮补充Arg对氧化应激仔猪肝脏IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA表达的影响
由图1可见,氧化应激显著降低仔猪肝脏IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA的相对表达量(P<0.05)。补充0.8% Arg能显著增加因氧化应激导致的IGF-Ⅰ mRNA相对表达量的下降(P<0.05),补充1.6% Arg能显著增加因氧化应激导致的IGF-Ⅰ和IGFBP3 mRNA相对表达量的下降(P<0.05)。
| 表5 饲粮补充Arg对氧化应激仔猪肝脏一氧化氮合成酶活性的影响 Table 5 Effects of arginine supplementation on liver activities of NOS of piglets under oxidative stress |
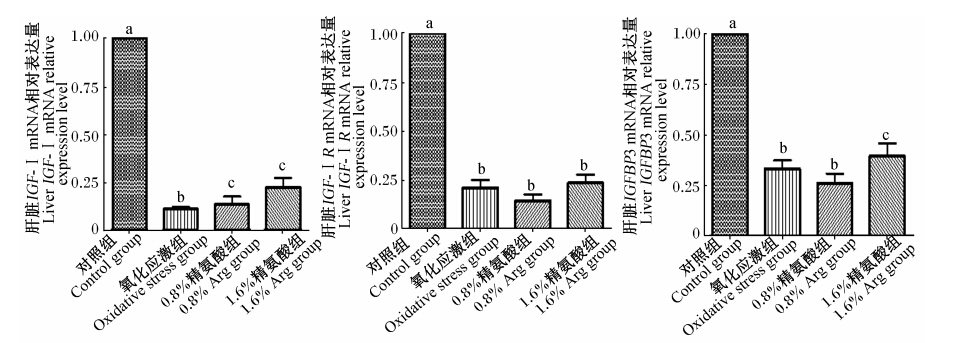 | 图1 饲粮补充Arg对氧化应激仔猪肝脏IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA表达的影响Fig. 1 Effects of arginine supplementation on liver expression of IGF-Ⅰ,IGF-ⅠR and IGFBP3 mRNA of piglets under oxidative stress |
2.5 饲粮补充Arg对氧化应激仔猪肌肉IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA表达的影响
由图2可见,氧化应激对仔猪肌肉IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA的相对表达量无显著 影响(P>0.05)。补充1.6% Arg能显著增加氧化应激仔猪肌肉IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA的相对表达量(P<0.05)。
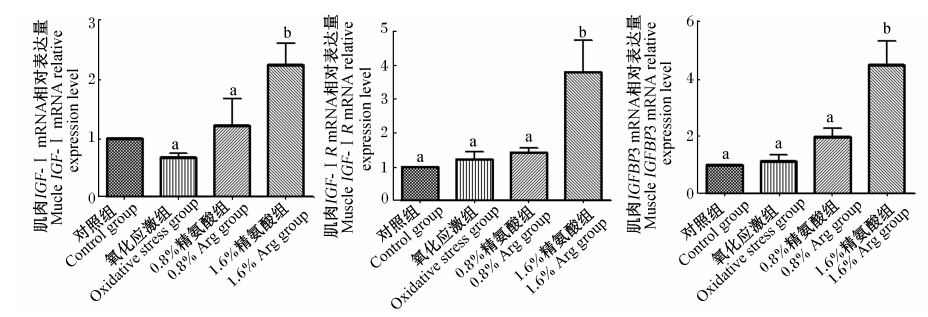 | 图2 饲粮补充Arg对氧化应激仔猪肌肉IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA表达的影响Fig. 2 Effects of arginine supplementation on muscle expression of IGF-Ⅰ,IGF-ⅠR and IGFBP3 mRNA of piglets under oxidative stress |
3 讨 论
生产上许多因素会使仔猪遭遇氧化应激,导致生产性能降低,甚至继发感染各种疾病。研究发现,Arg具有抗氧化应激的作用[20]。本实验室之前的研究也表明,饲粮补充Arg能缓解仔猪氧化应激[12],本试验在此基础上进一步研究了Arg抗仔猪氧化应激的作用机制。
应激和内分泌有着重要的关系。Wright等[21]研究发现,内毒素(LPS)增加了公猪的血液皮质醇和肿瘤坏死因子-α(TNF-α)的浓度,降低了血液中IGF-Ⅰ的浓度。血液中胰岛素和IGF-Ⅰ的浓度与仔猪生长性能有着重要的关系。在生长性能很差的仔猪上发现,血液循环中IGF-Ⅰ和IGFBP3的浓度都很低[22]。本试验中,Diquat诱导的仔猪氧化应激可显著降低仔猪血浆胰岛素和IGF-Ⅰ的浓度。Priego等[23]报道,注射LPS可降低大鼠血浆IGF-Ⅰ浓度和肝脏IGF-Ⅰ mRNA的表达,本试验结果与此一致,氧化应激显著降低了应激后24 h的血浆IGF-Ⅰ浓度以及肝脏IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA的相对表达量。此外,本试验中对照组仔猪应激后96 h的血浆胰岛素浓度较其他时间点大幅度的降低,而96 h血浆IGF-Ⅰ浓度则大幅度的升高,分析其原因可能是应激会导致胰岛素分泌的下降,连续的采血对猪只也是一种应激;IGF-Ⅰ浓度升高的原因可能与机体的自我修复功能有关,具体的原因有待进一步的研究。
Arg可通过刺激胰岛素和生长激素的分泌,调节应激状态下动物的代谢,增加蛋白质的合成代谢,降低蛋白质的分解代谢[24, 25]。Liu等[26]研究认为,补充Arg能减低LPS应激断奶仔猪蛋白质分解代谢,维持骨骼肌蛋白质的沉积率,从而缓解LPS应激导致的仔猪生长受阻。本试验结果显示,补充Arg可显著增加氧化应激仔猪的血浆胰岛素和IGF-Ⅰ浓度以及肝脏IGF-Ⅰ mRNA的相对表达量;同时,本实验室之前的研究结果显示,补充Arg可缓解氧化应激导致的仔猪生产性能的下降[12]。肌肉是IGF-Ⅰ转录的重要场所,本试验结果显示,补充Arg显著增加了氧化应激条件下仔猪肌肉IGF-Ⅰ基因的表达,说明Arg通过上调氧化应激条件下仔猪肌肉中IGF-Ⅰ、IGF-ⅠR和IGFBP3 mRNA的表达,从而影响氧化应激仔猪肌肉蛋白质的合成。研究发现,IGF-Ⅰ是一种潜在的抗氧化剂,能显著降低体外培养大鼠软骨细胞内活性氧的水平[27],通过稳定线粒体保护成年大鼠缺血/再灌注模型的心肌细胞免受活性氧损伤[28],保护肠上皮细胞免受氧化应激诱导的细胞凋亡[29]。补充Arg可上调氧化应激仔猪肝脏IGF-Ⅰ mRNA的表达,也可能是Arg调控机体抵御氧化应激的另一种机制。以上结果说明,Arg抗氧化应激的作用途径之一是通过对内分泌的调控作用,上调氧化应激仔猪胰岛素和IGF-Ⅰ的浓度,从而缓解仔猪氧化应激。
此外,有研究显示,Arg可通过NO途径促进激素的分泌[5, 30]。本试验中,补充Arg能改善氧化应激仔猪肝脏一氧化氮合成酶的活性,显著提高肝脏IGF-ⅠmRNA的表达。研究表明,血液循环中的IGF-Ⅰ主要来源于肝脏的生物合成[31]。因此,结合本试验结果,推测给氧化应激仔猪补充Arg可活化肝脏一氧化氮合成酶活性,并通过NO途径调控肝脏IGF-Ⅰ的合成,进而影响循环中IGF-Ⅰ的浓度。同时,血液指标结果显示,补充Arg对氧化应激仔猪血浆NOS的活性无显著影响,这说明Arg-NO途径可能存在组织特异性,氧化应激仔猪补充Arg促进激素分泌可能通过依赖和不依赖NO的2种途径。
综上所述,Arg缓解仔猪氧化应激的途径之一是通过促进胰岛素、IGF-Ⅰ的分泌,进而调控生长相关因子基因表达,其具体的作用机制是否依赖于NO途径还有待于进一步的研究。 4 结 论
Arg通过促进胰岛素、IGF-Ⅰ的分泌进而上调生长相关因子基因表达是其缓解仔猪氧化应激的途径之一。
| [1] | LOSCALZO J.L-arginine and atherothrombosis[J].The Journal of Nutrition, 2004, 134(Suppl.10):2798S-2800S. ( 1) 1)
|
| [2] | YANG Z H, MING X F.Recent advances in understanding endothelial dysfunction in atherosclerosis[J].Clinical Medicine and Research, 2006, 4(1):53-65. ( 1) 1)
|
| [3] | ASKEW E W.Environmental and physical stress and nutrient requirements[J]. American Journal of Clinical Nutrtion, 1995, 61(Suppl.3):631S-637S. ( 1) 1)
|
| [4] | BREDT D S, SNYDER S H.Nitric oxide:a physiological messenger molecule[J].Annual Review of Biochemistry, 1994, 63:175-195. ( 1) 1)
|
| [5] | WU G Y, BAZER F W, DAVIS T A, et al.Important roles for the arginine family of amino acids in swine nutrition and production[J]. Livestock Science, 2007, 112(1/2):8-22. ( 2) 2)
|
| [6] | SIASOS G, TOUSOULIS D, ANTONIADES C, et al.L-arginine, the substrate for no synthesis:an alternative treatment for premature atherosclerosis?[J]. International Journal of Cardiology, 2007, 116(3):300-308. ( 1) 1)
|
| [7] | BARRY D P, ASIM M, LEWIS N D, et al.Increased L-arginine availability enhances Helicobacter pylori colonization in the chronic mouse model[J]. Gastroenterology, 2009, 136(5):A-104. ( 1) 1)
|
| [8] | HERNANDEZ A, HANSEN C F, MULLAN B P, et al.L-arginine supplementation of milk liquid or dry diets fed to pigs after weaning has a positive effect on production in the first three weeks after weaning at 21 days of age[J]. Animal Feed Science and Technology, 2009, 154(1/2):102-111. ( 1) 1)
|
| [9] | BERARD J, BEE G.Effects of dietary L-arginine supplementation to gilts during early gestation on foetal survival, growth and myofiber formation[J]. Animal, 2010, 4(10):1680-1687. ( 1) 1)
|
| [10] | KONG X F, TAN B, YIN Y L, et al.L-arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells[J]. The Journal of Nutritional Biochemistry, 2012, 23(9):1178-1183. ( 1) 1)
|
| [11] | 吴琛, 刘俊锋, 孔祥峰, 等.日粮添加精氨酸对环江香猪肉质及抗氧化功能的影响[C]//姚军虎.第六次全国饲料营养学术研讨会论文集.北京:中国畜牧兽医学会动物营养学分会, 2010.( 1) 1)
|
| [12] | ZHENG P, YU B, HE J, et al.Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets[J]. British Journal of Nutrition, 2013, 109(12):2253-2260. ( 4) 4)
|
| [13] | RYTWO G, TROPP D, SERBAN C.Adsorption of diquat, paraquat and methyl green on sepiolite:experimental results and model calculations[J]. Applied Clay Science, 2002, 20(6):273-282. ( 1) 1)
|
| [14] | ROGERS L K, BATES C M, WELTY S E, et al.Diquat induces renal proximal tubule injury in glutathione reductase-deficient mice[J]. Toxicology and Applied Pharmacology, 2006, 217(3):289-298. ( 1) 1)
|
| [15] | BENZICK A E, REDDY S L, GUPTA S, et al.Diquat- and acetaminophen-induced alterations of biliary efflux of iron in rats[J]. Biochemical Pharmacology, 1994, 47(11):2079-2085. ( 1) 1)
|
| [16] | BLAKEMAN D P, RYAN T P, JOLLY R A, et al.Diquat-dependent protein carbonyl formation:identification of lipid-dependent and lipid-independent pathways[J]. Biochemical Pharmacology, 1995, 50(7):929-935. ( 1) 1)
|
| [17] | SPALDING D J M, MITCHELL J R, JAESCHKE H, et al.Diquat hepatotoxicity in the fischer-344 rat:the role of covalent binding to tissue proteins and lipids[J]. Toxicology and Applied Pharmacology, 1989, 101(2):319-327. ( 1) 1)
|
| [18] | NRC.Nutrient requirements of swine[S]. 10th ed.Washington, D.C.:National Academy Press, 1998. ( 1) 1)
|
| [19] | PFAFFL M W.A new mathematical model for relative quantification in real-time RT-PCR[J]. Nucleic Acids Research, 2001, 29(9):e45. ( 1) 1)
|
| [20] | SUSCHEK C V, SCHNORR O, HEMMRICH K, et al.Critical role of L-arginine in endothelial cell survival during oxidative stress[J]. Circulation, 2003, 107(20):2607-2614. ( 1) 1)
|
| [21] | WRIGHT K J, BALAY R, HILL C M, et al.Integrated adrenal, somatotropic, and immune responses of growing pigs to treatment with lipopolysaccharide[J]. Journal of Animal Science, 2000, 78(7):1892-1899. ( 1) 1)
|
| [22] | SALERI R, BARATTA M, MAINARDI G L, et al.IGF-1, IGFBP-2 and -3 but not GH concentration are different in normal and poor growing piglets[J]. Reproduction Nutrition Development, 2001, 41(2):163-172. ( 1) 1)
|
| [23] | PRIEGO T, DE CÁCERES I I, MARTIN A I, et al.No plays a role in LPS-induced decreases in circulating IGF-Ⅰ and IGFBP-3 and their gene expression in the liver[J]. American Journal of Physiology:Endocrinology and Metabolism, 2004, 286(1):E50-E56. ( 1) 1)
|
| [24] | FRANK J W, ESCOBAR J, NGUYEN H V, et al.Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets[J]. The Journal of Nutrition, 2007, 137(2):315-319. ( 1) 1)
|
| [25] | WU G, MEININGER C J, KNABE D A, et al.Arginine nutrition in development, health and disease[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2000, 3(1):59-66. ( 1) 1)
|
| [26] | LIU Y L, HUANG J J, HOU Y Q, et al.Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs[J]. British Journal of Nutrition, 2008, 100(3):552-560. ( 1) 1)
|
| [27] | JALLALI N, RIDHA H, THRASIVOULOU C, et al.Modulation of intracellular reactive oxygen species level in chondrocytes by IGF-1, FGF, and TGF-β1[J]. Connective Tissue Research, 2007, 48(3):149-158. ( 1) 1)
|
| [28] | PI Y, GOLDENTHAL M J, MARIN-GARCIA J.Mitochondrial involvement in IGF-1 induced protection of cardiomyocytes against hypoxia/reoxygenation injury[J]. Molecular and Cellular Biochemistry, 2007, 301(1/2):181-189. ( 1) 1)
|
| [29] | BAREGAMIAN N, SONG J, JESCHKE M G, et al.IGF-1 protects intestinal epithelial cells from oxidative stress-induced apoptosis[J]. Journal of Surgical Research, 2006, 136(1):31-37. ( 1) 1)
|
| [30] | ZHAO J P, JIAO H C, SONG Z G, et al.Effects of L-arginine supplementation on glucose and nitric oxide (no) levels and activity of no synthase in corticosterone-challenged broiler chickens (Gallus gallus)[J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2009, 150(4):474-480. ( 1) 1)
|
| [31] | DELAFONTAINE P, SONG Y H, LI Y.Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels[J]. Arteriosclerosis Thrombosis and Vascular Biology, 2004, 24(3):435-444. ( 1) 1)
|




