动物体的微生物种类包括细菌(数量最多)、古菌、真菌、病毒等,其数量多达1013~1014个,是动物体本身细胞总数的10倍之多,其中大部分定植在胃肠道中[1]。单胃动物肠道菌群的分布具有空间特异性,其数量级从近端至远端消化道呈依次递增趋势,以结肠中的细菌数量最多(为1011~1012个)[2]。在肠道内,从上皮细胞到肠腔内,细菌的数量和种类依次递增,且肠腔内的菌群与附着在黏液层以及上皮隐窝中的菌群种类明显不同[3]。这些共生微生物在促进肠道相关淋巴组织(gut-associated lymphoid tissues, GALTs)的发育和抵抗病原体入侵等方面发挥着重要作用[4]。动物肠道菌群的结构和分布可受遗传[5]、饲粮[6]、环境[7]、抗生素的使用[8]等因素影响,其直接结果是使菌群结构发生相应改变。微生物群落间的动态平衡受到干扰,必然导致宏基因组功能的变化。动物的肠道不仅是营养物质消化吸收的场所,同时也是机体的第1道防线,是重要的免疫器官之一。而宿主肠道黏膜中存在许多与免疫功能相关的受体或信号分子,细菌作为免疫识别最为重要的对象之一,菌群结构的变化势必引起宿主肠道免疫系统的快速响应,这种免疫应答反应又反过来调控肠道菌群的组成和分布[9]。因此肠道菌群与机体免疫之间可能存在复杂的相互作用,但目前大多研究仅停留在表面,对具体哪些特定细菌影响何种免疫细胞的增殖分化及具体机制知之甚少。为此, 本文综合国内外最新相关研究结果,系统综述了单胃动物肠道菌群与宿主肠道免疫功能之间的相互作用及其可能的互作机制。
1 肠道菌群对宿主肠道免疫系统的调控单胃动物的肠道免疫系统由三大屏障组成,即由肠黏膜上皮细胞和杯状细胞等构成的机械屏障、由肠道免疫细胞及其分泌的免疫因子构成的免疫屏障以及由肠道正常菌群构成的生物屏障[10]。越来越多的研究表明, 肠道菌群能调控各种免疫细胞的分化和功能。肠道共生菌的存在对肠道和组织淋巴结构发育有至关重要的作用。
1.1 介导GALTs的发育GALTs包括集合淋巴小结(Peyer’s patches, PP)、孤立淋巴滤泡(isolated lymphoid follicles, ILF)和肠系膜淋巴结(mesenteric lymph nodes, MLN)[11]。研究表明,无菌小鼠GALTs的发育存在明显缺陷,尤其是PP和ILF[12]。在胎儿时期,淋巴组织诱导细胞(lymphoid tissue inducer, LTi)能在无菌条件下诱导PP的发育[13],而ILFs的发育则需要微生物的介导[14]。对无菌兔(GF-APX)的研究发现,将含1×109 CFU的6种细菌[枯草芽孢杆菌(B. subtilis)、地衣芽孢杆菌(B. licheniformis)、短小芽孢杆菌(B. pumilus)、脆弱拟杆菌(B. fragilis)、表皮葡萄球菌(S. epidermidis)、近端梭菌(C. subterminale)]混合悬浮液注入GF-APX肠腔内能通过诱导B细胞的增殖促进GALTs的发育。为进一步确定具体是哪一种细菌能诱导GALTs发育,作者将它们单独或两两接种至GF-APX肠道内,发现除了单独或两两接种枯草芽孢杆菌与脆弱拟杆菌能诱导GALTs发育外,其他4种菌单独或两两接种均不能诱导GALTs发育,暗示单一的某种细菌也许不能完全诱导GALTs的发育,保证肠道内细菌的种类和多样性对肠道免疫系统的充分发育来说是必需的[15]。
1.2 介导肠道辅助性T(helper T, Th)细胞的增殖分化Th17细胞是一种特殊的CD4+ Th细胞,对宿主的防御至关重要。Th17细胞通过产生促炎性细胞因子白细胞介素-17A(IL-17A)、白细胞介素-17F(IL-17F)和白细胞介素-22(IL-22) 对自身免疫性疾病的发展起作用[16]。研究表明,Th17细胞的数量在经过抗生素治疗的动物或无菌动物的结肠中大大减少[17-18],说明微生物对Th17细胞发育有重要作用。随后Gaboriau等[19]发现肠道中存在一种共生梭状芽胞杆菌,即分节丝状菌(segmented filamentous bacteria, SFB),其对Th17细胞的发育至关重要;同时,还发现缺乏SFB的C57BL/6成年小鼠的小肠固有层中Th17细胞数量较低,但植入SFB 2周后Th17细胞数量显著增加,表明SFB能诱导小鼠小肠中Th17细胞的生成。此外,在无菌小鼠肠道中接种ASF(altered Schaedler flora)——含8种细菌的一种特定的菌群后,其结肠固有层中Th17细胞的数量也显著提高,但其调控效果弱于SFB[20]。其他研究发现,对3~4周龄的无菌小鼠灌胃200 μL成人粪便样品混悬液后同样能诱导Th17细胞的增殖[21],由于成人肠道类无SFB定植[22],暗示动物肠道中可能存在大量其他种类的共生菌, 这些共生菌能特异性诱导Th17细胞的增殖分化。
1.3 介导调节性T(regulatory T, Treg)细胞的增殖分化叉头/翼状螺旋转录因子3+(forkhead box P3+, FOXP3+)Treg细胞也是CD4+ Th细胞的亚群,在维持肠道功能稳态中发挥重要作用。研究发现,在抗生素处理或无菌小鼠的肠道中虽然仍能检测到Treg细胞,但其数量在小肠固有层中明显下降,表明微生物有维持Treg细胞数量或促进Treg细胞分化的作用[23]。目前已有几种共生菌被证明具有诱导Treg细胞增殖分化的活性。对无菌小鼠灌胃46株梭杆菌属细菌的混悬液可导致结肠固有层Treg细胞数量显著增加,经分离鉴定,这些细菌分属于梭状芽胞杆菌的2个类簇——梭状芽胞杆菌Ⅳ簇和ⅪⅤa簇[23]。此外,接种ASF也可引起无菌小鼠结肠固有层Treg细胞的增殖,值得一提的是ASF中包含3株属于梭状芽胞杆菌ⅪⅤa簇的细菌[20]。Atarashi等[24]进一步分离鉴定出人类肠道中的17株细菌(分属于梭状芽胞杆菌的Ⅳ簇、ⅪⅤa簇和ⅩⅧ簇)也能诱导肠道中Treg细胞的生成。此外,人类肠道中的共生菌脆弱拟杆菌也能促进小鼠结肠中Treg细胞的增殖和细胞因子白细胞介素-10(IL-10) 的产生[25]。
1.4 介导肠黏膜B细胞的增殖分化早期B细胞的发育不仅发生在胎儿的肝脏和骨髓上,还发生在肠黏膜上[26]。微生物和肠道特异性B细胞之间存在密切的关系,B细胞通过产生免疫球蛋白A(IgA)来防止微生物感染[27],反之微生物能诱导肠道B细胞的胞外信号调节受体[26]。无菌小鼠体内因缺乏微生物介导的信号,导致PP的生发中心发育不成熟,从而降低B细胞的生成数量[28]。因此,在消化道内,B细胞是通过共生菌群的刺激在PP中成熟的,但其具体机制尚属未知。此外,小鼠结肠固有层的树突状细胞(dendritic cells, DCs)中,共生菌的鞭毛蛋白能通过促进维甲酸的合成来诱导不同B细胞的分化[29]。
1.5 介导先天淋巴细胞(innate lymphoid cells, ILCs)的增殖分化ILCs作为一种固有免疫细胞越来越受到关注,它的功能特性与T细胞相似[30]。淋巴前体可分化成3个ILCs亚型:T转录因子ILCs(T-bet+ ILCs, ILC1s)、GATA结合蛋白-3 ILCs[GATA-binding protein 3 (GATA3+) ILCs, ILC2s]、维甲酸相关孤核受体γT ILCs[retinoicacid receptor-relaedorphan receptor-γt (RORγt+) ILCs, ILC3s][31]。微生物对ILCs的发展和功能作用一直饱受争议。研究发现,无菌小鼠体内RORγt+NKp46+CD127+NK1.1-ILCs或RORγt+NKp46+CD127+NK1.1int ILCs的数量显著降低,说明微生物对ILC3s的分化是必需的[32]。在缺乏肠道菌群时,由ILCs分泌的IL-22的含量显著降低,表明共生菌还能调控ILCs的免疫功能[33]。相反,有研究发现肠道菌群对ILC3s的分化并无显著影响,且会抑制IL-22的分泌[34]。
2 肠道菌群调控宿主肠道免疫系统的可能机制 2.1 调控GALTs发育肠道菌群刺激GALTs发育主要是由DCs对细菌及其代谢产物的识别而实现的,细菌通过激活DCs上的各种模式识别受体(pattern recognition receptors, PRRs)来诱发这一过程[35]。常见的PRRs包括Toll样受体家族(Toll-like receptors, TLRs)、NOD样受体家族(nucleotide-binding oligomerizationdomain-like receptor, NLRs)等。TLRs识别细菌或其代谢产物后,进一步激活髓样分化蛋白88(MYD88) 接头蛋白样蛋白(MAL)-MYD88和含Toll/白细胞介素-1受体(TLR)结构域能诱导β型干扰素(IFN-β)的接头分子(TRIF)相关接头分子(TRAM)-TRIF信号通路,激活DCs[36]。活化后的DCs能诱导PP生发中心的T细胞增殖,促进B细胞分泌IgA;通过淋巴血管到达MLN,诱导效应T细胞增殖,使隐窝小结发育为成熟的ILFs。Bouskra等[12]研究发现ILFs还可通过NOD1(nucleotide-binding oligomerization domain 1) 与细菌细胞壁上的聚肽糖相结合后而诱发其发育成熟。
2.2 调控Th17细胞增殖分化越来越多的研究证明肠道菌群是通过LP的单核吞噬细胞包括DCs和巨噬细胞,来促进肠道Th17细胞发育的。上文提到,SFB能特异性的诱导Th17细胞的分化,其具体的机制是,SFB通过刺激肠道上皮细胞分泌血清淀粉样蛋白A(serum amyloid A, SAA),SAA能促进回肠固有层CD11c+ DCs分泌细胞因子白细胞介素-6(IL-6) 和白细胞介素-23(IL-23),IL-6和IL-23是诱导Th17细胞分化的关键因素[37]。与Th17细胞分化有关的另一个关键细胞因子是白细胞介素-1β(IL-1β),Shaw等[38]研究发现,无菌小鼠肠道中LP巨噬细胞分泌IL-1β减少,且敲除了IL-1β受体的小鼠肠道中Th17细胞数量显著减少,表明共生菌还可能通过诱导IL-1β的产生来促进肠道Th17细胞的发育;进一步研究得出,MyD88敲除小鼠肠道中IL-1β和Th17细胞的含量都显著降低[38],推测共生菌是通过TLR-MyD88信号通路诱导肠道LP巨噬细胞产生IL-1β,进而促进Th17细胞分化的。
2.3 调控Treg细胞增殖分化由于相关研究极少,目前尚不清楚特定肠道菌群诱导Treg细胞发育的机制。有限的研究表明,β型转化生长因子(transfoming growth factor β, TGF-β)参与了肠道菌群对Treg细胞的诱导分化。梭状芽孢杆菌Ⅳ簇和ⅪⅤa簇能通过刺激结肠上皮细胞产生TGF-β,诱导Treg细胞的分化[23]。除上皮细胞外,肠道LP DCs的某些亚群也能参与Treg细胞的诱导分化,如CD103+CD11b+CD11c+ LP DCs和CD103+CD11b-CD11c+ LP DCs能优先诱导CD4+T细胞分化为Treg细胞[39]。CD103+ LP DCs能表达驱动Treg细胞分化的相关因子,如TGF-β和视黄酸脱氢酶(retinoic acid dehydrogenase, RALDH),视黄酸(retinoic acid, RA)也能有效诱导Treg细胞的分化[40]。此外,在TGF-β存在时,LP CD11b+CD11c-巨噬细胞能通过产生RA来诱导肠道Treg细胞的分化[41]。脆弱拟杆菌产生的多糖A(polysaccharide A, PSA)也能通过激活TLR2-MyD88信号通路来刺激Treg细胞的分化及IL-10的分泌[42]。
2.4 调控B细胞增殖分化肠道共生菌可通过不同途径来调控B细胞的分化。研究发现,MyD88缺失的小鼠肠道中CD11b+IgA+B细胞数量显著下降[43],说明共生菌能通过激活LP DCs或滤泡DCs上的MyD88信号,促进IgA+B细胞的产生。在受到细菌刺激后,PPs中滤泡DCs通过分泌TGF-β、趋化因子CXCL13、B细胞活化因子(B-cell activating factor, BAFF)来促进B细胞的分化和IgA+的生成[44];LP DCs能通过分泌TGF-β、RA、肿瘤坏死因子α(tumor necrosis factor α, TNF-α)、BAFF、诱生型一氧化氮合酶(inducible nitric oxidesynthase, iNOS)和增殖诱导配体(a proliferation-inducing ligand, APRIL)来促进IgA+B细胞的生成[45]。
2.5 调控ILCs功能上文提到肠道菌群对ILCs的发育和功能同时存在正面与负面效应,一方面,共生菌能促进RORγt+ ILCs分泌细胞因子IL-22产生相应的免疫功能[46];另一方面,共生菌能诱导肠道上皮细胞分泌白细胞介素-25(IL-25),IL-25作用于固有层IL-17RB+ DCs来抑制ILC3s分泌IL-22[47]。但其中的具体机制目前尚不清楚。
除上述的直接调控(图 1)外,肠道菌群的多种代谢产物均能对宿主肠道免疫系统进行间接调控,如梭状芽胞杆菌Ⅳ簇和ⅪⅤa簇的代谢产物丁酸能诱导CD4+T细胞分化为Treg细胞[48];双歧杆菌的代谢产物维生素D能促进Th细胞分化[49]等。
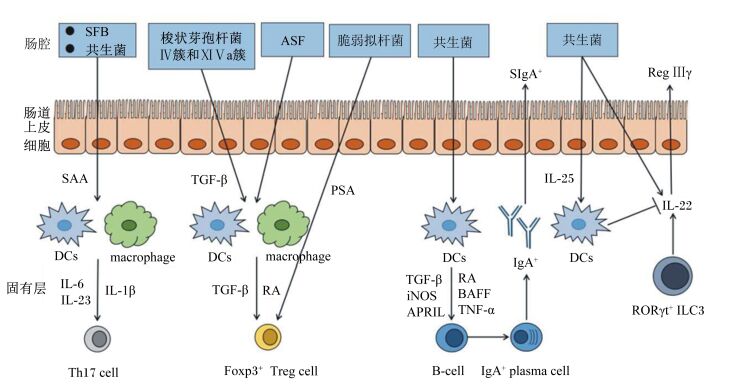
|
SFB:分节丝状菌segmented filamentous bacteria;ASF:含8种细菌的一种特定的菌群altered Schaedler flora;IgA+:免疫球蛋白A+ immunoglobulin A+;SAA:血清淀粉样蛋白A serum amyloid A;TGF-β:β型转化生长因子transfoming growth factor β;PSA:多糖A polysaccharide A;DCs:树突状细胞dendritic cells;Th17 cell:辅助性T细胞17 T helper 17 cell;Treg:调节性T细胞regulatory T cell;RA:视黄酸retinoic acid;BAFF:B细胞活化因子B-cell activating factor;Foxp3+:叉头/翼状螺旋转录因子3+ forkhead/winged helix transcription factor 3+;TNF-α:肿瘤坏死因子α tumor necrosis f-actor α;APRIL:增殖诱导配体a proliferation-inducing ligand;iNOS:诱生型一氧化氮合酶inducible nitric oxidesynthase;SIgA+:分泌型免疫球蛋白A+ secretory immunoglobulin A+;plasma cell:浆细胞;macrophage:巨噬细胞;RORγt+:维甲酸相关孤核受体γt retinoid related orphan receptor γt;IL:白细胞介素interleukin;Reg Ⅲγ:胰岛再生源蛋白3γ regenerating islet-derived protein Ⅲγ。 图 1 肠道微生物介导的肠道免疫系统发育(根据文献[37-49]总结) Figure 1 The gut microbiota-mediated development of the intestinal immune system (summarized according to references[37-49]) |
如前所述,共生菌群对宿主肠道免疫系统的发育有重要作用,但微生物的过度刺激也可能导致肠道中免疫细胞不适当的激活和肠内炎症的发生。肠道黏膜屏障由肠黏膜表面的黏液层、肠上皮本身及其紧密连接、黏膜下固有层等组成,其作为物理屏障是抵御肠腔微生物的第1道防线,能减少微生物与小肠上皮的直接接触[50],有效阻止细菌穿透黏膜。此外,胰岛再生源蛋白Ⅲγ(regenerating islet-derived protein Ⅲγ, RegⅢγ)可作为限制细菌渗透进肠黏膜的另一道屏障[51],ILC3s能分泌细胞因子IL-22,IL-22作用于上皮细胞,激活p38-丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)或信号传导子及转录激活子3(signal transducer and activator of transcrip-tion3, STAT3) 信号通路促进RegⅢγ的生成[52]。Zheng等[53]研究发现IL-22能够通过诱导小鼠小肠上皮细胞中RegⅢγ的表达来抑制鼠类柠檬酸杆菌(Citrobacter rodentium)感染结肠,ILC3s数量减少则会增加共生菌木糖氧化产碱菌(Alcaligenes xylosoxidians)进入肠腔的机会,从而导致宿主肠道损伤和全身炎症[46]。肠黏膜表面的分泌型IgA(secretory immunoglobulin A, SIgA)也能与共生菌[阴沟肠杆菌(E. cloacae)或野生型大肠杆菌(wild-type E. coli)]特异性结合,防止其穿透上皮屏障[54]。就此而言,宿主肠道免疫系统对共生菌有一种制约作用,在正常情况下限制共生菌进入肠道上皮细胞,避免微生物对肠道的过度刺激,维持肠道微生物与宿主正常的共生关系。
3.2 影响共生菌群的组成和功能免疫因子IgA能通过对肠道菌群组成和功能的调控来维持宿主和微生物之间的共生关系。研究发现,小鼠体内活化诱导胞嘧啶核苷脱氨酶(activation-induced cytidine deaminase, AICD)的缺失或突变可导致肠道IgA的应答缺陷,使细菌数量扩增(尤其是厌氧菌),从而引起肠道菌群的组成发生改变[55]。抑制性协同受体程序性死亡蛋白-1(inhibitory co-receptor programmed death-1) 的缺失能降低了IgA与细菌的结合能力,导致小鼠肠道菌群结构的改变,与野生型小鼠相比其共生菌的数量如双歧杆菌属(Bifidobacterium)和多型拟杆菌属(Bacteroides)等的细菌数量无明显变化,而肠杆菌科(Enterobacteriaceae)的细菌数量则增加近400倍[56],暗示这种菌群结构的改变可促进某些条件性病原菌的增殖,使其从丰度较低的常驻菌转变为对宿主有害致病菌。除了改变细菌群落结构外,一项在小鼠上的试验发现,IgA与共生菌多形拟杆菌(Bacteroides thetaiotaomicron)结合后能影响其基因表达,导致编码亚硝酸盐还原酶的操纵子(BT1414~1418)、参与一氧化氮代谢的基因(BT0687) 和编码细胞色素D泛醇氧化酶亚基的操纵子(与细菌的耐氧性有关)表达量显著上升[57]。ILC3s的功能缺陷时,IL-22分泌量减少,导致SFB异常扩增,使肠道Th17细胞免疫应答增加[58]。
此外,某些免疫基因的缺失也可能影响肠道菌群结构,如转录因子T-bet(由Tbx21基因编码)能够调控参与先天性和适应性免疫反应的细胞的炎症应答,小鼠缺失Tbx21基因可导致肠道内潜在致病菌克雷白氏杆菌(Klebsiella pneumoniae)、奇异变形杆菌(Proteus mirabilis)和幽门螺旋杆菌(Helicobacter typhlonius)累积,易患溃疡性结肠炎[59]。上皮细胞NLRP6炎性体缺陷小鼠的炎性因子白细胞介素-18(IL-18) 产量减少,菌群结构发生改变,普雷沃氏菌科(Prevotellaceae)细菌的数量大幅增加[60]。
上述研究均说明了肠道免疫系统能通过各种途径调节共生菌在宿主肠道中的分布、组成和功能,但目前只对少数免疫细胞和因子与共生菌的关系做了初步研究,更多的免疫细胞因子对宿主肠道微生物的影响及其具体机制还有待进一步探索。
4 小结大量研究初步揭示了单胃动物肠道菌群与宿主肠道免疫系统之间的复杂互作关系,这种互作机制保障了肠道内环境的稳定。然而,研究尚需深入,很多问题仍不清楚,如消化道中的其他共生微生物(如真菌、病毒)是否与肠道免疫系统间也存在互作?具体机制是什么?与细菌-宿主免疫系统互作有无异同?肠道微生物与宿主免疫系统之间的互作是否也遵循“肠-脑-肠”轴这一经典规则?从营养学角度出发,探究能否通过营养调控手段来改善肠道菌群结构,甚至靶向调控某一类或几类特殊菌群,达到提高动物机体免疫力的效果,将是动物营养学研究的全新领域,相关研究结果将为丰富动物抗病营养学理论提供基础数据。
| [1] | SEKIROV I, RUSSELL S L, ANTUNES L C M, et al. Gut microbiota in health and disease[J]. Physiological Reviews, 2010, 90(3): 859–904. DOI: 10.1152/physrev.00045.2009 |
| [2] | O'HARA A M, SHANAHAN F. The gut flora as a forgotten organ[J]. EMBO Reports, 2006, 7(7): 688–693. DOI: 10.1038/sj.embor.7400731 |
| [3] | SWIDSINSKI A, LOENING-BAUCKE V, LOCHS H, et al. Spatial organization of bacterial flora in normal and inflamed intestine:a fluorescence in situ hybridization study in mice[J]. World Journal of Gastroenterology, 2005, 11(8): 1131–1140. DOI: 10.3748/wjg.v11.i8.1131 |
| [4] | KAU A L, AHERN P P, GRIFFIN N W, et al. Human nutrition, the gut microbiome and the immune system[J]. Nature, 2011, 474(7351): 327–336. DOI: 10.1038/nature10213 |
| [5] | DOMINGUEZBELLO M G, COSTELLO E K, CONTRERAS M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(26): 11971–11975. DOI: 10.1073/pnas.1002601107 |
| [6] | SCOTT K P, GRATZ S W, SHERIDAN P O, et al. The influence of diet on the gut microbiota[J]. Pharmacological Research, 2013, 69(1): 52–60. DOI: 10.1016/j.phrs.2012.10.020 |
| [7] | BENSON A K, KELLY S A, LEGGE R, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(44): 18933–18938. DOI: 10.1073/pnas.1007028107 |
| [8] | JERNBERG C, LÖFMARK S, EDLUND C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota[J]. Microbiology, 2010, 156(11): 3216–3223. DOI: 10.1099/mic.0.040618-0 |
| [9] | CLARKE G, STILLING R M, KENNEDY P J, et al. Minireview:gut microbiota:the neglected endocrine organ[J]. Molecular Endocrinology, 2014, 28(8): 1221–1238. DOI: 10.1210/me.2014-1108 |
| [10] | MAGRONE T, JIRILLO E. The interplay between the gut immune system and microbiota in health and disease:nutraceutical intervention for restoring intestinal homeostasis[J]. Current Pharmaceutical Design, 2013, 19(7): 1329–42. |
| [11] | KOBOZIEV I, KARLSSON F, GRISHAM M B. Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation[J]. Annals of the New York Academy of Sciences, 2010, 1207(Suppl.3): E86–E93. |
| [12] | BOUSKRA D, BRÉZILLON C, BÉRARD M, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis[J]. Nature, 2008, 456(7221): 507–510. DOI: 10.1038/nature07450 |
| [13] | MOREAU M C, CORTHIER G. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3H/HeJ mice[J]. Infection & Immunity, 1988, 56(10): 2766–2768. |
| [14] | PABST O, HERBRAND H, FRIEDRICHSEN M, et al. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling[J]. Journal of Immunology, 2006, 177(10): 6824–6832. DOI: 10.4049/jimmunol.177.10.6824 |
| [15] | RHEE K J, SETHUPATHI P, DRIKS A, et al. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire[J]. The Journal of Immunology, 2004, 172(2): 1118–1124. DOI: 10.4049/jimmunol.172.2.1118 |
| [16] | LITTMAN D R, RUDENSKY A Y. Th17 and regulatory T cells in mediating and restraining inflammation[J]. Cell, 2010, 140(6): 845–858. DOI: 10.1016/j.cell.2010.02.021 |
| [17] | IVANOV Ⅱ, DE LLANOS FRUTOS R, MANEL N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine[J]. Cell Host & Microbe, 2008, 4(4): 337–349. |
| [18] | ATARASHI K, NISHIMURA J, SHIMA T, et al. ATP drives lamina propria TH17 cell differentiation[J]. Nature, 2008, 455(7214): 808–812. DOI: 10.1038/nature07240 |
| [19] | GABORIAU-ROUTHIAU V, RAKOTOBE S, LÉCUYER E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses[J]. Immunity, 2009, 31(4): 677–689. DOI: 10.1016/j.immuni.2009.08.020 |
| [20] | GEUKING M B, CAHENZLI J, LAWSON M A, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses[J]. Immunity, 2011, 34(5): 794–806. DOI: 10.1016/j.immuni.2011.03.021 |
| [21] | CHUNG H, PAMP S J, HILL J A, et al. Gut immune maturation depends on colonization with a host-specific microbiota[J]. Cell, 2012, 149(7): 1578–1593. DOI: 10.1016/j.cell.2012.04.037 |
| [22] | 王宇. 健康人群肠道分节丝状菌(SFB)的调查以及雌马酚转化的研究[D]. 硕士学位论文. 杭州: 浙江师范大学, 2013. |
| [23] | ATARASHI K, TANOUE T, SHIMA T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species[J]. Science, 2011, 331(6015): 337–341. DOI: 10.1126/science.1198469 |
| [24] | ATARASHI K, TANOUE T, OSHIMA K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota[J]. Nature, 2013, 500(7461): 232–236. DOI: 10.1038/nature12331 |
| [25] | ROUND J L, MAZMANIAN S K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(27): 12204–12209. DOI: 10.1073/pnas.0909122107 |
| [26] | WESEMANN D R, PORTUGUESE A J, MEYERS R M, et al. Microbial colonization influences early B-lineage development in the gut lamina propria[J]. Nature, 2013, 501(7465): 112–115. DOI: 10.1038/nature12496 |
| [27] | MACPHERSON A J, GEUKING M B, MCCORY K D. Homeland security:IgA immunity at the frontiers of the body[J]. Trends in Immunology, 2012, 33(4): 160–167. DOI: 10.1016/j.it.2012.02.002 |
| [28] | FAGARASAN S, KAWAMOTO S, KANAGAWA O, et al. Adaptive immune regulation in the gut:T cell-dependent and T cell-independent IgA synthesis[J]. Annual Review of Immunology, 2010, 28(28): 243–273. |
| [29] | MORA J R, IWATA M, EKSTEEN B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells[J]. Science, 2006, 314(5802): 1157–1160. DOI: 10.1126/science.1132742 |
| [30] | WALKER J A, BARLOW J L, MCKENZIE A N. Innate lymphoid cells-how did we miss them?[J]. Nature Reviews Immunology, 2013, 13(2): 75–87. DOI: 10.1038/nri3349 |
| [31] | SPITS H, DI SANTO J P. The expanding family of innate lymphoid cells:regulators and effectors of immunity and tissue remodeling[J]. Nature Immunology, 2011, 12(1): 21–27. DOI: 10.1038/ni.1962 |
| [32] | SATOH-TAKAYAMA N, VOSSHENRICH C A, LESJEAN-POTTIER S, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense[J]. Immunity, 2008, 29(6): 958–970. DOI: 10.1016/j.immuni.2008.11.001 |
| [33] | SANOS S L, BUI V L, MORTHA A, et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells[J]. Nature Immunology, 2009, 10(1): 83–91. DOI: 10.1038/ni.1684 |
| [34] | SAWA S, LOCHNER M, SATOH-TAKAYAMA N, et al. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota[J]. Nature Immunology, 2011, 12(4): 320–326. DOI: 10.1038/ni.2002 |
| [35] | MAYNARD C L, ELSON C O, HATTON R D, et al. Reciprocal interactions of the intestinal microbiota and immune system[J]. Nature, 2012, 489(7415): 231–241. DOI: 10.1038/nature11551 |
| [36] | 王珊珊, 王佳堃, 刘建新. 肠道微生物对宿主免疫系统的调节及其可能机制[J]. 动物营养学报, 2015, 27(2) :375–382. |
| [37] | IVANOV Ⅱ, ATARASHI K, MANEL N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria[J]. Cell, 2009, 139(3): 485–498. DOI: 10.1016/j.cell.2009.09.033 |
| [38] | SHAW M H, KAMADA N, KIM Y G, et al. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine[J]. Journal of Experimental Medicine, 2012, 209(2): 251–258. DOI: 10.1084/jem.20111703 |
| [39] | COOMBES J L, SIDDIQUI K R R, ARANCIBIA-CÁRCAMO C V, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β-and retinoic acid-dependent mechanism[J]. Journal of Experimental Medicine, 2007, 204(8): 1757–1764. DOI: 10.1084/jem.20070590 |
| [40] | MUCIDA D, PARK Y, KIM G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid[J]. Science, 2007, 317(5835): 256–260. DOI: 10.1126/science.1145697 |
| [41] | DENNING T L, WANG Y C, PATEL S R. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses[J]. Nature Immunology, 2007, 8(10): 1086–1094. DOI: 10.1038/ni1511 |
| [42] | ROUND J L, LEE S M, LI J, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota[J]. Science, 2011, 332(6032): 974–977. DOI: 10.1126/science.1206095 |
| [43] | KUNISAWA J, GOHDA M, HASHIMOTO E, et al. Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice[J]. Nature Communications, 2013, 4: 1772. DOI: 10.1038/ncomms2718 |
| [44] | SUZUKI K, MARUYA M, KAWAMOTO S, et al. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin a generation in the gut[J]. Immunity, 2010, 33(1): 71–83. DOI: 10.1016/j.immuni.2010.07.003 |
| [45] | UEMATSU S, FUJIMOTO K, JANG M H, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5[J]. Nature Immunology, 2008, 9(7): 769–776. DOI: 10.1038/ni.1622 |
| [46] | SONNENBERG G F, MONTICELLI L A, ALENGHAT T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria[J]. Science, 2012, 336(6086): 1321–1325. DOI: 10.1126/science.1222551 |
| [47] | VONARBOURG C, MORTHA A, BUI V L, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes[J]. Immunity, 2010, 33(5): 736–751. DOI: 10.1016/j.immuni.2010.10.017 |
| [48] | FURUSAWA Y, OBATA Y, FUKUDA S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells[J]. Nature, 2013, 504(7480): 446–450. DOI: 10.1038/nature12721 |
| [49] | MYSZKA M, KLINGER M. The immunomodulatory role of vitamin D[J]. Postępy Higieny i Medycyny Do'swiadczalnej, 2014, 68(68): 865–878. |
| [50] | HOOPER L V, LITTMAN D R, MACPHERSON A J. Interactions between the microbiota and the immune system[J]. Science, 2012, 336(6086): 1268–1273. DOI: 10.1126/science.1223490 |
| [51] | VAISHNAVA S, BEHRENDT C L, ISMAIL A S, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(52): 20858–20863. DOI: 10.1073/pnas.0808723105 |
| [52] | SEKIKAWA A, FUKUI H, SUZUKI K, et al. Involvement of the IL-22/REG Iα axis in ulcerative colitis[J]. Laboratory Investigation, 2010, 90(3): 496–505. DOI: 10.1038/labinvest.2009.147 |
| [53] | ZHENG Y, VALDEZ P A, DANILENKO D M, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens[J]. Nature Medicine, 2008, 14(3): 282–289. DOI: 10.1038/nm1720 |
| [54] | MACPHERSON A J, GATTO D, SAINSBURY E, et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria[J]. Science, 2000, 288(5474): 2222–2226. DOI: 10.1126/science.288.5474.2222 |
| [55] | WEI M, SHINKURA R, DOI Y, et al. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense[J]. Nature Immunology, 2011, 12(3): 264–270. DOI: 10.1038/ni.1991 |
| [56] | KAWAMOTO S, TRAN T H, MARUYA M, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut[J]. Science, 2012, 336(6080): 485–489. DOI: 10.1126/science.1217718 |
| [57] | PETERSON D A, MCNULTY N P, GURUGE J L, et al. IgA response to symbiotic bacteria as a mediator of gut homeostasis[J]. Cell Host & Microbe, 2007, 2(5): 328–339. |
| [58] | QIU J, GUO X H, CHEN Z M, et al. Group 3 innate lymphoid cells inhibit T-Cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora[J]. Immunity, 2013, 39(2): 386–399. DOI: 10.1016/j.immuni.2013.08.002 |
| [59] | POWELL N, WALKER A W, STOLARCZYK E, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells[J]. Immuniy, 2012, 37(4): 674–684. DOI: 10.1016/j.immuni.2012.09.008 |
| [60] | ELINAV E, STROWIG T, KAU A L, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis[J]. Cell, 2011, 145(5): 745–757. DOI: 10.1016/j.cell.2011.04.022 |




