脂多糖(lipopolysaccharide,LPS)也被称为内毒素,是革兰氏阴性菌外膜的主要成分,被认为是与革兰氏阴性菌感染相关的内毒素休克发病机制中的一个关键分子[1],多在细胞分裂、死亡或抗生素治疗细菌感染的过程中释放出[2]。LPS对细菌生存而言必不可少,它通过建立一个有效的渗透性屏障来阻止各种抗菌化合物入侵,包括疏水性抗生素、抗菌肽等[3]。抗菌肽是一种具有生物活性的带正电荷及具备疏水性和两亲性的小分子多肽。研究证实天然的阳离子抗菌肽可以防止细菌、病毒和寄生虫等多种感染[4-5],还能直接中和LPS,抑制炎性细胞因子的过度产生和释放从而控制炎症反应,并通过免疫调节减少炎性损伤,在炎症过程中具有重要的作用[6-7]。近段时间以来,抗菌肽因其抗感染性能可作为治疗人类以及动植物疾病的替代药物而备受关注[8]。本文综述了近年来LPS诱导炎症产生的机制及抗菌肽抑制LPS诱导炎症反应的作用机理。
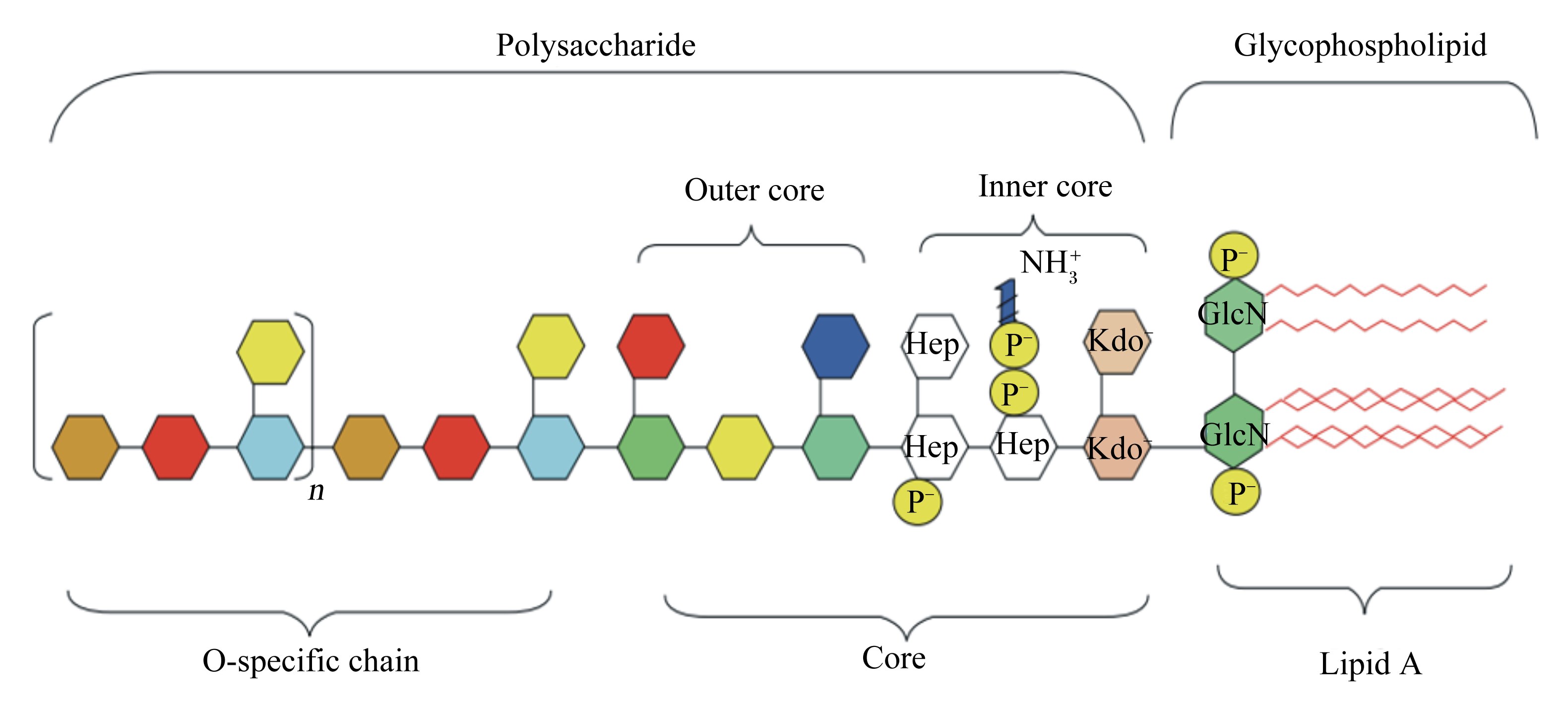
1 LPS诱导炎症反应 1.1 LPS的化学结构LPS是革兰氏阴性杆菌细胞壁结构与功能的重要组成部分,由O-特异性多糖链、核心寡聚糖及类脂A构成,是带有负电荷磷酸基团的疏水性长链脂肪酸(图 1)[9]。其中类脂A是LPS结构中最保守的部分,在LPS的生物活性中起主要调控作用[10]。LPS的核心寡聚糖和磷酸基团都带有负电荷,提示LPS对阳性离子具有极高的亲和性[1]。
|
Polysaccharide:多糖;Glycophospholipid:磷脂;Core:核心寡聚糖;Outer core:外核心寡聚糖;Inner core:内核心寡聚糖;O-specific chain:O-特异性多糖链;Lipid A:类脂A;Hep:L-甘油基-D-甘露庚糖 L-glycero-D-manno-heptose;Kdo:2-酮基-3-脱氧辛酸 2-keto-3-deoxyoctulosonic acid;GlcN:氨基葡萄糖 glucosamine;波浪线为脂肪酸 Zig-zag lines mean fatty acids。 图 1 LPS的化学结构 Figure 1 Chemical structure of LPS[9] |
免疫细胞可识别病原体相关分子模式(pathogen-associated molecular patterns,PAMPs),在炎症反应中起重要作用[11]。感染时,PAMPs之一的LPS与免疫细胞表面最主要的受体之一——CD14结合并通过脂多糖结合蛋白(lipopolysaccharide binding protein,LBP)形成LPS-LBP-CD14三联复合物作用于Toll样受体4(Toll-like receptors 4,TLR4)从而启动跨膜信号转导,发挥致炎效应[12]。LBP是一种介导LPS与靶细胞表面受体CD14结合的关键载体蛋白。LBP的N端是LBP与LPS的结合位点,尤其是富含疏水性氨基酸和碱性氨基酸的LBP109~133位残基容易与LPS结合;而C端则是其与CD14的结合位点。Toll样受体(Toll-like receptors,TLR)是第1个被发现并明确特征的模式识别受体(pattern recognition receptor,PRR),TLR4已被确认为可识别LPS的受体[13]。
LPS参与细胞介导的炎症反应时有2条不同的信号转导通路。第1条是髓样分化因子88(myeloid differentiation factor 88,MyD88)依赖途径,第2条是含有Toll/(白细胞介素-1)IL-1受体(TIR)结构域诱导干扰素β(TRIF)依赖途径,两者都由LPS和TLR4的关联触发[14]。盛金良等[15]的试验结果表明,LPS能够很快诱导TLR4的表达水平升高,在LPS作用20 min后即达到较高水平,且在整个试验过程中保持较高水平。
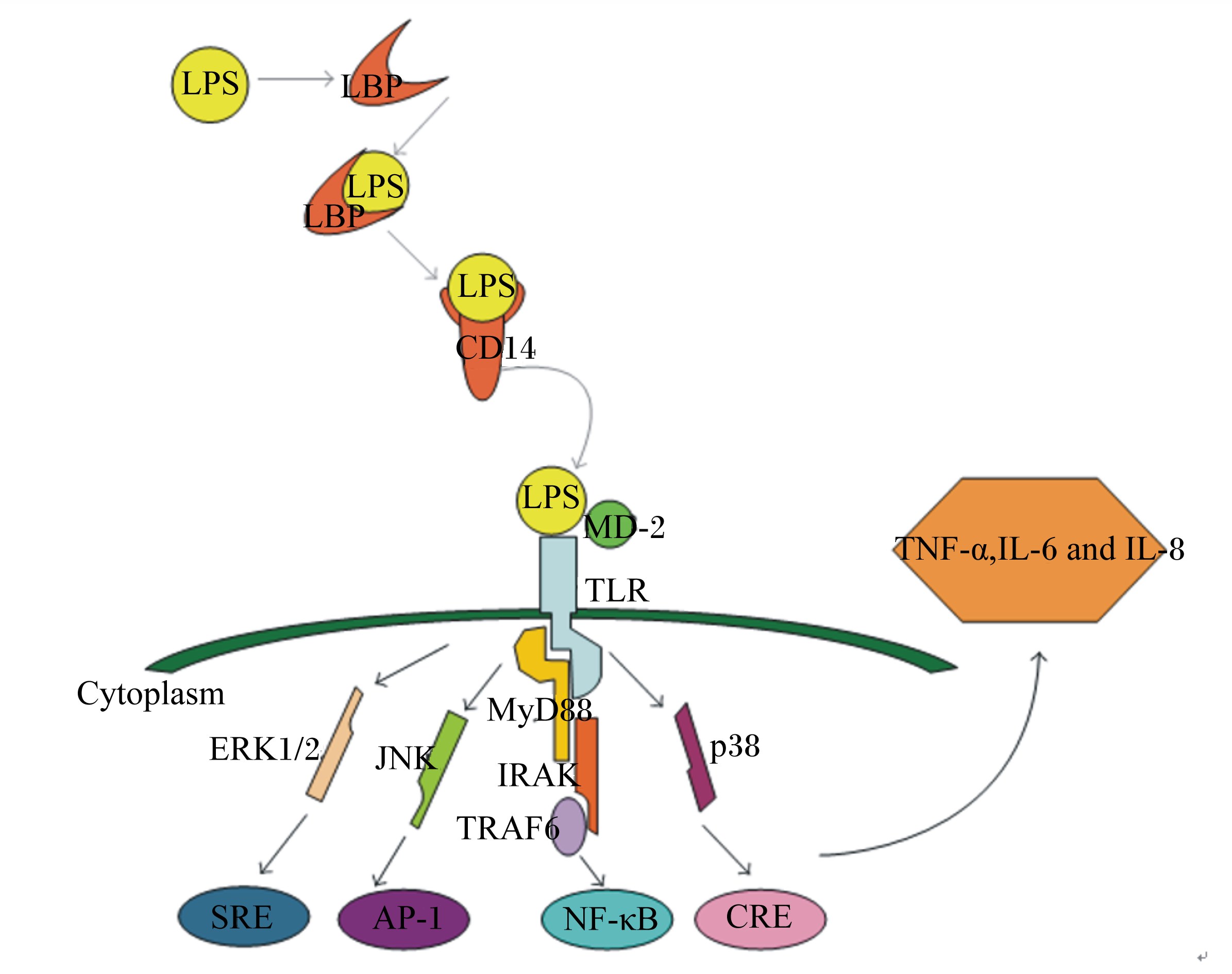
LPS/TLR4信号通道的激活受到多种信号蛋白或受体的调控(图 2)[16]。髓样分化蛋白-2(myeloid differentiation protein-2,MD-2)是一种特殊的外分泌蛋白,能帮助TLR4识别LPS。首先,MD-2与TLR4在免疫细胞表面形成TLR4-MD-2复合体,当LPS进入血液循环时,LBP作为载体蛋白将LPS传递给CD14,后者进一步凝集LPS并将其呈递给TLR4-MD-2复合体。最后,MD-2识别并与LPS结合形成TLR4-MD-2-LPS三聚体,激活TLR4跨膜信号通路。MyD88依赖信号通路的激活导致丝裂原活化蛋白激酶(mitogen-activated protein kinases,MAPKs)、抑制性κB激酶(inhibitor of nuclear factor kappa-B kinase,IKK)和核转录因子-κB(nuclear factor-κB,NF-κB)磷酸化,最终导致促炎细胞因子如肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)、白细胞介素-6(IL-6)、诱导型一氧化氮合酶(iNOS)、环氧合酶-2(COX-2)等的表达[17]。MAPKs通路主要包括p38、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)和细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)[18]。许多研究表明,抑制p38、JNK和ERK 3条通路的磷酸化可达到抗炎目的。p38的激活是促炎细胞因子表达的先决条件,抑制p38通路已被证实可在人体内毒素血症中发挥抗炎作用[19]。
|
LPS:脂多糖 lipopolysaccharide;LBP:脂多糖结合蛋白 lipopolysaccharide binding protein;MD-2:髓样分化蛋白-2 myeloid differentiation protein-2;TLR:Toll样受体 Toll-like receptor;Cytoplasm:细胞浆;ERK1/2:细胞外调节蛋白激酶 extracellular regulated protein kinases;JNK:c-Jun氨基末端激酶 c-Jun N-terminal kinase;MyD88:髓样分化因子88 myeloid differentiation factor 88;IRAK:白细胞介素-1受体相关激酶 interleukin-1 receptor-associated kinase;TRAF6:肿瘤坏死相关因子6 tumor necrosis factor receptor-associated factor 6;SRE:血清反应组件 serum response element;AP-1:激活子蛋白-1 activator protein-1;NF-κB:核转录因子-κB nuclear factor-κB;CRE;cAMP反应组件 cAMP response element;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;IL-6:白细胞介素-6 interleukin-6;IL-8:白细胞介素-8 interleukin-8。 图 2 LPS的信号转导途径 Figure 2 Signal transduction pathway of LPS[16] |
当PAMPs与PRR相互作用时,免疫细胞分泌趋化因子及防御素α和LL-37等抗菌肽[20]。抗菌肽可通过诱导肥大细胞发生脱颗粒作用,释放组胺和前列腺素D2,引起血管舒张并导致血液中免疫细胞的释放,进而诱导巨噬细胞凋亡和淋巴细胞活化来调节宿主免疫力。此外,抗菌肽还可以增强成纤维细胞的趋化性,促进内皮细胞和淋巴细胞的增殖,加速伤口愈合。例如,人抗菌肽LL-37可与甲酰肽受体1(FPR1)相互作用使单核细胞、中性粒细胞、淋巴细胞和T淋巴细胞趋化[21]。研究表明经过活化的FPR1和LL-37不仅对白细胞有趋化作用,还能增强白细胞的黏附力、吞噬能力,促进氧中间产物的释放,增强对细菌的杀伤力,从而增强免疫力[22]。
3 抗菌肽与LPS相互作用时结构与活性的关系针对抗菌肽的结构与活性关系的详细研究表明,有许多抗菌肽都具备中和LPS的活性,但是不同结构的肽中和LPS的活性存在着显著的差异。Heinbockel等[23]研究了Hbγ-35和Pep19-2.5 2种抗菌肽对LPS聚合构造的影响。Hbγ-35作用时LPS被分解为立方体结构,且Hbγ-35增加了LPS诱导的人体单核细胞分泌TNF-α。与之相反的是,Pep19-2.5使LPS从立方体结构转变成多层结构,从而抑制了TNF-α分泌。
Kaconis等[24]研究了一系列合成肽对LPS的中和作用,表明LPS形成多层结构的能力与抑制LPS刺激产生细胞因子直接相关,且合成肽与LPS的结合在抗菌肽的抗菌活性和抗炎活性均起着重要的作用。rBPI21是中性粒细胞N末端的杀菌/通透性增加蛋白(bactericidal/permeability-increasing protein,BPI)片段,它能够选择性的抑制革兰氏阴性菌,且对LPS有很强的亲和力。rBPI21在与LPS相互作用时可引起含有丰富的磷脂酰胆碱的革兰阴性细菌膜泄漏[25]。
事实上,许多在结构上不够优化的天然抗菌肽,可以通过适当地进行氨基酸取代改善其活性[26]。例如Nan等[27]将抗菌肽LL-37的类似物a4中5、15位的苯丙氨酸替换为色氨酸后显著提高了它中和LPS的活性。
Singh等[28]通过一系列来源于S1肽酶的抗菌肽来探究静电作用在抗菌肽与LPS相互作用时发挥的作用,证实在很大程度上抗菌肽与磷脂膜结合是依赖于肽的两亲性构象,而与LPS结合则取决于抗菌肽的净电荷及疏水性。同样,Andrä等[29]报道LPS与抗菌肽NK-2结合时不仅依靠静电作用,疏水作用也非常关键。Lee等[30]根据牛源抗菌肽BAMP-27合成了一种含18个氨基酸残基的肽,BAMP-18以及其类似物(BAMP-18-W、BAMP-18-L、BAMP-18-I和BAMP-18-F)。结果证实BAMP-18及类似物能明显抑制TNF-α和一氧化氮(NO)。尽管BMAP-18-W疏水性低于BMAP-18-L,其中和LPS的能力却更强。说明抗菌肽中和LPS的活性不仅与携带的正电荷数及疏水性有关,还存在其他的影响因素。
4 抗菌肽直接中和LPS的作用机制抗菌肽来源众多,包括来源于昆虫的天蚕素与蜂毒素的混合抗菌肽CEMA、人抗菌肽LL-37、牛源抗菌肽BAMP-27、合成的小分子阳离子抗菌肽等,其中大多数抗菌肽都被证实能显著抑制LPS致炎症反应,并防止内毒素血症[31]。
Fang等[32]试验表明,成熟的U937细胞在受到LPS刺激后会激活NF-κB通路并释放TNF-α。当U937细胞与LBP、LPS共同处理时,NF-κB活化的程度及TNF-α的表达量均显著升高。证实LBP能加剧LPS诱导的炎症反应。而多肽P1和MP12对LPS-LBP复合物诱导的NF-κB的活化和TNF-α表达的具有明显的抑制作用,均较LPS单独处理时更低。证实多肽P1和MP12可以和CD14与LBP结合位点结合,阻止LPS和CD14通过LBP结合,抑制LPS释放炎症因子。
Nagaoka等[33]研究表明,抗菌肽CAP18、CAP11以及LL-37均可抑制LPS与CD14结合从而抑制LPS诱导巨噬细胞表达产生TNF-α。表明抗菌肽不仅可以结合LPS,也可以和免疫细胞膜受体CD14结合从而此抑制LPS诱导炎症。最近,该团队的研究显示,LL-37通过抑制LPS受体复合物的集合以及受体CD14、TLR4和磷酸化的JNK来阻止LPS诱导内皮细胞凋亡[34]。应当指出的是,LL-37对TNF-α的表达调控机制是有待进一步研究的。一方面,LL-37显示出很强的预防内毒素休克小鼠模型合成TNF-α的能力;另一方面,Babolewska等[35]最近的研究表明LL-37可诱导大鼠结缔组织中成熟的肥大细胞脱粒与迁移,并刺激这些细胞合成多种细胞因子,其中包括TNF-α。
Liu等[36]报道,CLP-19通过与LPS竞争结合LBP从而抑制LPS诱导炎症反应信号通路,明显减少细胞因子TNF-α的释放。而当抗菌肽、LPS和LBP三者同时存在时,抗菌肽能够成功阻止LPS结合LBP但很少分解LPS-LBP复合物。Monisha[37]发现,预处理后的抗菌肽MBI-28与吞噬细胞共同培养1 h,除去上清液后在新的培养基中加入LPS,MBI-28仍然能够抑制巨噬细胞表达产生的TNF-α,提示抗菌肽还存在另外一个机制抑制LPS诱导的炎症,具体的作用机制还需进一步的研究。
最近的研究表明LL-37对细胞凋亡拥有惊人的抑制能力,败血性休克的另一个特点就是LPS和ATP通过促进半胱氨酸特异性蛋白水解酶1(cystein aspartate-specific proteases 1,caspase-1)的过表达导致细胞死亡,大量的促炎性细胞因子包括IL-1β在级联反应的作用下细胞外释放,其中IL-1β是引起全身性休克组织损伤的主要因子。Hu等[38]就证实了LL-37不仅可以抑制LPS结合CD14和TLR4,还能够抑制ATP驱动的P2X7受体激活以及caspase-1的形成。
Swangchan等[39]试验证明,牛子宫内膜细胞在受到LPS刺激时,舌抗菌肽(LAP)、气管抗菌肽(TAP)、S100A8、S100A9和S100A12等的mRNA含量会增加,表明发生炎症时,抗菌肽的基因表达将会被上调。同时也证实了抗菌肽的一些基因(TAP和S100A9等)可作为子宫炎症的诊断标志物。
Kim等[40]在原有的抗菌肽GNU7基础上加入几种设计合理的LPS靶向肽,包括乳铁蛋白28~34位的氨基酸(Lf28~34),BPI的84~99位氨基酸(BPI84~99)以及从头合成肽(Syn),从而合成了Lf-GNU7、BPI-GNU7、Syn-GNU7 3种合成抗菌肽。试验结果证明,与GNU7相比,合成肽的抗菌活性提升了8~32倍,其中SYN-GNU7具有最强的中和LPS及抗炎活性。说明在原有抗菌肽的基础上加入少量LPS靶向肽可显著提高抗菌肽的抗炎活性。
抗菌肽抗炎的作用模式比较复杂,包含多个分子机制。较为重要的有如下3种:1)通过阻塞LPS脂质A的抗原表位来防止LPS与LBP结合从而避免NF-κB通路激活;2)通过抗菌肽的吸附作用使巨噬细胞和单核细胞膜的正电荷积聚,反过来使膜与LPS结合;3)抗菌肽引发的吞噬作用,中和电荷以及破坏LPS集合物[41]。
5 小 结近年来的研究证实抗菌肽具备良好的抑制炎症以及治疗LPS引起的败血性休克的潜能。此外,抗菌肽还可以调节炎症相关信号通路和转录因子、调节免疫活性相关分子,调节机体免疫功能来实现抗炎作用。虽然抗菌肽的应用前景广阔,但就目前而言大规模应用还暂时不可行,部分阳离子抗菌肽的细胞毒性问题[42]、抗菌肽的不稳定性[43-44]等都亟待解决。随着对抗菌肽研究的不断深入,不断开发合适的抗菌肽剂型,将为其在炎症的临床治疗带来更为广阔的应用前景,有望作为新的抗炎药物应用于各种炎症性疾病的治疗。
| [1] | RAETZ C R H, WHITFIELD C. Lipopolysaccharide endotoxins[J]. Annual Review of Biochemistry, 2002 , 71 : 635 –700. DOI: 10.1146/annurev.biochem.71.110601.135414 |
| [2] | HANCOCK R E, SCOTT M G. The role of antimicrobial peptides in animal defenses[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000 , 97 (16) : 8856 –8861. DOI: 10.1073/pnas.97.16.8856 |
| [3] | ROSENFELD Y, SHAI Y. Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions:role in bacterial resistance and prevention of sepsis[J]. Biochimica et Biophysica Acta (BBA):Biomembranes, 2006 , 1758 (9) : 1513 –1522. DOI: 10.1016/j.bbamem.2006.05.017 |
| [4] | RAJANBABU V, CHEN J Y. Antiviral function of tilapia hepcidin 1-5 and its modulation of immune-related gene expressions against infectious pancreatic necrosis virus (IPNV) in Chinook salmon embryo (CHSE)-214 cells[J]. Fish & Shellfish Immunology, 2011 , 30 (1) : 39 –44. |
| [5] | ARNETT E, LEHRER R I, PRATIKHYA P, et al. Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes[J]. Cellular Microbiology, 2011 , 13 (4) : 635 –651. DOI: 10.1111/cmi.2011.13.issue-4 |
| [6] | BROWN K L, POON G F, BIRKENHEAD D, et al. Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses[J]. The Journal of Immunology, 2011 , 186 (9) : 5497 –5505. DOI: 10.4049/jimmunol.1002508 |
| [7] | CHANDRA A, SRIVASTAVA R K, KASHYAP M P, et al. The anti-inflammatory and antibacterial basis of human omental defense:selective expression of cytokines and antimicrobial peptides[J]. PLoS One, 2011 , 6 (5) : e20446 . DOI: 10.1371/journal.pone.0020446 |
| [8] | MANGONI M L, EPAND R F, ROSENFELD Y, et al. Lipopolysaccharide,a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization[J]. Journal of Biological Chemistry, 2008 , 283 (34) : 22907 –22917. DOI: 10.1074/jbc.M800495200 |
| [9] | CAROFF M, KARIBIAN D, CAVAILLON J M, et al. Structural and functional analyses of bacterial lipopolysaccharides[J]. Microbes and Infection, 2002 , 4 (9) : 915 –926. DOI: 10.1016/S1286-4579(02)01612-X |
| [10] | COHEN J. The immunopathogenesis of sepsis[J]. Nature, 2002 , 420 (6917) : 885 –891. DOI: 10.1038/nature01326 |
| [11] | BERNARDINO A L F, MYERS T A, ALVAREZ X, et al. Toll-like receptors:insights into their possible role in the pathogenesis of lyme neuroborreliosis[J]. Infection and Immunity, 2008 , 76 (10) : 4385 –4395. DOI: 10.1128/IAI.00394-08 |
| [12] | ZWEIGNER J, SCHUMANN R R, WEBER J R. The role of lipopolysaccharide-binding protein in modulating the innate immune response[J]. Microbes and Infection, 2006 , 8 (3) : 946 –952. DOI: 10.1016/j.micinf.2005.10.006 |
| [13] | KAYAGAKI N, WONG M T, STOWE I B, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4[J]. Science, 2013 , 341 (6151) : 1246 –1249. DOI: 10.1126/science.1240248 |
| [14] | KAWAI T, AKIRA S. The role of pattern-recognition receptors in innate immunity:update on Toll-like receptors[J]. Nature Immunology, 2010 , 11 (5) : 373 –384. DOI: 10.1038/ni.1863 |
| [15] | 盛金良, 陈创夫, 杨霞, 等. 绵羊Toll样受体家庭在肺泡巨噬细胞的分布及脂多糖(LPS)刺激对TLR2、TLR4表达的影响[J]. 华北农学报, 2010 , 25 (1) :30 –35. |
| [16] | SUN Y, SHANG D. Inhibitory effects of antimicrobial peptides on lipopolysaccharide-induced inflammation[J]. Mediators of Inflammation, 2014 , 2015 : 167572 . |
| [17] | WANG D H, XU N N, ZHANG Z B, et al. Sophocarpine displays anti-inflammatory effect via inhibiting TLR4 and TLR4 downstream pathways on LPS-induced mastitis in the mammary gland of mice[J]. International Immunopharmacology, 2016 , 35 : 111 –118. DOI: 10.1016/j.intimp.2016.03.026 |
| [18] | CARNEIRO D M V F, DOMINGUES P F, VAZ A K. Innate immunity of the bovine mammary gland:response to infection[J]. Ciência Rural, 2009 , 39 (6) : 1934 –1943. DOI: 10.1590/S0103-84782009005000106 |
| [19] | LI D P, FU Y H, ZHANG W, et al. Salidroside attenuates inflammatory responses by suppressing nuclear factor-κB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice[J]. Inflammation Research, 2013 , 62 (1) : 9 –15. DOI: 10.1007/s00011-012-0545-4 |
| [20] | KARAPETYAN A V, KLYACHKIN Y M, SELIM S, et al. Bioactive lipids and cationic antimicrobial peptides as new potential regulators for trafficking of bone marrow-derived stem cells in patients with acute myocardial infarction[J]. Stem Cells and Development, 2013 , 22 (11) : 1645 –1656. DOI: 10.1089/scd.2012.0488 |
| [21] | SCOTT M G, DULLAGHAN E, MOOKHERJEE N, et al. An anti-infective peptide that selectively modulates the innate immune response[J]. Nature Biotechnology, 2007 , 25 (4) : 465 –472. DOI: 10.1038/nbt1288 |
| [22] | LEE H Y, BAE Y S. The anti-infective peptide,innate defense-regulator peptide,stimulates neutrophil chemotaxis via a formyl peptide receptor[J]. Biochemical and Biophysical Research Communications, 2008 , 369 (2) : 573 –578. DOI: 10.1016/j.bbrc.2008.02.046 |
| [23] | HEINBOCKEL L, PALACIOS-CHAVES L, ALEXANDER C, et al. Mechanism of Hbγ-35-induced an increase in the activation of the human immune system by endotoxins[J]. Innate Immunity, 2015 , 21 (3) : 305 –313. DOI: 10.1177/1753425914535957 |
| [24] | KACONIS Y, KOWALSKI I, HOWE J, et al. Biophysical mechanisms of endotoxin neutralization by cationic amphiphilic peptides[J]. Biophysical Journal, 2011 , 100 (11) : 2652 –2661. DOI: 10.1016/j.bpj.2011.04.041 |
| [25] | CHEN C Z, OU C Y, WANG R H, et al. The role of bactericidal/permeability-increasing protein in men with chronic obstructive pulmonary disease[J]. Journal of Chronic Obstructive Pulmonary Disease, 2012 , 9 (2) : 197 –202. DOI: 10.3109/15412555.2011.654143 |
| [26] | HILPERT K, ELLIOTT M R, VOLKMER-ENGERT R, et al. Sequence requirements and an optimization strategy for short antimicrobial peptides[J]. Chemistry & Biology, 2006 , 13 (10) : 1101 –1107. |
| [27] | NAN Y H, BANG J K, JACOB B, et al. Prokaryotic selectivity and LPS-neutralizing activity of short antimicrobial peptides designed from the human antimicrobial peptide LL-37[J]. Peptides, 2012 , 35 (2) : 239 –247. DOI: 10.1016/j.peptides.2012.04.004 |
| [28] | SINGH S, KASETTY G, SCHMIDTCHEN A, et al. Membrane and lipopolysaccharide interactions of C-terminal peptides from S1 peptidases[J]. Biochimica et Biophysica Acta:Biomembranes, 2012 , 1818 (9) : 2244 –2251. DOI: 10.1016/j.bbamem.2012.03.017 |
| [29] | ANDRÄ J, KOCH M H J, BARTELS R, et al. Biophysical characterization of endotoxin inactivation by NK-2,an antimicrobial peptide derived from mammalian NK-lysin[J]. Antimicrobial Agents and Chemotherapy, 2004 , 48 (5) : 1593 –1599. DOI: 10.1128/AAC.48.5.1593-1599.2004 |
| [30] | LEE E K, KIM Y C, NAN Y H, et al. Cell selectivity,mechanism of action and LPS-neutralizing activity of bovine myeloid antimicrobial peptide-18 (BMAP-18) and its analogs[J]. Peptides, 2011 , 32 (6) : 1123 –1130. DOI: 10.1016/j.peptides.2011.03.024 |
| [31] | MOOKHERJEE N, HANCOCK R E W. Cationic host defence peptides:innate immune regulatory peptides as a novel approach for treating infections[J]. Cellular and Molecular Life Sciences, 2007 , 64 (7/8) : 922 –933. |
| [32] | FANG L, XU Z, WANG G S, et al. Directed evolution of an LBP/CD14 inhibitory peptide and its anti-endotoxin activity[J]. , 2014 , 9 (7) : e101406 . |
| [33] | NAGAOKA I, HIROTA S, NIYONSABA F, et al. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-α by blocking the binding of LPS to CD14+ cells[J]. The Journal of Immunology, 2001 , 167 (6) : 3329 –3338. DOI: 10.4049/jimmunol.167.6.3329 |
| [34] | SUZUKI K, MURAKAMI T, KUWAHARA-ARAI K, et al. Human anti-microbial cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of endothelial cells[J]. International Immunology, 2011 , 23 (3) : 185 –193. DOI: 10.1093/intimm/dxq471 |
| [35] | BABOLEWSKA E, BRZEZINSKA-BŁASZCZYKE E. Human-derived cathelicidin LL-37 directly activates mast cells to proinflammatory mediator synthesis and migratory response[J]. Cellular Immunology, 2015 , 293 (2) : 67 –73. DOI: 10.1016/j.cellimm.2014.12.006 |
| [36] | LIU Y, NI B, REN J D, et al. Cyclic Limulus anti-lipopolysaccharide (LPS) factor-derived peptide CLP-19 antagonizes LPS function by blocking binding to LPS binding protein[J]. Biological and Pharmaceutical Bulletin, 2011 , 34 (11) : 1678 –1683. DOI: 10.1248/bpb.34.1678 |
| [37] | MONISHA G S.Comparison of structurally related peptides in their antimicrobial and anti-endotoxic activity[D].Master Thesis.Vancouver:University of British Columbia,1998. |
| [38] | HU Z S, MURAKAMI T, SUZUKI K, et al. Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism[J]. PLoS One, 2014 , 9 (1) : e85765 . DOI: 10.1371/journal.pone.0085765 |
| [39] | SWANGCHAN-UTHAI T, LAVENDER C R M, CHENG Z R, et al. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide[J]. Biology of Reproduction, 2012 , 87 (6) : 135 . DOI: 10.1095/biolreprod.112.102376 |
| [40] | KIM H, JANG J H, KIM S C, et al. Enhancement of the antimicrobial activity and selectivity of GNU7 against gram-negative bacteria by fusion with LPS-targeting peptide[J]. Peptides, 2016 , 82 : 60 –66. DOI: 10.1016/j.peptides.2016.05.010 |
| [41] | SCHMIDTCHEN A, MALMSTEN M. (Lipo)polysaccharide interactions of antimicrobial peptides[J]. Journal of Colloid and Interface Science, 2015 , 449 : 136 –142. DOI: 10.1016/j.jcis.2014.11.024 |
| [42] | LI H, WANG Y Y, ZHANG H Q, et al. Yohimbine enhances protection of berberine against LPS-induced mouse lethality through multiple mechanisms[J]. PLoS One, 2012 , 7 (12) : e52863 . DOI: 10.1371/journal.pone.0052863 |
| [43] | MICHALOPOULOS A, FALAGAS M E. Colistin and polymyxin B in critical care[J]. Critical Care Clinics, 2008 , 24 (2) : 377 –391. DOI: 10.1016/j.ccc.2007.12.003 |
| [44] | ALBADA H B, PROCHNOW P, BOBERSKY S, et al. Short antibacterial peptides with significantly reduced hemolytic activity can be identified by a systematic L-to-D exchange scan of their amino acid residues[J]. ACS Combinatorial Science, 2013 , 15 (11) : 585 –592. DOI: 10.1021/co400072q |




