断奶是猪生产周期中最大的应激源,包括将小猪从母猪中分离和仔猪饮食中去除母乳,这会导致母仔关联的断裂[1]。断奶仔猪还可能暴露于其他应激源,包括自然 (畜舍、运输) 和社会 (陌生的同伴) 环境的变化[2]。这种饲粮和环境的突然变化通常会导致仔猪消化功能紊乱,出现胃肠疾病和腹泻,使生长受阻[3-4],产生断奶应激。因此,许多功能性物质被作为控制断奶动物腹泻的生长促进剂应用于断奶饲粮中[5-6]。其中,壳聚糖作为天然线性杂多糖,是一种无毒的营养补充剂,且通常被认为是一种安全的化合物[7]。壳聚糖衍生自甲壳素,主要存在于甲壳类动物如虾或蟹的外骨骼及真菌的细胞壁中。壳聚糖是葡糖胺[β-(1, 4)-2-氨基-2-脱氧-D-葡萄糖]和N-乙酰葡糖胺 (2-乙酰氨基-2-脱氧-D-葡萄糖) 的共聚物[8]。近期的研究显示,壳聚糖具有抗肿瘤[9-10]、降低胆固醇[11]、增强免疫[12]、抗糖尿病[13]、促进伤口愈合[14]、抗真菌和抗微生物[15]等多种生物功能,并能改善幼龄动物的生长性能[16]等。本研究旨在探讨壳聚糖对断奶仔猪生长性能、粪便评分以及血清激素和T淋巴细胞亚群的影响,为壳聚糖在养猪业中推广应用提供科学依据。
1 材料与方法 1.1 试验材料壳聚糖:脱乙酰度为85.09%,黏度为45 cps,购于山东济南海德贝海洋生物工程有限公司。
1.2 试验设计选取60头健康无病的杜×大×长三元杂交断奶仔猪[初始体重为 (8.85±1.52) kg],随机分为5个组,每组12头猪,公母各占1/2。5组试验猪所喂试验饲粮分别是在基础饲粮中添加0(对照组)、250、500、1 000和2 000 mg/kg的壳聚糖配制而成。基础饲粮参照NRC (1998) 猪营养需求标准配制成全价粉料,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平 (风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
试验猪只于保育舍内网床 (2.0 m×2.2 m) 上饲养,各组猪舍环境条件及饲养管理均保持一致。仔猪于 (28±2) 日龄断奶,断奶后转入保育舍,饲喂7 d的过渡饲粮,而后开始正式试验,正式试验期间饲喂试验饲粮,自由采食,自由饮水。试验期为14 d。
1.4 样品采集及指标测定 1.4.1 生长性能试验开始和结束当天早晨,仔猪空腹称重,并记录每头仔猪每日的给料量和剩料量,计算平均日增重 (ADG)、平均日采食量 (ADFI) 和料重比 (F/G)。
1.4.2 粪便评分根据Hu等[17]介绍的5分法对仔猪粪便进行主观视觉评分,即于每天14:00观察仔猪粪便形态外观,评为1~5分 (粪便评分标准见表 2),并记录粪便评分。
|
|
表 2 粪便评分标准 Table 2 Standard of fecal score |
于试验结束当天清晨,每组随机挑选6头体况相近的仔猪,真空采血管于前腔静脉采血10 mL,静置20 min后,3 000 r/min离心15 min,分离血清置于-20 ℃冰箱冻存待测。按照试剂盒说明书进行血清生长激素释放激素 (GHRH)、生长抑制素 (SS)、生长激素 (GH)、类胰岛素生长因子-Ⅰ(IGF-Ⅰ)、瘦素 (LP)、促肾上腺皮质激素释放激素 (CRH)、促肾上腺皮质激素 (ACTH)、皮质醇 (COR) 以及T淋巴细胞亚群中可溶性CD3(sCD3)、可溶性CD4(sCD4)、可溶性CD8(sCD8) 浓度的测定。CRH和GHRH测定试剂盒购自南京博尔迪生物科技有限公司,其余指标测定试剂盒购自南京建成生物工程研究所。
1.5 数据统计分析试验数据经Excel 2007整理后,采用SAS 9.0统计软件进行回归统计分析,并进行Duncan氏法多重比较 (粪便评分除外)。粪便评分进行双因子互作方差分析,分析主效应 (时间和饲粮) 以及二者的互作效应。P < 0.05为差异显著。
2 结果与分析 2.1 壳聚糖对断奶仔猪生长性能的影响由表 3可知,随着壳聚糖添加量的增加,断奶仔猪的ADG呈现显著的线性和二次曲线增加效应 (P < 0.05),F/G则呈现显著的线性和二次曲线降低效应 (P < 0.05)。各壳聚糖添加组的ADG显著高于对照组 (P < 0.05),F/G显著低于对照组 (P < 0.05)。但饲粮中添加壳聚糖对断奶仔猪试验末重和ADFI均无显著影响 (P>0.05)。
|
|
表 3 壳聚糖对断奶仔猪生长性能的影响 Table 3 Effects of chitosan on growth performance of weaned piglets |
由图 1可知,在第2天,500和2 000 mg/kg壳聚糖添加组断奶仔猪粪便评分显著低于对照组 (P < 0.05);在第3天,250和2 000 mg/kg壳聚糖添加组断奶仔猪粪便评分显著低于对照组 (P < 0.05);在第5、10和12天,1 000 mg/kg壳聚糖添加组断奶仔猪粪便评分显著低于对照组 (P < 0.05);在第11天,各壳聚糖添加组断奶仔猪粪便评分均显著低于对照组 (P < 0.05)。在整个试验期内,饲粮和时间对断奶仔猪粪便评分均有显著影响 (P < 0.05),但二者对断奶仔猪的粪便评分没有显著的互作效应 (P>0.05)。
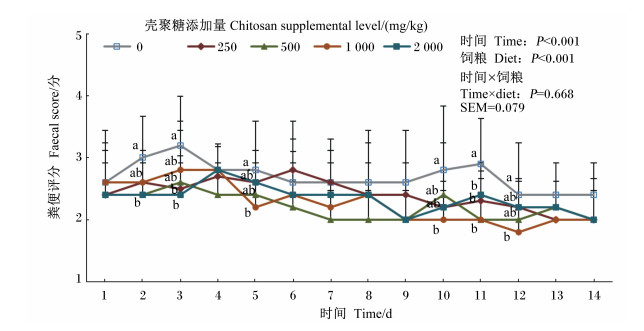
|
在同一抽样日数据点标注不同字母表示组间显著差异 (P < 0.05)。重叠数据点字母标注相同,未重复标出。 Data points in the same sampling day with different letters mean significant difference among groups (P < 0.05). Overlapping data points had the same letters, and were not labeled repeatedly. 图 1 壳聚糖对断奶仔猪粪便评分的影响 Figure 1 Effects of chitosan on fecal score of weaned piglets |
由表 4可知,随着壳聚糖添加量的增加,断奶仔猪的血清GHRH和GH浓度呈现显著的二次曲线增加效应 (P < 0.05),而血清LP浓度则呈现显著的线性增加效应 (P < 0.05)。各壳聚糖添加组血清GHRH浓度均显著高于对照组 (P < 0.05)。500和1 000 mg/kg壳聚糖添加组的血清GH浓度显著高于对照组 (P < 0.05),2 000 mg/kg壳聚糖添加组的血清LP浓度显著高于对照组 (P < 0.05)。
|
|
表 4 壳聚糖对断奶仔猪血清生长轴激素浓度的影响 Table 4 Effects of chitosan on serum growth axis hormone concentrations of weaned piglets |
由表 5可知,随着壳聚糖添加量的增加,断奶仔猪的血清CRH浓度呈现二次曲线降低趋势 (P=0.088),各壳聚糖添加组血清CRH浓度均显著低于对照组 (P < 0.05)。断奶仔猪的血清ACTH和COR浓度呈现显著的二次曲线降低效应 (P < 0.05)。500和1 000 mg/kg壳聚糖添加组的血清ACTH浓度显著低于对照组 (P < 0.05),各壳聚糖添加组的血清COR浓度均显著低于对照组 (P < 0.05)。
|
|
表 5 壳聚糖对断奶仔猪血清应激激素浓度的影响 Table 5 Effects of chitosan on serum stress hormone concentrations of weaned piglets |
由表 6可知,随着壳聚糖添加量的增加,断奶仔猪的血清sCD8浓度呈现显著的二次曲线降低效应 (P < 0.05),且500、1 000和2 000 mg/kg壳聚糖添加组的血清sCD8浓度显著低于对照组 (P < 0.05),血清中sCD3、sCD4浓度及sCD4/sCD8均未与壳聚糖添加量呈现显著的线性或二次曲线效应 (P>0.05)。
|
|
表 6 壳聚糖对断奶仔猪血清T淋巴细胞亚群的影响 Table 6 Effects of chitosan on T lymphocyte subset of weaned piglets |
动物受到环境、营养和免疫应激的影响时,其各种代谢过程受到负面影响,导致消化障碍、腹泻、生长性能降低和死亡率增加。特别是仔猪断奶期,仔猪的消化系统和免疫系统尚不完善,腹泻伴随着脱水甚至死亡是常见的。资料显示,壳聚糖作为一种天然碱性多糖,具有促进幼龄动物生长的特性。Liu等[18]报道,饲粮中补充0.01%或0.02%的壳聚糖对断奶仔猪的采食量、体增重和饲料效率均产生了积极影响。Zhou等[19]进行的一项评估补充壳聚糖对断奶猪生长性能、营养素消化率和腹泻发病率的影响的研究显示,0.20%壳聚糖可改善生长性能,增强干物质和氮总表观消化率,减少腹泻的发生率。本试验结果显示,饲粮中添加壳聚糖改善了断奶仔猪的生长性能,这可能是血清中GHRH和GH浓度的增加所致。Tang等[20]的研究结果证明壳聚糖可增加断奶仔猪血浆中GH和IGF-Ⅰ的浓度以及增加肝脏和肌肉中IGF-Ⅰ mRNA的丰度,推测膳食补充壳聚糖可能通过增加血浆中GH和IGF-Ⅰ的浓度来提高生长性能和饲料转化效率,这与本研究结果一致。另外,本试验还发现,饲粮添加2 000 mg/kg壳聚糖显著增加了断奶仔猪血清LP浓度。LP是由动物白色脂肪组织所分泌的一种蛋白质类激素,可参与动物的脂肪代谢调控[21]。大量研究指出,LP作用于脑信号中枢,可抑制进食量、增加能量消耗以及抑制脂肪合成,促进脂肪分解[22-23]。本研究中,饲粮添加2 000 mg/kg壳聚糖显著增加了断奶仔猪血清LP浓度,但并未对生长性能产生负面影响。这可能是因为断奶仔猪以骨骼发育和肌肉沉积为主,并不主要沉积脂肪,血清LP浓度的增加并不会大幅度影响生长性能。
断奶期仔猪肠道免疫学和行为变化最快的时期之一。在这段时间内,断奶仔猪经历肠道结构和功能 (酶活性和吸收或分泌) 的生理变化[3, 24-25]。这些改变影响小肠的消化、吸收和分泌能力,也会对肠屏障功能产生不利影响[24, 26-28]。当肠黏膜屏障被破坏时,肠道上皮通透性增加,毒素、细菌或饲料相关抗原穿过肠黏膜上皮,导致炎症、吸收不良、腹泻,并降低生长性能。其中,大肠杆菌被认为是导致仔猪断奶后腹泻的最重要原因之一。因此,大肠杆菌群落的减少可以降低断奶仔猪腹泻的发生率[29]。在本研究中,与对照组相比,壳聚糖添加组断奶仔猪的粪便评分降低。结合本课题组前期的研究[30],壳聚糖的补充减少了断奶仔猪肠道大肠杆菌的数量。Liu等[18]也发现,补饲0.01%或0.02%的壳聚糖可减少粪便中大肠杆菌的数量,增加乳杆菌的数量,降低腹泻的发生率。另外,壳聚糖可以结合某些类型的细菌,并且可能干扰它们对宿主动物的肠组织的黏附[18, 31],降低肠黏膜损伤;壳聚糖还可以延迟食糜通过肠道的速率,并且具有吸收水的能力[32],从而降低粪便评分,减少腹泻的发生。
仔猪断奶后,血清中CRH和COR浓度增加,表明断奶应激诱导CRH受体介导的肠功能障碍的通路的激活[33]。本试验结果显示,断奶仔猪饲粮中添加壳聚糖可以降低血清CRH、ACTH和COR浓度。CRH通过中枢的下丘脑-垂体-肾上腺轴或外周以CRH为基础的旁分泌系统的激活来调节胃肠道功能[34]。CRH、ACTH和COR等神经内分泌因子异常释放可以导致肠道细胞因子失衡,损害肠道屏障功能,增加肠道上皮的通透性[35-36],导致细菌和抗原等能够通过上皮屏障而进入体内,进而引起炎症和腹泻。Chen等[37]也发现壳聚糖降低免疫应激仔猪血液COR浓度。这表明壳聚糖能够缓解仔猪的断奶应激,并保护肠道屏障的完整性,减少腹泻。此外,CRH由下丘脑室旁核小细胞部神经元产生,于垂体前叶与CRH受体结合诱导ACTH释放,进而刺激肾上腺糖皮质激素释放[38],而糖皮质激素是调节炎症及免疫反应的重要物质,通过这些应激激素的作用,应激对免疫功能具有不利影响,包括降低自然杀伤 (NK) 细胞活性以及淋巴细胞亚群、淋巴细胞增殖等[39],导致机体的免疫力降低,增加疾病的易感性。在本试验中,饲粮添加壳聚糖降低了断奶仔猪血清中CRH、ACTH和COR浓度,反映了壳聚糖对仔猪断奶应激的缓解作用,并且可能对断奶仔猪的免疫功能有一定的正面影响。
细胞免疫应答在宿主对细胞内病原体的反应中通过抑制病原体复制和加速感染细胞的清除发挥重要作用。外周血中的sCD3、sCD4和sCD8是可溶形式的CD3+、CD4+和CD8+,其与T淋巴细胞的活化相关[40]。这些可溶形式的CD3+、CD4+和CD8+已被鉴定为T淋巴细胞活化和疾病或感染发生的重要标志物。在本研究中,饲粮中添加壳聚糖降低断奶仔猪血清sCD8浓度,这一结果表明壳聚糖可以调节T淋巴细胞的免疫功能。本课题组前期的研究表明壳聚糖对淋巴细胞功能具有抑制作用[41]。因此,免疫功能的抑制可能是壳聚糖提高生长性能的途径之一。这是因为免疫激活伴随着代谢活动的改变,导致营养物质重新分配,优先支持机体防御外来抗原[42]。此外,本研究以及本课题组前期的研究[43]表明,壳聚糖可以提高断奶仔猪的生长性能,这可能是壳聚糖改善免疫功能的反映。
总体而言,饲粮中添加不同剂量的壳聚糖均能够不同程度地促进断奶仔猪的生长,减少腹泻的发生,减缓应激,增强细胞免疫。但从试验数值上看,以添加500 mg/kg壳聚糖的作用效果最明显,随着添加量的继续增加,这种效果则不那么明显,原因可能是:壳聚糖作为一种难被哺乳动物消化酶消化的带正电荷的黏多糖,能够增加肠内容物黏度[11],加之其具有的阴离子交换性质,能够影响胆汁酸循环,增加脂肪排泄量。高剂量的壳聚糖影响营养物质,特别是脂肪及脂溶性维生素的消化吸收,引起机体内的营养再分配。所以,壳聚糖对断奶仔猪生长和免疫的调节作用呈现剂量效应。
4 结论① 饲粮中添加壳聚糖改善断奶仔猪的生长性能,增加断奶仔猪血清GHRH和GH浓度,高剂量壳聚糖 (2 000 mg/kg) 增加血清LP浓度。
② 饲粮中添加壳聚糖降低断奶仔猪的粪便评分,降低断奶仔猪血清CRH、ACTH、COR和sCD8浓度。
③ 总之,断奶仔猪饲粮中添加适宜剂量的壳聚糖能够促进断奶仔猪的生长,降低腹泻,缓解断奶应激。
| [1] | KELLEY K W. Stress and immune function:a bibliographic review[J]. Annals of Veterinary Research, 1980, 11(4): 445–478. |
| [2] | VAN DER MEULEN J, KOOPMANS S J, DEKKER R A, et al. Increasing weaning age of piglets from 4 to 7 weeks reduces stress, increases post-weaning feed intake but does not improve intestinal functionality[J]. Animal, 2010, 4(10): 1653–1661. DOI: 10.1017/S1751731110001011 |
| [3] | PLUSKE J R, HAMPSON D J, WILLIAMS I H. Factors influencing the structure and function of the small intestine in the weaned pig:a review[J]. Livestock Production Science, 1997, 51(1/2/3): 215–236. |
| [4] | MAO X F, PIAO X S, LAI C H, et al. Effects of β-glucan obtained from the Chinese herb Astragalus membranaceus and lipopolysaccharide challenge on performance, immunological, adrenal, and somatotropic responses of weanling pigs[J]. Journal of Animal Science, 2005, 83(12): 2775–2782. DOI: 10.2527/2005.83122775x |
| [5] | CORREA-MATOS N J, DONOVAN S M, ISAACSON R E, et al. Fermentable fiber reduces recovery time and improves intestinal function in piglets following Salmonella typhimurium infection[J]. The Journal of Nutrition, 2003, 133(6): 1845–1852. |
| [6] | WANG Y Z, SHAN T Z, XU Z R, et al. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs[J]. Animal Feed Science and Technology, 2007, 135(3/4): 263–272. |
| [7] | THANOU M, VERHOEF J C, JUNGINGER H E. Oral drug absorption enhancement by chitosan and its derivatives[J]. Advanced Drug Delivery Reviews, 2001, 52(2): 117–126. DOI: 10.1016/S0169-409X(01)00231-9 |
| [8] | BALDRICK P. The safety of chitosan as a pharmaceutical excipient[J]. Regulatory Toxicology and Pharmacology, 2010, 56(3): 290–299. DOI: 10.1016/j.yrtph.2009.09.015 |
| [9] | JEON Y J, KIM S K. Antitumor activity of chitosan oligosaccharides produced in ultrafiltration membrane reactor system[J]. Journal of Microbiology and Biotechnology, 2002, 12(3): 503–507. |
| [10] | QIN C Q, DU Y M, XIAO L, et al. Enzymic preparation of water-soluble chitosan and their antitumor activity[J]. International Journal of Biological Macromolecules, 2002, 31(1/2/3): 111–117. |
| [11] | GALLAHER C M, MUNION J, HESSLINK R, Jr, et al. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats[J]. The Journal of Nutrition, 2000, 130(11): 2753–2759. |
| [12] | YIN Y L, TANG Z R, SUN Z H, et al. Effect of galacto-mannan-oligosaccharides or chitosan supplementation on cytoimmunity and humoral immunity in early-weaned piglets[J]. Asian-Australasian Journal of Animal Sciences, 2008, 21(5): 723–731. DOI: 10.5713/ajas.2008.70408 |
| [13] | HAYASHI K, ITO M. Antidiabetic action of low molecular weight chitosan in genetically obese diabetic KK-Ay mice[J]. Biological & Pharmaceutical Bulletin, 2002, 25(2): 188–192. |
| [14] | PORPORATTO C, BIANCO I D, RIERA C M, et al. Chitosan induces different L-arginine metabolic pathways in resting and inflammatory macrophages[J]. Biochemical and Biophysical Research Communications, 2003, 304(2): 266–272. DOI: 10.1016/S0006-291X(03)00579-5 |
| [15] | QIN C Q, LI H R, XIAO Q, et al. Water-solubility of chitosan and its antimicrobial activity[J]. Carbohydrate Polymers, 2006, 63(3): 367–374. DOI: 10.1016/j.carbpol.2005.09.023 |
| [16] | SWIATKIEWICZ S, SWIATKIEWICZ M, ARCZEWSKA-WLOSEK A, et al. Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition[J]. Journal of Animal Physiology and Animal Nutrition, 2015, 99(1): 1–12. DOI: 10.1111/jpn.2014.99.issue-1 |
| [17] | HU C H, GU L Y, LUAN Z S, et al. Effects of montmorillonite-zinc oxide hybrid on performance, diarrhea, intestinal permeability and morphology of weanling pigs[J]. Animal Feed Science and Technology, 2012, 177(1/2): 108–115. |
| [18] | LIU P, PIAO X S, KIM S W, et al. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs[J]. Journal of Animal Science, 2008, 86(10): 2609–2618. DOI: 10.2527/jas.2007-0668 |
| [19] | ZHOU T X, CHO J H, KIM I H. Effects of supplementation of chito-oligosaccharide on the growth performance, nutrient digestibility, blood characteristics and appearance of diarrhea in weanling pigs[J]. Livestock Science, 2012, 144(3): 263–268. DOI: 10.1016/j.livsci.2011.12.009 |
| [20] | TANG Z R, YIN Y L, NYACHOTI C M, et al. Effect of dietary supplementation of chitosan and galacto-mannan-oligosaccharide on serum parameters and the insulin-like growth factor-Ⅰ mRNA expression in early-weaned piglets[J]. Domestic Animal Endocrinology, 2005, 28(4): 430–441. DOI: 10.1016/j.domaniend.2005.02.003 |
| [21] | 曾俊, 杨刚毅. 脂肪细胞因子与胰岛素抵抗的关系及其机制研究新进展[J]. 成都医学院学报, 2011, 6(1) :78–82. |
| [22] | TRAYHURN P. Hypoxia and adipose tissue function and dysfunction in obesity[J]. Physiological Reviews, 2013, 93(1): 1–21. DOI: 10.1152/physrev.00017.2012 |
| [23] | GE J F, QI C C, ZHOU J N. Imbalance of leptin pathway and hypothalamus synaptic plasticity markers are associated with stress-induced depression in rats[J]. Behavioural Brain Research, 2013, 249: 38–43. DOI: 10.1016/j.bbr.2013.04.020 |
| [24] | BOUDRY G, PÉRON V, LE HUËROU-LURON I, et al. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine[J]. The Journal of Nutrition, 2004, 134(9): 2256–2262. |
| [25] | LACKEYRAM D, YANG C B, ARCHBOLD T, et al. Early weaning reduces small intestinal alkaline phosphatase expression in pigs[J]. The Journal of Nutrition, 2010, 140(3): 461–468. DOI: 10.3945/jn.109.117267 |
| [26] | MOESER A J, RYAN K A, NIGHOT P K, et al. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2007, 293(2): G413–G421. DOI: 10.1152/ajpgi.00304.2006 |
| [27] | SPREEUWENBERG M A M, VERDONK J M A J, GASKINS H R, et al. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning[J]. The Journal of Nutrition, 2001, 131(5): 1520–1527. |
| [28] | SMITH F, CLARK J E, OVERMAN B L, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2010, 298(3): G352–G363. DOI: 10.1152/ajpgi.00081.2009 |
| [29] | FAIRBROTHER J M, NADEAU É, GYLES C L. Escherichia coli in postweaning diarrhea in pigs:an update on bacterial types, pathogenesis, and prevention strategies[J]. Animal Health Research Reviews, 2005, 6(1): 17–39. DOI: 10.1079/AHR2005105 |
| [30] | 徐元庆, 史彬林, 李俊良, 等. 壳聚糖对断奶仔猪肠道菌群的影响[J]. 饲料研究, 2012(10) :54–56. DOI: 10.3969/j.issn.1002-2813.2012.10.020 |
| [31] | OFEK I, HASTY D L, SHARON N. Anti-adhesion therapy of bacterial diseases:prospects and problems[J]. FEMS Immunology & Medical Microbiology, 2003, 38(3): 181–191. |
| [32] | WALSH A M, SWEENEY T, BAHAR B, et al. The effect of chitooligosaccharide supplementation on intestinal morphology, selected microbial populations, volatile fatty acid concentrations and immune gene expression in the weaned pig[J]. Animal, 2012, 6(10): 1620–1626. DOI: 10.1017/S1751731112000481 |
| [33] | MOESER A J, KLOK C V, RYAN K A, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2007, 292(1): G173–G181. |
| [34] | PASCHOS K A, KOLIOS G, CHATZAKI E. The corticotropin-releasing factor system in inflammatory bowel disease:prospects for new therapeutic approaches[J]. Drug Discovery Today, 2009, 14(13/14): 713–720. |
| [35] | SANTOS J, ALONSO C, VICARIO M, et al. Neuropharmacology of stress-induced mucosal inflammation:implications for inflammatory bowel disease and irritable bowel syndrome[J]. Current Molecular Medicine, 2008, 8(4): 258–273. DOI: 10.2174/156652408784533788 |
| [36] | GAREAU M G, SILVA M A, PERDUE M H. Pathophysiological mechanisms of stress-induced intestina damage[J]. Current Molecular Medicine, 2008, 8(4): 274–281. DOI: 10.2174/156652408784533760 |
| [37] | CHEN Y J, KIM I H, CHO J H, et al. Effects of chitooligosaccharide supplementation on growth performance, nutrient digestibility, blood characteristics and immune responses after lipopolysaccharide challenge in weanling pigs[J]. Livestock Science, 2009, 124(1/2/3): 255–260. |
| [38] | LIGHTMAN S L. The neuroendocrinology of stress:a never ending story[J]. Journal of Neuroendocrinology, 2008, 20(6): 880–884. DOI: 10.1111/jne.2008.20.issue-6 |
| [39] | MARKETON J I W, GLASER R. Stress hormones and immune function[J]. Cellular Immunology, 2008, 252(1/2): 16–26. |
| [40] | UEHARA S, GOTHOH K, HANDA H, et al. Immune function in patients with acute pancreatitis[J]. Journal of Gastroenterology and Hepatology, 2003, 18(4): 363–370. DOI: 10.1046/j.1440-1746.2003.02979.x |
| [41] | LI J L, SHI B L, YAN S M, et al. Effects of dietary supplementation of chitosan on humoral and cellular immune function in weaned piglets[J]. Animal Feed Science and Technology, 2013, 186(3/4): 204–208. |
| [42] | SPURLOCK M E. Regulation of metabolism and growth during immune challenge:an overview of cytokine function[J]. Journal of Animal Science, 1997, 75(7): 1773–1783. DOI: 10.2527/1997.7571773x |
| [43] | XU Y Q, SHI B L, YAN S M, et al. Effects of chitosan supplementation on the growth performance, nutrient digestibility, and digestive enzyme activity in weaned pigs[J]. Czech Journal of Animal Science, 2014, 59(4): 156–163. |




