鱼类通过摄食为自身机体提供营养和能量,促进生长发育[1]。摄食调控复杂且精细,各种外周食欲相关物理或化学信号通过神经系统或体液传递途径产生饥饿或饱腹信号到达中枢神经系统调节摄食[2]。促皮质激素释放激素(corticotropin releasing factor,CRF)作为中枢调控的饱腹感信号因子(厌食欲因子),其成熟肽含有41个氨基酸,可激活下丘脑-垂体-肾上腺(hypothalamus-hypophysis-interrenal,HPI)轴,引起垂体释放促肾上腺皮质激素(adrenocorticotropic hormone,ATCH),影响动物的摄食行为[3-4]。CRF已成为动物摄食和能量代谢领域的研究热点之一,目前在哺乳动物上关于CRF的摄食调控有大量的研究报道,而鱼类上较少。因此本文依据CRF在哺乳动物和部分鱼类中的研究现状,阐述了CRF的发现历史、分子结构、对鱼类摄食的调控作用及其机制,重点综述了CRF作为外源多肽通过不同方式注射或作为内源调节子通过不同方式处理对鱼类摄食的影响,以及CRF与受体、皮质醇和其他食欲因子之间的互作。这将为今后鱼类摄食调控和生长的研究以及生产提供理论依据。
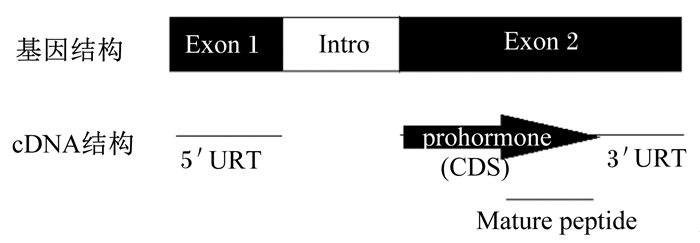
1 CRF的发现历史和结构1955年Saffran等[5]体外培养大鼠垂体,通过下丘脑提取液刺激显示ACTH的释放增加,故此命名为CRF。直至1981年Vale等[4]从绵羊下丘脑处提取纯化出含41个氨基酸残基的CRF多肽。在此基础上,1983年Furutani等[6]进一步验证了CRF基因的结构。CRF主要位于下丘脑处,可刺激垂体分泌ACTH。Vandenborne等[7]依据哺乳动物和鱼类的报道以及在家鸡上的鉴定,总结了CRF基因结构,它含2个外显子和1个内含子,外显子1含5′非编码区,外显子2包含编码区和3′非编码区(图 1)。鱼类上CRF基因cDNA长为0.9~1.0 kbp,编码区一般为0.4~0.5 kbp。CRF编码区编码的氨基酸结构包括了疏水信号肽,中间1段未证明功能的保守肽,以及C端含41个氨基酸残基的成熟肽,激活C末端的2个位点(酰胺基团)可使CRF具有活性。在白亚口鱼(Catostomus commersonii)[8]、金鱼(Carassius auratus)[9]、红大马哈鱼(Oncorhynchus nerka)[10]、虹鳟(Oncorhynchus mykiss)[11]和鲤鱼(Cyprinus carpio)[12]上发现CRF基因含2个亚型(CRF1和CRF2),而罗非鱼(Oreochromis mossambicus)[13]、比目鱼(Platichthys flesus)[14]、斑马鱼(Barchydanio rerio var)[15]和齐口裂腹鱼(Schizothorax prenanti)[16]上CRF基因无亚型。
|
prohormone:激素原;Mature peptide:成熟肽;Exon 1:外显子1;Exon 2:外显子2;Intro:内含子;5′URT:5′非编码区5′ noncoding region;3′URT:3′非编码区3′noncoding region;CDS:编码区序列coding sequence。 图 1 CRF基因结构 Figure 1 The structure of CRF gene[7] |
依据结构和分布,CRF在鱼类的食欲调控内分泌系统中起着重要的调控作用。通过CRF作为外源多肽通过不同方式注射探讨其对鱼类摄食影响,或通过不同处理下鱼类内源CRF基因的表达水平和摄食的变化探究CRF的调控机制,结果显示CRF可调控鱼类摄食。
2.1 外源性CRF调控鱼类摄食 2.1.1 中枢注射CRF调控鱼类摄食对哺乳动物中枢(脑室)注射CRF探究其摄食量的变化有研究报道,结果均证明CRF通过中枢注射可抑制哺乳动物摄食[17-18]。在鱼类上,中枢注射CRF引起的摄食调控作用目前仅限金鱼。De Pedro等[19]对金鱼饥饿处理24 h后中枢注射CRF,注射后2 h摄食量减少,提示CRF可能通过中枢系统抑制金鱼摄食。Berinier等[20]在金鱼上证实CRF受体拮抗剂αh-CRF(9-41)预处理可使CRF注射效果逆转。综上,中枢注射CRF对金鱼有抑制摄食的作用,而通过αh-CRF(9-41)预处理可使中枢CRF的作用效果逆转。
2.1.2 外周注射CRF调控鱼类摄食现有少量研究表明在哺乳动物和鱼类上,外周注射CRF摄食量无显著变化,但是有下降趋势[19, 21]。在鱼类上,De Pedro等[19]对金鱼腹腔注射CRF,摄食量呈下降趋势,提示腹腔注射CRF可能影响金鱼的摄食量。以上试验为只持续1 d的单次外周注射,无长期外周注射的报道,无法明确长期外周注射CRF对鱼类摄食是否抑制。
虽然外周注射CRF对摄食调控的报道极少,但是外周CRF及CRF相关肽分布于啮齿动物的胃肠道系统中有报道,报道显示CRF及CRF相关肽少量分布于外周组织中[22-23]。Taché等[24]认为通过对啮齿动物的胃肠道直接或间接刺激,可使其外周CRF及CRF相关肽的水平上升,从而抑制啮齿动物摄食,提示啮齿动物上的外周CRF及CRF相关肽在应激引起相关肠动力改变过程中有重要的调控作用。在鱼类上,Pepels等[25]检测出应激处理的尼罗罗非鱼(Oreochromis niloticus)的血液中存在CRF,提示CRF可能也存在于鱼类机体外周组织中。
2.2 内源性CRF调控鱼类摄食 2.2.1 不同摄食状况对CRF的调控在啮齿动物上,禁食后CRF基因表达水平降低[26]。而在鱼类上目前报道仅限于金鱼和齐口裂腹鱼。Maruyama等[27]对金鱼分3组试验7 d,结果显示与正常投喂组相比,禁食组的脑CRF基因的表达水平呈下降趋势,但差异不显著,而过量投喂组则显著上升。Wang等[16]对齐口裂腹鱼短期(1 d)禁食处理,显示下丘脑CRF基因表达水平无显著变化,而长期(7 d)禁食后,下丘脑CRF基因表达水平显著下降,复投喂第9天则回升。综上,长期禁食可使鱼类CRF作为厌食欲因子抑制鱼类摄食。
2.2.2 环境因子对CRF的调控影响鱼类生长发育的所栖息的环境因子主要包括氧饱和度、氨气含量和渗透性能。氧气饱和程度越低、氨气增多以及渗透压上升可抑制鱼类摄食,CRF基因表达水平上升,提示CRF在不同外界环境处理下可作为厌食欲因子调控鱼类摄食。
短期缺氧下可使CRF在鱼类上发挥厌食欲因子的作用,而长期缺氧下,CRF的厌食欲调节作用降低。Bernier等[28]使虹鳟处于50%或35%氧饱和度下24 h,结果显示前脑CRF基因表达水平和摄食量均下降,并与缺氧处理程度呈正相关,进一步在长期(72 h)缺氧处理后,前脑CRF基因表达水平下降幅度及其厌食欲调节作用呈降低的趋势。
在氨气处理中,尽管Wood[29]认为低水平的外源性氨气对鱼类摄食无显著影响,但是Ortega等[30]长期增加水中的氨气可引起虹鳟摄食量呈剂量依赖性减少,CRF基因表达水平增加。Craig等[31]发现和淡水区域的相比,海水区域的虹鳟脑CRF表达水平的上升,摄食量下降,提示渗透压上升CRF可作为厌食欲因子。
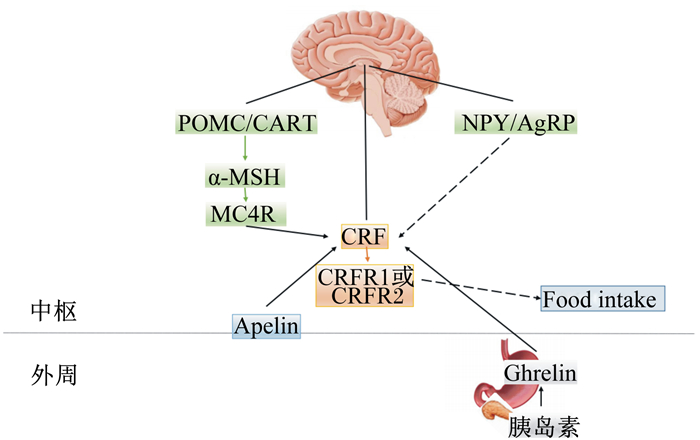
3 CRF调控鱼类摄食的作用机制CRF调控动物包括鱼类的摄食作用机制复杂,主要包括以下3种:1)CRF与受体结合发挥食欲调控作用;2)CRF通过HPI轴激活下游皮质醇的释放调控摄食;3)CRF通过和其他食欲调节子结合直接或间接调控摄食(图 2)。这3种机制之间也可相互作用共同影响CRF。
|
CRF:促皮质激素释放激素corticoliberin;CRFR1:促皮质激素释放激素受体1 corticoliberin receptor 1;CRFR2:促皮质激素释放激素受体2 corticoliberin receptor;POMC:阿黑皮素原proopiomelanocortin;CART:可卡因和安非他明调节转录子Cocaine and amphetamine regulation transcripton;NPY:神经肽Y neuropeptide Y;AgRP:刺鼠相关蛋白agouti-related protein;α-MSH:α-促黑激素α melanocyte stimulaing hormone;MC4R:黑皮素4受体melanocortin 4 receptor;Ghrelin:饥饿素;Food intake:采食。实线箭头表示促进作用solid arrow indicated promotion effect;虚线箭头表示抑制作用dashed arrow indicated inhibition effect。 图 2 CRF调控摄食机制 Figure 2 The mechanisms of CRF regulation on the feeding |
哺乳动物上,CRF可直接和CRF受体2(CRFR2)结合发挥食欲抑制作用,但是与CRF受体1(CRFR1)结合对摄食调控无影响。而鱼类上,CRF系统可能存在不同通路调控鱼类摄食。Vaughan等[32]报道CRF相关肽硬骨鱼紧张肽Ⅰ(urotensin Ⅰ,UⅠ)和CRF均可与CRFR2亲和发挥抑制摄食的作用,并且亲和力度同等。Bernier等[33]认为金鱼上中枢注射UⅠ,和中枢注射CRF组相比,更有效地减少机体摄食量,提示了在鱼类上CRF和UⅠ无选择性亲和CRFR2发挥厌食欲作用,或者CRF可能与CRFR1或CRFR2亲和调节鱼类摄食。
CRF与受体结合的区域主要位于下丘脑视前核(NPO)和结节外侧核(NLT)[34]。而Arai等[35]在鲶鱼(Silurus asotus)脑干中也发现CRFR1少量存在,提示中枢系统CRF可通过参与调节胃肠运动的脑干神经元回路影响摄食。Cardoso等[36]在河豚(Fugu rubripes)肠道发现微量CRFR1,Martínez等[37]对小鼠外周注射CRF,均证明胃肠道系统存在微量CRF受体,并与CRF结合刺激肠动力变化调控机体摄食。
3.2 CRF激活HPI轴上的末端产物皮质醇来调控鱼类摄食在HPI轴上,由下丘脑释放的CRF刺激垂体释放ACTH,进一步激活肾上腺的糖皮质激素皮质醇直接调控鱼类摄食,或通过和其他内分泌调节途径互作以及胃肠道营养吸收间接调节鱼类摄食。
一些研究报道皮质醇作为糖皮质激素可抑制摄食。Gregory等[38]研究表明升高虹鳟血浆皮质醇水平,可抑制其摄食量。而皮质醇的增多可负反馈于CRF,使CRF引起的厌食欲的效果减小。进一步有研究报道显示,CRF可通过和其他内分泌途径互作调控皮质醇对摄食的影响。Bernier等[39]在金鱼上进行适量且慢性增加血浆皮质醇处理时,可使摄食量增加,前脑CRF基因表达水平降低,神经肽Y(NPY)基因表达水平升高,进一步增加皮质醇浓度的分解可使CRF基因表达水平降低,而对NPY基因表达无影响,摄食量有下降趋势。CRF引起糖皮质激素皮质醇的释放,可间接通过肠道营养吸收来影响摄食。Ducouret等[40]研究表明,糖皮质激素受体在鱼类胃肠道中存在,Vllette等[41]发现皮质醇对肠道钠/钾-ATP酶活性有提高作用。
3.3 CRF与其他食欲调节子结合调控鱼类摄食CRF与受体结合后可通过和中枢食欲调控神经系统互作,或通过血液循环和外周食欲调节因子互作传递饱腹感信号。CRF与中枢食欲调控神经系统调节因子包括下丘脑神经元阿黑皮素原(POMC)/可卡因和安非他明调节转录子(CART)、NPY/刺鼠相关蛋白(AgRP)、POMC多肽加工处理的可调控动物摄食的片段α-促黑激素(α-MSH)及黑皮素4受体(MC4R)相互作用调控鱼类摄食。CRF与外周食欲调控因子包括胃肠道分泌的饥饿素(ghrelin)和胰腺分泌的胰岛素三者之间互作调控鱼类摄食。CRF也可与脑肠肽(apelin)互作调控鱼类摄食。
3.3.1 CRF与POMC/CART或NPY/AgRP互作调控摄食在大鼠上通过体外和体内试验说明通过CRF系统可上调POMC/CART参与厌食欲作用,并且POMC/CART通过CRF系统激活HPI轴[42]。而NPY/AgRP可下调CRF调节摄食,NPY/AgRP与CRF之间的互作也和糖皮质激素紧密相关。Heinrichs等[43]对大鼠中枢共注射NPY和CRF受体拮抗剂αh-CRF(9-41),注射部位为下丘脑室旁核区域,摄食量增加;随后对通过糖皮质激素地塞米松处理后的大鼠,中枢注射NPY,结果显示室旁核的CRF基因表达下调,摄食量增加。在鱼类上的报道还较缺乏,目前仅在金鱼上有研究。在金鱼上,Bernier等[39]缓慢适量增加血浆皮质醇浓度,刺激NPY释放,抑制CRF基因表达,摄食量增加。
3.3.2 CRF与α-MSH和MC4R互作调控摄食CRF作为整合厌食欲信号的神经多肽,整合了POMC多肽加工处理的可调控动物摄食的片段α-MSH及其受体MC4R调控摄食[44]。CRF与黑皮素系统之间的互作在鱼类上目前仅在金鱼中有报道。Matsuda等[45]对金鱼中枢共注射α-MSH兴奋剂(MT Ⅱ)和αh-CRF(9-41),结果显示MT Ⅱ引起的食欲抑制效果减小。然而,共注射CRF和MC4R拮抗剂HS024,发现对金鱼摄食量无显著影响。进一步通过免疫组化分析检测出CRF和MSH神经元位于大脑处,其中α-MSH包含的神经纤维末梢和下丘脑区域的CRF神经元紧密结合。综上,提示了在鱼类上,α-MSH和MC4R通过CRF信号通路发挥厌食欲作用。
3.3.3 CRF与ghrelin和胰岛素互作调控摄食通过CRF信号途径可使外周食欲调节因子ghrelin抑制食欲,又因ghrelin参与糖代谢,所以CRF、ghrelin和胰岛素之间互作对动物摄食产生影响。ghrelin和CRF共同作用可使胰岛素敏感性降低。Solomon等[46]对大鼠预处理静脉注射0.5 mL ghrelin特异性抗体和非特异性抗体,然后对皮下组织单次注射生理盐水、胰岛素和2-脱氧葡萄糖,通过免疫组化方法检测出,胰岛素和ghrelin抗体共处理组的CRF阳性神经元比胰岛素处理组的更高,并且与无特异性抗体预处理组之间进行比较,胰岛素处理组和2-脱氧葡萄糖处理组的CRF c-fos(1种即刻早期基因)阳性神经元均显著高于生理盐水对照组,而摄食量与对照组相比却显著增加。在CRF厌食欲信号中通过胰岛素处理出现反常的现象,说明低血液葡萄糖应激可能导致HPI轴激活补偿葡萄糖水平,刺激少量ACTH和皮质醇的生成,少量皮质醇可刺激动物摄食。
在鱼类上,Jönsson等[47]对虹鳟幼鱼分4组进行中枢注射,ghrelin组显著降低摄食量,αh-CRF(9-41)组对摄食量无显著影响,共注射组的摄食量和ghrelin组比较有升高的趋势,并与对照组的摄食量相当。此结果提示ghrelin通过CRF系统抑制鱼类摄食。
3.3.4 CRF与apelin互作调控摄食CRF与apelin对摄食调控的互作有少量报道。apelin可刺激CRF的释放,并通过CRF系统抑制哺乳动物摄食[48-49]。Lv等[49]禁食处理雄性小鼠24 h,分别中枢注射0.3、1.0和3.0 μg/kg apelin-13,对照组注射生理盐水,检测出试验组摄食量和对照组的比较呈剂量依赖性抑制;进一步对小鼠分成4组,与注射生理盐水对照组相比,αh-CRF(9-41)处理组的4 h摄食量无显著变化,apelin-13处理组的累积摄食量则极显著减少,而apelin-13和αh-CRF(9-41)共注射处理组与对照组比较摄食量无显著差异,与apelin-13处理组比较摄食量显著增加。CRF与脑肠肽apelin之间的互作目前在鱼类上还未见报道。
4 小结鱼类通过摄食为自身机体提供营养和能量,促进其生长发育和繁殖。摄食调控复杂且精细,通过中枢或者外周的厌食欲因子和增食欲因子互作调控机体摄食。CRF作为重要的中枢调控厌食欲因子,是动物摄食和能量代谢领域的研究热点之一。目前,CRF关于摄食调控和机制的研究主要集中在哺乳动物上,而鱼类上研究少。由于鱼类所处环境差异达,生理结构和机能差异较大,所以未来应在借鉴哺乳动物研究结果的基础上,深入探讨CRF对不同鱼类的摄食调节机制,为鱼类摄食调控和生产应用提供理论依据。
| [1] |
SAPER C B, CHOU T C, ELMQUISTl J K. The need to feed:homeostatic and hedonic control of eating[J]. Neuron, 2002, 36(2): 199-211. DOI:10.1016/S0896-6273(02)00969-8 |
| [2] |
田娟, 何艮, 麦康森, 等. 鱼类食欲调控研究进展[J]. 动物营养学报, 2016, 28(4): 984-998. |
| [3] |
VOLKOFF H. The neuroendocrine regulation of food intake in fish:a review of current knowledge[J]. Frontiers in Neuroscience, 2016, 10: 540. |
| [4] |
VALE W, SPIESS J, RIVIER C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin[J]. Science, 1981, 213(4514): 1394-1397. DOI:10.1126/science.6267699 |
| [5] |
SAFFRAN M, SCHALLY A V. The release of corticotrophin by anterior pituitary tissue in vitro[J]. Canadian Journal of Biochemistry and Physiology, 1955, 33(3): 408-415. DOI:10.1139/o55-054 |
| [6] |
FURUTANI Y, MORIMOTO Y, SHIBAHARA S, et al. Cloning and sequence analysis of cDNA for ovine corticotropin-releasing factor precursor[J]. Nature, 1983, 301(5900): 537-540. DOI:10.1038/301537a0 |
| [7] |
VANDENBORNE K, DE GROEF B, GEELISSEN S M, et al. Molecular cloning and developmental expression of corticotropin-releasing factor in the chicken[J]. Endocrinology, 2005, 146(1): 301-308. DOI:10.1210/en.2004-0608 |
| [8] |
OKAWARA Y, MORLEY S D, BURZIO L O, et al. Cloning and sequence analysis of cDNA for corticotropin-releasing factor precursor from the teleost fish Catostomus commersoni[J]. Proceedings of the National Academy of Sciences of the United States of America, 1988, 85(22): 8439-8443. DOI:10.1073/pnas.85.22.8439 |
| [9] |
BERNIER N J, LIN X W, PETER R E. Differential expression of corticotropin-releasing factor (CRF) and urotensin Ⅰ precursor genes, and evidence of CRF gene expression regulated by cortisol in goldfish brain[J]. General and Comparative Endocrinology, 1999, 116(3): 461-477. DOI:10.1006/gcen.1999.7386 |
| [10] |
ANDO H, HASEGAWA M, ANDO J, et al. Expression of salmon corticotropin-releasing hormone precursor gene in the preoptic nucleus in stressed rainbow trout[J]. General and Comparative Endocrinology, 1999, 113(1): 87-95. DOI:10.1006/gcen.1998.7182 |
| [11] |
DOYON C, GILMOUR K, TRUDEAU V, et al. Corticotropin-releasing factor and neuropeptide Y mRNA levels are elevated in the preoptic area of socially subordinate rainbow trout[J]. General and Comparative Endocrinology, 2003, 133(2): 260-271. DOI:10.1016/S0016-6480(03)00195-3 |
| [12] |
HUISING M O, METZ J R, VAN SCHOOTEN C, et al. Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response[J]. Journal of Molecular Endocrinology, 2004, 32(3): 627-648. DOI:10.1677/jme.0.0320627 |
| [13] |
VAN ENCKEVORT F H J, PEPELS P P L M, LEUNISSEN J A M, et al. Oreochromis mossambicus (tilapia) corticotropin-releasing hormone:cDNA sequence and bioactivity[J]. Journal of Neuroendocrinology, 2000, 12(2): 177-186. |
| [14] |
LU W Q, DOW L, GUMUSGOZ S, et al. Coexpression of corticotropin-releasing hormone and urotensin Ⅰ precursor genes in the caudal neurosecretory system of the euryhaline flounder (Platichthys flesus):a possible shared role in peripheral regulation[J]. Endocrinology, 2004, 145(12): 5786-5797. DOI:10.1210/en.2004-0144 |
| [15] |
CHANDRASEKAR G, LAUTER G, HAUPTMANN G. Distribution of corticotropin-releasing hormone in the developing zebrafish brain[J]. Journal of Comparative Neurology, 2007, 505(4): 337-351. DOI:10.1002/(ISSN)1096-9861 |
| [16] |
WANG T, ZHOU C W, YUAN D Y, et al. Schizothorax prenanti corticotropin-releasing hormone (CRH):molecular cloning, tissue expression, and the function of feeding regulation[J]. Fish Physiology and Biochemistry, 2014, 40(5): 1407-1415. DOI:10.1007/s10695-014-9935-6 |
| [17] |
MATSUDA K, MORIMOTO N, HASHIMOTO K, et al. Changes in the distribution of corticotropin-releasing factor (CRF)-like immunoreactivity in the larval bullfrog brain and the involvement of CRF in the cessation of food intake during metamorphosis[J]. General and Comparative Endocrinology, 2010, 168(2): 280-286. DOI:10.1016/j.ygcen.2010.01.004 |
| [18] |
MATSUDA K. Regulation of feeding behavior and psychomotor activity by corticotropin-releasing hormone (CRH) in fish[J]. Frontiers in Neuroscience, 2013, 7: 91. |
| [19] |
DE PEDRO N, ALONSO-GÓMEZ A L, GANCEDO B, et al. Role of corticotropin-releasing factor (CRF) as a food intake regulator in goldfish[J]. Physiology & Behavior, 1993, 53(3): 517-520. |
| [20] |
BERNIER N J, PETER R E. Appetite-suppressing effects of urotensin Ⅰ and corticotropin-releasing hormone in goldfish (Carassius auratus)[J]. Neuroendocrinology, 2001, 73(4): 248-260. DOI:10.1159/000054642 |
| [21] |
PARROTT R. Central administration of corticotropin releasing factor in the pig:effects on operant feeding, drinking and plasma cortisol[J]. Physiology & Behavior, 1990, 47(3): 519-524. |
| [22] |
YUAN P Q, WU S V, ELLIOTT J, et al. Expression of corticotropin releasing factor receptor type 1(CRF 1) in the human gastrointestinal tract and upregulation in the colonic mucosa in patients with ulcerative colitis[J]. Peptides, 2012, 38(1): 62-69. DOI:10.1016/j.peptides.2012.07.028 |
| [23] |
COLOMBO E, SANGIOVANNI E, DELL'AGLI M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract[J]. Evidence-Based Complementary and Alternative Medicine, 2013, 2013: 247145. |
| [24] |
TACHÉ Y, PERDUE M H. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function[J]. Neurogastroenterology & Motility, 2004, 16(Suppl.1): 137-142. |
| [25] |
PEPELS P P, VAN Helvoort H, Bonga S W E, et al. Corticotropin-releasing hormone in the teleost stress response:rapid appearance of the peptide in plasma of tilapia (Oreochromis mossambicus)[J]. Journal of Endocrinology, 2004, 180(3): 425-438. DOI:10.1677/joe.0.1800425 |
| [26] |
YADAWA A K, CHATURVEDI C M. Expression of stress hormones AVP and CRH in the hypothalamus of Mus musculus following water and food deprivation[J]. General and Comparative Endocrinology, 2016, 239: 13-20. DOI:10.1016/j.ygcen.2016.03.005 |
| [27] |
MARUYAMA K, MIURA T, UCHIYAMA M, et al. Relationship between anorexigenic action of pituitary adenylate cyclase-activating polypeptide (PACAP) and that of corticotropin-releasing hormone (CRH) in the goldfish, Carassius auratus[J]. Peptides, 2006, 27(7): 1820-1826. DOI:10.1016/j.peptides.2006.01.013 |
| [28] |
BERNIER N J, CRAIG P M. CRF-related peptides contribute to stress response and regulation of appetite in hypoxic rainbow trout[J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2005, 289(4): R982-R990. DOI:10.1152/ajpregu.00668.2004 |
| [29] |
WOOD C M. Dogmas and controversies in the handling of nitrogenous wastes:is exogenous ammonia a growth stimulant in fish?[J]. Journal of Experimental Biology, 2004, 207(12): 2043-2054. DOI:10.1242/jeb.00990 |
| [30] |
ORTEGA V A, RENNER K J, BERNIER N J. Appetite-suppressing effects of ammonia exposure in rainbow trout associated with regional and temporal activation of brain monoaminergic and CRF systems[J]. Journal of Experimental Biology, 2005, 208(10): 1855-1866. DOI:10.1242/jeb.01577 |
| [31] |
CRAIG P M, AL-TIMIMI H, ERNIER N J. Differential increase in forebrain and caudal neurosecretory system corticotropin-releasing factor and urotensin Ⅰ gene expression associated with seawater transfer in rainbow trout[J]. Endocrinology, 2005, 146(9): 3851-3860. DOI:10.1210/en.2005-0004 |
| [32] |
VAUGHAN J, DONALDSON C, BITTENCOURT J, et al. Urocortin, a mammalian neuropeptide related to fish urotensin Ⅰ and to corticotropin-releasing factor[J]. Nature, 1995, 378(6554): 287-292. DOI:10.1038/378287a0 |
| [33] |
BERNIER N J, PETER R E. The hypothalamic-pituitary-interrenal axis and the control of food intake in teleost fish[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2001, 129(2/3): 639-644. |
| [34] |
BERTHOUD H R. Multiple neural systems controlling food intake and body weight[J]. Neuroscience & Biobehavioral Reviews, 2002, 26(4): 393-428. |
| [35] |
ARAI M, ASSIL I Q, ABOU-SAMRA A B. Characterization of three corticotropin-releasing factor receptors in catfish:a novel third receptor is predominantly expressed in pituitary and urophysis[J]. Endocrinology, 2001, 142(1): 446-454. DOI:10.1210/endo.142.1.7879 |
| [36] |
CARDOSO J C R, POWER D M, ELGA R, et al. Isolation and characterisation of the corticotropin releasing factor receptor 1(CRFR1) gene in a teleost fish, Fugu rubripes[J]. DNA Sequence, 2003, 14(3): 215-218. DOI:10.1080/1042517031000112624 |
| [37] |
MARTÍNEZ V, WANG L X, RIVIER J E, et al. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortinⅡ, and urocortin Ⅲ on gastric emptying and colonic transit in mice:role of CRF receptor subtypes 1 and 2[J]. Journal of Pharmacology and Experimental Therapeutics, 2002, 301(2): 611-617. DOI:10.1124/jpet.301.2.611 |
| [38] |
GREGORY T R, WOOD C M. The effects of chronic plasma cortisol elevation on the feeding behaviour, growth, competitive ability, and swimming performance of juvenile rainbow trout[J]. Physiological and Biochemical Zoology, 1999, 72(3): 286-295. DOI:10.1086/316673 |
| [39] |
BERNIER N J, BEDARD N, PETER R E. Effects of cortisol on food intake, growth, and forebrain neuropeptide Y and corticotropin-releasing factor gene expression in goldfish[J]. General and Comparative Endocrinology, 2004, 135(2): 230-240. DOI:10.1016/j.ygcen.2003.09.016 |
| [40] |
DUCOURET B, TUJAGUE M, ASHRAF J, et al. Cloning of a teleost fish glucocorticoid receptor shows that it contains a deoxyribonucleic acid-binding domain different from that of mammals[J]. Endocrinology, 1995, 136(9): 3774-3783. DOI:10.1210/endo.136.9.7649084 |
| [41] |
VLLETTE P A, YOUNG G. Tissue culture of sockeye salmon intestine:functional response of Na+-K+-ATPase to cortisol[J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2005, 288(6): R1598-R1605. DOI:10.1152/ajpregu.00741.2004 |
| [42] |
SMITH S M, VAUGHAN J M, DONALDSON C J, et al. Cocaine-and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism[J]. Endocrinology, 2004, 145(11): 5202-5209. DOI:10.1210/en.2004-0708 |
| [43] |
HEINRICHS S C, MENZAGHI F, PICH E M, et al. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y[J]. Brain Research, 1993, 611(1): 18-24. DOI:10.1016/0006-8993(93)91771-J |
| [44] |
BAZHAN N, ZELENA D. Food-intake regulation during stress by the hypothalamo-pituitary-adrenal axis[J]. Brain Research Bulletin, 2013, 95: 46-53. DOI:10.1016/j.brainresbull.2013.04.002 |
| [45] |
MATSUDA K, KOJIMA K, SHIMAKURA S I, et al. Corticotropin-releasing hormone mediates α-melanocyte-stimulating hormone-induced anorexigenic action in goldfish[J]. Peptides, 2008, 29(11): 1930-1936. DOI:10.1016/j.peptides.2008.06.028 |
| [46] |
SOLOMON A, DE FANTI B A, MARTÍNEZ A M. Peripheral ghrelin participates in glucostatic feeding mechanisms and in the anorexigenic signalling mediated by CART and CRF neurons[J]. Nutritional Neuroscience, 2005, 8(5/6): 287-295. |
| [47] |
JÖNSSON E, KAIYA H, BJÖRNSSON B T. Ghrelin decreases food intake in juvenile rainbow trout (Oncorhynchus mykiss) through the central anorexigenic corticotropin-releasing factor system[J]. General and Comparative Endocrinology, 2010, 166(1): 39-46. DOI:10.1016/j.ygcen.2009.11.001 |
| [48] |
TAHERI S, MURPHY K, COHEN M, et al. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats[J]. Biochemical and Biophysical Research Communications, 2002, 291(5): 1208-1212. DOI:10.1006/bbrc.2002.6575 |
| [49] |
LV S Y, YANG Y J, QIN Y J, et al. Central apelin-13 inhibits food intake via the CRF receptor in mice[J]. Peptides, 2012, 33(1): 132-138. DOI:10.1016/j.peptides.2011.11.011 |




