2. 陕西省商洛市畜牧产业发展中心, 商洛 726000
2. The Development Centre of Animal Husbandry in Shangluo City of Shanxi Province, Shangluo 726000, China
奶牛围产期包括围产前期(产前21 d)和围产后期(产后21 d)2个阶段,是奶牛泌乳周期中的关键时期。此阶段奶牛经历妊娠-分娩-泌乳的生理转变,涉及多个组织的协调变化,营养需要量显著增加,而干物质采食量(dry matter intake,DMI)急剧下降,营养摄入严重不足,奶牛往往处于多种营养物质的负平衡状态,其中能量负平衡(negative energy balance, NEB)尤为突出[1]。围产期奶牛通过动员体脂缓解NEB,产生的非酯化脂肪酸(nonesterified fatty acids,NEFA)主要进入肝脏进行代谢,主要有3条代谢途径:1)完全氧化形成CO2和H2O,高效供能;2)不完全氧化生成酮体,供能效率低,并易诱发酮病;3)酯化反应形成甘油三酯(triglycerides, TG),其在肝脏蓄积可导致脂肪肝的发生[2]。酮病和脂肪肝严重威胁奶牛围产期健康,并继发一系列营养代谢病,造成奶牛过早淘汰。蛋氨酸(methionine,Met)是维持动物生长发育和各项生理活动的必需氨基酸,也是机体重要的甲基供体,在肝脏一碳单位循环及相关代谢过程中发挥重要作用[3]。研究表明,Met可调控围产期奶牛能量和脂质代谢,维持肝脏健康[4],改善机体抗氧化和免疫功能[5-6],且母体的部分调控效应可在犊牛中有所体现[7]。本文概述了Met理化性质和生物学功能及瘤胃降解特性,综述了过瘤胃蛋氨酸(rumen-protected methionine, RPM)调控奶牛围产期相关代谢的研究进展,并探讨了围产期奶牛饲粮RPM的适宜添加量,旨在为Met在奶牛围产期的基础研究及应用提供科学依据和技术参考。
1 Met营养概述Met的分子式为C5H11O2NS,化学结构式如图 1,相对分子质量149.21,是人类和动物体内的含硫必需氨基酸。Met与细胞信号转导、核酸和蛋白质合成等许多生理生化过程密切相关。Met营养平衡对奶牛生长发育、生理代谢、机体健康和泌乳性能的高效发挥至关重要,除构成细胞蛋白质外,还具有以下功能:1)作为底物和调控物质,参与奶牛机体蛋白质合成,其中泌乳牛乳腺蛋白质合成尤其关键。Met和赖氨酸(lysine, Lys)是奶牛泌乳最重要的2种限制性氨基酸,其限制性排位由基础饲粮的类型决定[8];2)其代谢产物S-腺苷甲硫氨酸(S-adenosylmethionine, SAM)是机体重要的甲基供体,参与和调控多种生理生化过程[9];3)与胆碱类似,Met可促进肝脏极低密度脂蛋白(very-low-density lipoprotein, VLDL)和肉毒碱合成,调控肝细胞脂质代谢[2, 10];4)合成部分抗氧化物质(如牛磺酸),维持奶牛机体氧化还原状态[11];5)调控奶牛免疫细胞活性和功能,增强机体免疫力[11-12]。此外,对产毛动物而言,Met还可转化为半胱氨酸,促进绒毛生长,增加绒毛产量[13]。
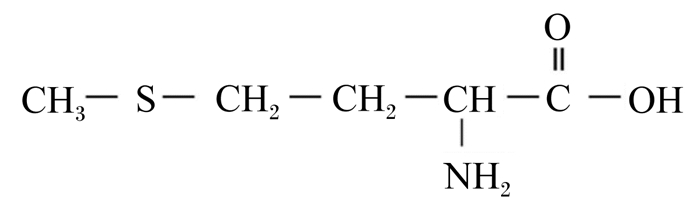
|
图 1 蛋氨酸的化学结构式 Figure 1 The chemical structure of methionine |
在瘤胃微生物的作用下,Met可在瘤胃大量降解,并在瘤胃参与相关代谢过程,到达小肠的Met较少,限制了奶牛对饲粮Met的利用[10]。在瘤胃中,Met一部分用于合成菌体蛋白,还有一部分进入其他代谢通路。例如,Met可作为甲基供体,与瘤胃微生物产生的氢结合,产生甲烷,造成能量和氨基酸损失。因此,需在奶牛饲粮中添加RPM,以保证充足的Met在小肠被吸收,随血液循环进入靶器官,发挥相应生理功能[3]。
2 Met对奶牛围产期生理代谢和健康的调控在奶牛体内,Met和胆碱的代谢过程相互关联,且二者间互相转化。以奶牛机体一碳单位循环为理论基础,Met和胆碱均具有促进围产期奶牛肝脏健康和代谢、增强抗氧化和免疫功能以及降低代谢性疾病的发生等功能[11, 14]。Met还可调控奶牛围产期消化道功能,提高饲粮氮素利用率,降低氮排放,改善产后泌乳性能[15-16],Met亦可作为底物和调控物质,通过哺乳动物雷帕霉素靶蛋白(mTOR)等通路促进奶牛机体蛋白质合成[8, 17]。
2.1 Met对围产期奶牛肝脏的调节功能RPM可调控围产期奶牛肝脏功能,促进肝细胞脂质和碳水化合物代谢,提高肝脏能量和其他物质的输出。脂质代谢异常或超载是威胁奶牛围产期肝脏健康和功能的主要诱因,通过营养调控促进NEFA完全氧化、TG转运和糖异生是保障肝脏能量高效转化和代谢以及重要蛋白质(如白蛋白和代谢酶类)合成的重要技术思路。
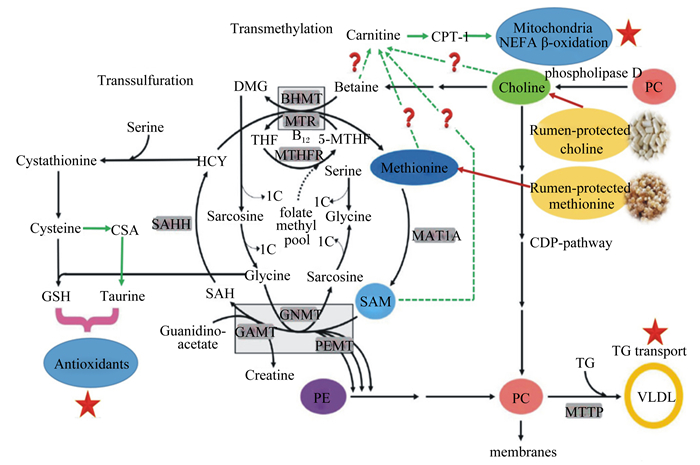
由于Met在一碳单位循环(图 2)和蛋白质合成中的重要作用,饲粮RPM对奶牛围产期肝脏功能和营养代谢的调控及其机理已成为研究热点。奶牛围产期饲粮添加RPM可上调肝脏过氧化物酶体增殖物激活受体α(peroxisome proliferator-activated receptor α, PPARα)的表达,进而提高丙酮酸羧化酶(pyruvate carboxylase, PC)、微粒体甘油三酯转运蛋白(microsomal triglyceride transfer protein, MTTP)和磷酸烯醇式丙酮酸激酶(phosphoenolpyruvate carboxykinase, PEPCK)的表达量,表明RPM可促进肝细胞脂蛋白组装,并增强糖异生[18]。进一步研究发现,这可能与一碳单位循环及某些基因的启动子甲基化有关[18-19]。Li等[4]研究发现,饲粮添加RPM提高了血液VLDL含量,且肝组织TG含量有所降低(4.70% vs. 3.40%,湿重基础),这说明RPM可促进TG转运,降低肝脏脂质沉积;同时,血液NEFA含量下降,这提示奶牛体脂动员减少,可能是由于NEB有所缓解。RPM可调控奶牛围产期肝脏脂质和能量代谢及转化,降低脂质沉积,但其信号网络、内分泌和其他可能机制等尚待阐明。一些关键通路和调控因子,如腺苷一磷酸激活的蛋白激酶[adenosine 5′-monophosphate (AMP)-activated protein, AMPK]、PPARα和固醇调节元件结合蛋白1c(sterol regulatory element binding protein 1c,SREBP-1c)等在饲粮甲基营养物质(Met、胆碱、甜菜碱和叶酸)调控奶牛围产期肝脏代谢和健康过程中扮演何种角色?其作用机理又是什么?尚待阐明。
|
Transsulfuration:转硫氢基反应;Transmethylation:转甲基反应;Carnitine:肉毒碱;CPT-1:肉毒碱棕榈酰转移酶1 carnitine palmitoyltransferase 1;Serine:丝氨酸;Glycine:甘氨酸;HCY:同型半胱氨酸homocysteine;DMG:二甲基甘氨酸dimethylglycine;MTR:5-甲基四氢叶酸-同型半胱氨酸甲基转移酶5-methyltetrahydrofolate-homocysteine methyltransferase;THF:四氢叶酸tetrahydrofolate;5-MTHF:5-甲基四氢叶酸5-methyltetrahydrofolate;MTHFR:亚甲基四氢叶酸还原酶methylenetetrahydrofolate reductase;SAM:S-腺苷甲硫氨酸S-adenosylmethionine;SAH:S-腺苷半胱氨酸S-adenocylhomocysteine;Cysteine:半胱氨酸;Choline:胆碱;Methionine:蛋氨酸;PC:磷脂酰胆碱phosphatidylcholine;VLDL:极低密度脂蛋白very low density lipoprotein;MTTP:微粒体甘油三酯转运蛋白microsomal triglyceride transfer protein;PE:磷脂酰乙醇胺phosphatidylethanolamine;Cystathionine:胱硫醚;GSH:谷胱甘肽glutathione;Antioxidants:抗氧化剂;Taurine:牛磺酸;CSA:半胱亚磺酸cysteine sulfinic acid;GNMT:甘氨酸-N-甲基转移酶glycine-N-methyltransferase;PEMT:磷脂酰乙醇胺N-甲基转移酶phosphatidylethanolamine N-methyltransferase;GAMT:胍基乙酸N-甲基转移酶guanidinoacetate N-methyltransferase;TG:甘油三酯triglyceride;BHMT:甜菜碱-同型半胱氨酸-S-甲基转移酶betaine-homocysteine S-methyltranferase;phoshphlipase D:磷酸脂酶D; Rumen-protected choline:过瘤胃胆碱;Rumen-protected methionine:过瘤胃蛋氨酸; CDP pathway:CDP通路; membranes:细胞膜。 图 2 甲基供体循环通路及其调控奶牛围产期代谢和健康的可能途径 Figure 2 Circulation of methyl donors and their potential roles in regulation of metabolism and health of dairy cows at perinatal period[3, 20] |
RPM通过奶牛机体一碳单位循环合成抗氧化物质,减少自由基对奶牛细胞的氧化损伤,并可增强机体免疫力,降低代谢性疾病和其他疾病的发生。研究表明,饲粮添加RPM可促进谷胱甘肽等抗氧化物质的从头合成,提高血浆氧自由基的清除能力,降低氧化应激和肝脏炎症反应,增强机体免疫功能[5-6]。Met供应与免疫反应密切相关,RPM影响奶牛免疫功能的原因有很多,如促进奶牛T淋巴细胞增殖[21],增强血液中性粒细胞的功能[22],降低氧化应激对免疫细胞的损伤[12],改变外周血T淋巴细胞亚群比例(CD4+/CD8+)等[3, 11]。此外,利用奶牛原代肝细胞培养技术,发现高含量NEFA和β-羟基丁酸(β-hydroxybutyric acid,BHBA)可导致肝细胞氧化应激,引起炎症反应,造成细胞损伤,降低肝细胞功能,并诱发细胞凋亡[3, 23-26]。可以推测,RPM可能通过缓解奶牛围产期NEB,减少体脂动员,增强肝脏功能,降低血液NEFA和BHBA含量,减轻NEFA和BHBA对免疫细胞的损伤,间接提高奶牛围产期免疫功能,这已在部分研究得到证实[27-29]。综上可知,Met在维持奶牛围产期机体氧化还原状态和免疫功能方面具有重要作用。
围产期奶牛饲粮添加RPM可提高肝脏甜菜碱-同型半胱氨酸-S-甲基转移酶(betaine-homocysteine S-methyltranferase, BHMT)、磷脂酰乙醇胺N-甲基转移酶(phosphatidylethanolamine N-methyltransferase, PEMT)、蛋氨酸腺苷转移酶1A(methionine adenosyltransferase 1A, MAT1A)、S-腺苷高半胱氨酸水解酶(S-adenosylhomocysteine hydrolase, SAHH)和胱硫醚β合成酶(cystathionine β-synthase, CBS)的基因表达量,降低5-甲基四氢叶酸-同型半胱氨酸甲基转移酶(5-methyltetrahydrofolate-homocysteine methyltransferas, MTR)的活性,表明RPM影响肝脏一碳单位循环,促进肝脏磷脂酰胆碱和一些抗氧化剂(牛磺酸和谷胱甘肽)的合成,这可能是RPM有利于奶牛肝脏和机体健康,并提高生产性能的重要原因[7, 19-20, 30]。还有研究发现,RPM影响肝脏全基因组和PPARα基因启动子区特定位点的甲基化,并上调PPARα等一系列能量和脂质代谢相关靶基因的表达,进而促进肝细胞脂质代谢和转运,降低TG沉积,并增强碳水化合物的代谢和转化[18]。
2.3 Met对犊牛生理的调控作用RPM经小肠消化、吸收进入奶牛体内后,Met可转化为SAM,提供游离甲基,引起肝脏和其他器官关键基因启动子区的甲基化,上调或下调部分基因和调控因子的表达,调控相关代谢,且部分变化可遗传给后代[7]。
奶牛围产前期补充RPM,可提高新生犊牛提高肝脏DNA甲基转移酶1[DNA (cytosine-5)-methyltransferase 1, DNMT1]基因的表达量[7],但这并不能证明母体肝脏的表观遗传学变化传代给犊牛,也可能是母体补充RPM提高了血液Met含量,进而通过胎盘血液循环进入胚胎,使得胚胎获得的Met或其他甲基供体增加,自身发生上述变化。在新生犊牛原代肝细胞培养试验中,提高培养基中Met和氯化胆碱含量,均可不同程度影响肝细胞甲基转移、转硫及相关过程,促进VLDL合成,并降低培养基中自由基积累[20]。因此,关于奶牛围产前期甲基供体供应对犊牛肝脏代谢及机体健康的影响,仍需进一步研究、确证,最终构建RPM调控奶牛围产期肝脏功能、主要营养物质的代谢和转化以及机体健康的机制网络,为甲基供体在奶牛上的营养实践提供理论基础。
3 围产期奶牛RPM的适宜添加量作为奶牛泌乳重要的限制性氨基酸之一,泌乳奶牛Met的相关研究较多,多集中于Met对奶牛乳腺上皮细胞蛋白质合成的调控及其机理,且一般与Lys一起研究[8, 15, 31-33],而对围产期奶牛的研究较少。制定泌乳奶牛Met需要量时,以保证饲粮代谢蛋白质(metabolizable protein, MP)满足需要为前提,在此基础上考虑不同氨基酸在MP中的含量及氨基酸间的比例关系,一般认为泌乳奶牛饲粮Lys : Met≈3 : 1,NRC(2001)推荐量为MP 7.2%和MP 2.4%。因此,通常根据基础饲粮MP、Lys和Met等含量的实测值,按照上述比例推算RPM添加量。现有营养标准和相关研究尚未给出奶牛围产期RPM建议添加量,基于本课题组研究并综合分析他人研究结果(表 1),建议围产期奶牛RPM添加量为10~20 g/d(以Met计)。
|
|
表 1 过瘤胃蛋氨酸的添加量及其对奶牛围产期代谢的影响 Table 1 Supplementary doses of RPM and their effects on the metabolism of transition dairy cows |
奶牛围产期的营养与管理对胎儿发育、奶牛健康、泌乳和繁殖性能十分关键,甚至影响奶牛整个泌乳生涯。RPM在调控奶牛围产期肝脏健康及营养代谢中发挥重要作用,其生理和分子机制并未完全明确,且添加量及添加形式尚无统一标准,有待研究。未来研究应主要关注以下4点:1)RPM精准添加量及添加方式的标准化。以饲粮MP、能氮和氨基酸平衡为基础,充分考虑Met形式、过瘤胃和利用率、效价等因素,规范添加方式;2)明确RPM调控奶牛围产期肝脏功能、代谢和健康的关键信号通路,并探寻神经内分泌和其他生理机制,系统解析其调控机制,并整合胆碱相关研究和机理,构建一碳单位调控奶牛围产期肝脏代谢的机理和技术网络;3)以核因子κB(nuclear factor kappa B, NF-κB)、Toll样受体4(Toll like receptor, TLR4)和红系衍生的核因子2相关因子2(nuclear factor erythroid 2-related factor 2, Nrf2)等通路为核心,研究RPM调控奶牛抗氧化和免疫功能的机理;4)进一步挖掘母体Met供应对胚胎发育、代谢和犊牛健康的影响,并探寻相关生理机制和信号传导,以及可能的表观遗传学机理。
| [1] |
余超. 生物素对围产期奶牛泌乳净能和代谢蛋白平衡及生产性能的影响[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10712-1016157689.htm
|
| [2] |
孙菲菲, 曹阳春, 李生祥, 等. 胆碱对奶牛围产期代谢的调控[J]. 动物营养学报, 2014, 26(1): 26-33. |
| [3] |
孙菲菲. 胆碱和蛋氨酸对奶牛围产期营养平衡和机体健康的影响及机制[D]. 博士学位论文. 杨凌: 西北农林科技大学, 2017.
|
| [4] |
LI C, BATISTEL F, OSORIO J S, et al. Peripartal rumen-protected methionine supplementation to higher energy diets elicits positive effects on blood neutrophil gene networks, performance and liver lipid content in dairy cows[J]. Journal of Animal Science and Biotechnology, 2016, 7(1): 18. DOI:10.1186/s40104-016-0077-9 |
| [5] |
OSORIO J, TREVISI E, JI P, et al. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart[J]. Journal of Dairy Science, 2014, 97(12): 7437-7450. DOI:10.3168/jds.2013-7679 |
| [6] |
OSORIO J, JI P, DRACKLEY J, et al. Smartamine M and MetaSmart supplementation during the peripartal period alter hepatic expression of gene networks in 1-carbon metabolism, inflammation, oxidative stress, and the growth hormone-insulin-like growth factor 1 axis pathways[J]. Journal of Dairy Science, 2014, 97(12): 7451-7464. DOI:10.3168/jds.2014-8680 |
| [7] |
JACOMETO C B, ZHOU Z, LUCHINI D, et al. Maternal supplementation with rumen-protected methionine increases prepartal plasma methionine concentration and alters hepatic mRNA abundance of 1-carbon, methionine, and transsulfuration pathways in neonatal Holstein calves[J]. Journal of Dairy Science, 2017, 100(4): 3209-3219. DOI:10.3168/jds.2016-11656 |
| [8] |
NAN X M, BU D P, LI X Y, et al. Ratio of lysine to methionine alters expression of genes involved in milk protein transcription and translation and mTOR phosphorylation in bovine mammary cells[J]. Physiological Genomics, 2014, 46(7): 268-275. DOI:10.1152/physiolgenomics.00119.2013 |
| [9] |
胡诚军, 江青艳, 孔祥峰. 畜禽蛋氨酸代谢及其生理功能研究进展[J]. 饲料工业, 2016, 37(15): 23-27. |
| [10] |
ARDALAN M, DEHGHAN-BANADAKY M, REZAYAZDI K, et al. The effect of rumen-protected methionine and choline on plasma metabolites of Holstein dairy cows[J]. The Journal of Agricultural Science, 2011, 149(5): 639-646. DOI:10.1017/S0021859610001292 |
| [11] |
SUN F F, CAO Y C, CAI C J, et al. Regulation of nutritional metabolism in transition dairy cows:energy homeostasis and health in response to post-ruminal choline and methionine[J]. PLoS One, 2016, 11(8): e0160659. DOI:10.1371/journal.pone.0160659 |
| [12] |
ZHOU Z, BULGARI O, VAILATI-RIBONI M, et al. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period[J]. Journal of Dairy Science, 2016, 99(11): 8956-8969. DOI:10.3168/jds.2016-10986 |
| [13] |
SAHOO A, SOREN N. Nutrition for wool production[J]. Webmedcentral Nutrition, 2011, 2(10): WMC002384. |
| [14] |
ZHOU Z, VAILATI-RIBONI M, TREVISI E, et al. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period[J]. Journal of Dairy Science, 2016, 99(11): 8716-8732. DOI:10.3168/jds.2015-10525 |
| [15] |
SINCLAIR K D, GARNSWORTHY P C, MANN G E, et al. Reducing dietary protein in dairy cow diets:implications for nitrogen utilization, milk production, welfare and fertility[J]. Animal, 2014, 8(2): 262-274. DOI:10.1017/S1751731113002139 |
| [16] |
WANG C, LIU H Y, WANG Y M, et al. Effects of dietary supplementation of methionine and lysine on milk production and nitrogen utilization in dairy cows[J]. Journal of Dairy Science, 2010, 93(8): 3661-3670. DOI:10.3168/jds.2009-2750 |
| [17] |
HUANG X, ZANG Y L, ZHANG M H, et al. Nuclear factor of κB1 is a key regulator for the transcriptional activation of milk synthesis in bovine mammary epithelial cells[J]. DNA and Cell Biology, 2017, 36(4): 295-302. DOI:10.1089/dna.2016.3610 |
| [18] |
OSORIO J S, JACOMETO C B, ZHOU Z, et al. Hepatic global DNA and peroxisome proliferator-activated receptor alpha promoter methylation are altered in peripartal dairy cows fed rumen-protected methionine[J]. Journal of Dairy Science, 2016, 99(1): 234-244. DOI:10.3168/jds.2015-10157 |
| [19] |
ZHOU Z, GARROW T A, DONG X W, et al. Hepatic activity and transcription of betaine-homocysteine methyltransferase, methionine synthase, and cystathionine synthase in periparturient dairy cows are altered to different extents by supply of methionine and choline[J]. The Journal of Nutrition, 2017, 147(1): 11-19. DOI:10.3945/jn.116.240234 |
| [20] |
CHANDLER T L, WHITE H M. Choline and methionine differentially alter methyl carbon metabolism in bovine neonatal hepatocytes[J]. PLoS One, 2017, 12(2): e0171080. DOI:10.1371/journal.pone.0171080 |
| [21] |
SODER K J, HOLDEN L A. Lymphocyte proliferation response of lactating dairy cows fed varying concentrations of rumen-protected methionine[J]. Journal of Dairy Science, 1999, 82(9): 1935-1942. DOI:10.3168/jds.S0022-0302(99)75429-9 |
| [22] |
OSORIO J, JI P, DRACKLEY J K, et al. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function[J]. Journal of Dairy Science, 2013, 96(10): 6248-6263. DOI:10.3168/jds.2012-5790 |
| [23] |
DENG Q, MA D, SHI Z, et al. Effects of β-hydroxybutyricacid on the synthesis and assembly of very low-density lipoprotein in bovine hepatocytes in vitro[J]. Journal of Animal Physiology and Animal Nutrition, 2016, 100(2): 331-336. DOI:10.1111/jpn.2016.100.issue-2 |
| [24] |
LI Y, DING H Y, WANG X C, et al. High levels of acetoacetate and glucose increase expression of cytokines in bovine hepatocytes, through activation of the NF-κB signalling pathway[J]. Journal of Dairy Research, 2016, 83(1): 51-57. DOI:10.1017/S0022029915000680 |
| [25] |
SHI X, LI X, LI D, et al. β-hydroxybutyrate activates the NF-κB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes[J]. Cellular Physiology and Biochemistry, 2014, 33(4): 920-932. DOI:10.1159/000358664 |
| [26] |
SHI X X, LI D D, DENG Q H, et al. NEFAs activate the oxidative stress-mediated NF-κB signaling pathway to induce inflammatory response in calf hepatocytes[J]. The Journal of Steroid Biochemistry and Molecular Biology, 2015, 145: 103-112. DOI:10.1016/j.jsbmb.2014.10.014 |
| [27] |
LACETERA N, FRANCI O, SCALIA D, et al. Effects of nonesterified fatty acids and β-hydroxybutyrate on functions of mononuclear cells obtained from ewes[J]. American Journal of Veterinary Research, 2002, 63(3): 414-418. DOI:10.2460/ajvr.2002.63.issue-3 |
| [28] |
LACETERA N, SCALIA D, FRANCI O, et al. Short communication:effects of nonesterified fatty acids on lymphocyte function in dairy heifers[J]. Journal of Dairy Science, 2004, 87(4): 1012-1014. DOI:10.3168/jds.S0022-0302(04)73246-4 |
| [29] |
LACETERA N, SCALIA D, BERNABUCCI U, et al. Lymphocyte functions in overconditioned cows around parturition[J]. Journal of Dairy Science, 2005, 88(6): 2010-2016. DOI:10.3168/jds.S0022-0302(05)72877-0 |
| [30] |
VAILATI-RIBONI M, OSORIO J S, TREVISI E, et al. Supplemental Smartamine M in higher-energy diets during the prepartal period improves hepatic biomarkers of health and oxidative status in Holstein cows[J]. Journal of Animal Science and Biotechnology, 2017, 8(1): 17. DOI:10.1186/s40104-017-0147-7 |
| [31] |
AWAWDEH M S. Rumen-protected methionine and lysine:effects on milk production and plasma amino acids of dairy cows with reference to metabolisable protein status[J]. Journal of Dairy Research, 2016, 83(2): 151-155. DOI:10.1017/S0022029916000108 |
| [32] |
ROBINSON P H. Impacts of manipulating ration metabolizable lysine and methionine levels on the performance of lactating dairy cows:A systematic review of the literature[J]. Livestock Science, 2010, 127(2/3): 115-126. |
| [33] |
ZANTON G I, BOWMAN G R, VÁZQUEZ-AÑÓN M, et al. Meta-analysis of lactation performance in dairy cows receiving supplemental dietary methionine sources or postruminal infusion of methionine[J]. Journal of Dairy Science, 2014, 97(11): 7085-7101. DOI:10.3168/jds.2014-8220 |
| [34] |
ACOSTA D A V, DENICOL A C, TRIBULO P, et al. Effects of rumen-protected methionine and choline supplementation on the preimplantation embryo in Holstein cows[J]. Theriogenology, 2016, 85(9): 1669-1679. DOI:10.1016/j.theriogenology.2016.01.024 |
| [35] |
DALBACH K F, LARSEN M, RAUN B M L, et al. Effects of supplementation with 2-hydroxy-4-(methylthio)-butanoic acid isopropyl ester on splanchnic amino acid metabolism and essential amino acid mobilization in postpartum transition Holstein cows[J]. Journal of Dairy Science, 2011, 94(8): 3913-3927. DOI:10.3168/jds.2010-3724 |
| [36] |
ARDALAN M, REZAYAZDI K, DEHGHAN-BANADAKY M. Effect of rumen-protected choline and methionine on physiological and metabolic disorders and reproductive indices of dairy cows[J]. Journal of Animal Physiology and Animal Nutrition, 2010, 94(6): e259-e265. DOI:10.1111/jpn.2010.94.issue-6 |
| [37] |
ORDWAY R S, BOUCHER S E, WHITEHOUSE N L, et al. Effects of providing two forms of supplemental methionine to periparturient Holstein dairy cows on feed intake and lactational performance[J]. Journal of Dairy Science, 2009, 92(10): 5154-5166. DOI:10.3168/jds.2009-2259 |
| [38] |
SOCHA M T, PUTNAM D E, GARTHWAITE B D, et al. Improving intestinal amino acid supply of pre-and postpartum dairy cows with rumen-protected methionine and lysine[J]. Journal of Dairy Science, 2005, 88(3): 1113-1126. DOI:10.3168/jds.S0022-0302(05)72778-8 |
| [39] |
PHILLIPS G J, CITRON T L, SAGE J S, et al. Adaptations in body muscle and fat in transition dairy cattle fed differing amounts of protein and methionine hydroxy analog[J]. Journal of Dairy Science, 2003, 86(11): 3634-3647. DOI:10.3168/jds.S0022-0302(03)73969-1 |




