2. 重庆市璧山区农业委员会, 重庆 402670;
3. 重庆市水产技术推广站, 重庆 401147
2. Bishan District Agriculture Commission, Chongqing 402670, China;
3. Fisheries Technology Extension Station of Chongqing, Chongqing 401147, China
蛋白质源是水产饲料重要的组成部分,严重影响饲料成本。因此,研究鱼类营养代谢机制,寻找鱼粉蛋白质替代源,是营养学家长期致力的方向。近年来,食品加工副产物引起了饲料加工者的广泛关注[1]。豆渣(SR)是豆浆或豆腐加工过程的副产品,在大豆消费量高的亚洲国家每年都有大量产出。目前,中国豆渣的年产量已超过280万t[2],仅重庆市梁平区礼让镇豆渣的年产量就在万吨以上。我国豆渣资源广阔,具有良好的开发利用前景。
豆渣富含蛋白质、脂肪、纤维、矿物质以及单糖和低聚糖,同时还富含膳食纤维[2],这些营养成分因大豆品种和加工方法不同而有所差异[3]。豆渣是人类优质食品,具有多种功效,如抗氧化活性[4]、预防心血管疾病[5]、减少肝脏脂肪沉积、维持机体健康[6-7]等。已有研究表明,豆渣可以作为反刍动物[8]、猪[9]和肉鸡[10]饲粮的蛋白质源。然而,有关豆渣在鱼类上应用的研究较少[11],可能是其干燥成本以及含有纤维、植酸和其他抗营养因子等因素所致[2]。研究发现,豆渣通过乳酸杆菌、芽孢杆菌、黄曲霉菌或酵母菌等发酵后可以提高其营养价值[12-13]。但迄今为止,未见发酵豆渣在建鲤(Cyprinus carpio var. Jian)上的研究报道。为此,本试验采用固态发酵豆渣,研究其对建鲤生长性能、体组成、血浆生化指标和抗氧化能力的影响,旨在为豆渣的利用提供理论依据。
1 材料与方法 1.1 试验饲料豆渣购自重庆市梁平区,为豆筋加工副产物。豆渣采用枯草芽孢杆菌、乳酸菌和酵母菌混合菌进行固态发酵(发酵pH为7.0,温度为28 ℃,时间为72 h),参考Tang等[14]和Li等[15]的方法制成发酵豆渣。豆渣和发酵豆渣的常规成分和氨基酸含量见表 1。
|
|
表 1 豆渣和发酵豆渣的常规成分和氨基酸含量(干物质基础) Table 1 Conventional component and amino acid contents of soybean residue and fermentedsoybean residue (DM basis) |
以鱼粉、豆粕、菜籽粕和棉籽蛋白为主要蛋白质源,以豆油为脂肪源配制基础饲料。在基础饲料中分别添加0(对照组)、6%、12%、18%和24%的发酵豆渣,通过调整豆粕的用量来平衡粗蛋白质含量,配制成5种等氮等脂的试验饲料(分别表示为FSR0、FSR6、FSR12、FSR18、FSR24),其组成及营养水平见表 2。各饲料原料粉碎过80目筛,采取逐级稀释法混合均匀,制成粒径为2.0 mm的颗粒饲料,风干后放入4 ℃冰箱中保存备用。
|
|
表 2 试验饲料组成及营养水平(风干基础) Table 2 Composition and nutrient levels of test diets (air-dry basis) |
选用当年培育的体质健壮、规格整齐的建鲤(平均体质量为8.49 g)450尾,随机分成5组,每组设3个重复,每个重复放养30尾。在室内淡水循环水族缸(有效体积为250 L)中饲养建鲤9周,日投饲率为体重的3%~5%,每天08:00、12:30、17:00各投喂1次。水源为曝气自来水,试验期间水温为(26.2±0.5)℃,pH为7.3±0.5,溶解氧浓度>6.8 mg/L,氨氮浓度<0.48 mg/L,亚硝酸盐氮浓度<0.06 mg/L。
1.3 样品制备与分析饲养试验结束后,禁食24 h后称重,每个重复随机取3尾鱼作为全鱼样品,用于体组成的测定;每个重复随机取4尾鱼,用MS-222进行麻醉,测体长、体高,分离出内脏、肝胰脏,用于形体指标的测定;每个重复随机取5尾鱼,用一次性无菌注射器取鱼体尾静脉血,用肝素和草酸钾-氟化钠[用于测定血浆葡萄糖(Glu)含量]抗凝,立即在4 ℃条件下1 000×g离心10 min,收集血浆,-20 ℃保存备用。
饲料原料及全鱼样品均在105 ℃烘干至恒重,然后采用凯氏定氮法测定粗蛋白质含量,索氏抽提法测定粗脂肪含量,高温(550 ℃)灼烧法测定粗灰分含量。植酸含量的测定参照Vaintraub等[16]的方法。胰蛋白酶抑制因子含量的测定参照Smith等[17]的方法。饲料样品用6 mol/L的盐酸于110 ℃下水解22 h,采用4.6 mm×60 mm分析柱,在日立8800氨基酸分析仪上测定各氨基酸含量。
血浆代谢指标采用日立7100全自动生化分析仪测定,包括丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)、碱性磷酸酶(ALP)活性以及葡萄糖、总胆固醇(TC)和甘油三酯(TG)含量。血浆总蛋白(TP)和丙二醛(MDA)含量以及超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和谷胱甘肽过氧化物酶(GPx)活性采用南京建成生物工程研究所生产的试剂盒进行测定。蛋白质含量采用考马斯亮蓝法测定。
1.4 计算公式

|
式中:Wt(g)和W0(g)分别为终末体重和初始体重;t(d)为养殖试验天数;F(g)为尾均摄食量;Fp(%)为饲料粗蛋白质含量;Nt(尾)和N0(尾)分别为终末尾数和初始尾数;L(cm)为体长;Wv(g)为内脏重;Wh(g)为肝胰脏重。
1.5 数据处理与分析采用SPSS 17.0对所得数据进行单因素方差分析(one-way ANOVA),若差异达到显著水平,则进行Tukey’s多重比较,显著性水平为P<0.05。试验数据均以平均值±标准误表示。
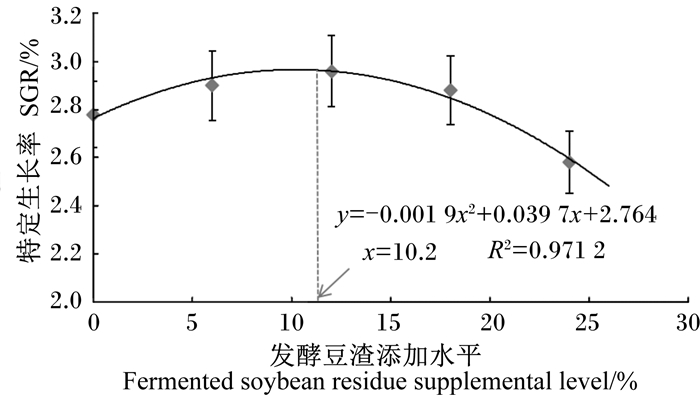
2 结果 2.1 发酵豆渣对建鲤生长性能的影响由表 3可知,随着发酵豆渣添加水平的增加,建鲤的终末体重、特定生长率和蛋白质效率呈先上升后下降的变化趋势,在发酵豆渣添加水平为12%时达到最大,而在添加水平为24%时达到最低,二者差异显著(P<0.05)。以二次曲线拟合发酵豆渣添加水平(x)与建鲤特定生长率(y)的关系(图 1),得到回归方程y=-0.001 9x2+0.039 7x+2.764(R2=0.971 2),由方程求得特定生长率最高时发酵豆渣添加水平为10.2%。相反,摄食率和饲料系数在发酵豆渣添加水平为12%时达到最低,显著低于其他各组(P<0.05)。各组建鲤的存活率均为100%。
|
|
表 3 发酵豆渣对建鲤生长性能的影响 Table 3 Effects of fermented soybean residue on growth performance of Jian carp |
|
图 1 饲料中发酵豆渣添加水平与建鲤特定生长率的关系 Figure 1 Relationship between dietary fermented soybean residue supplemental level and SGR of Jian carp |
由表 4可知,与对照组相比,发酵豆渣添加水平在12%及以上时,建鲤的脏体比显著降低(P<0.05)。随发酵豆渣添加水平的增加,肝体比呈先升高后下降趋势,在FSR12组有最大值,显著高于除FSR6组外的其他各组(P<0.05)。各组建鲤的肥满度以及全鱼水分、粗蛋白质、粗脂肪和粗灰分含量均无显著差异(P>0.05)。
|
|
表 4 发酵豆渣对建鲤形态指标和体组成的影响 Table 4 Effects of fermented soybean residue on morphological measurements and body composition of Jian carp |
由表 5可知,各组建鲤血浆丙氨酸氨基转移酶、天门冬氨酸氨基转移酶和碱性磷酸酶活性,以及葡萄糖和甘油三酯含量无显著差异(P>0.05)。FSR12组血浆总蛋白含量显著高于对照组、FSR6组和FSR24组(P<0.05),而与FSR18组无显著差异(P>0.05)。对照组血浆总胆固醇含量显著高于各试验组(P<0.05)。
|
|
表 5 发酵豆渣对建鲤血浆生化指标的影响 Table 5 Effects of fermented soybean residue on plasma biochemical indexes of Jian carp |
由表 6可知,各试验组血浆超氧化物歧化酶和过氧化氢酶活性显著高于对照组(P<0.05)。对照组血浆丙二醛含量显著于FSR12组、FSR18组和FSR24组(P<0.05),与FSR6组无显著差异(P>0.05)。FSR18组和FSR24组血浆过氧化氢酶/超氧化物歧化酶值显著高于对照组和其他试验组(P<0.05)。各试验组血浆谷胱甘肽过氧化物酶活性和谷胱甘肽过氧化物酶/超氧化物歧化酶值无显著差异(P>0.05)。
|
|
表 6 发酵豆渣对建鲤血浆抗氧化指标的影响 Table 6 Effects of fermented soybean residue on plasma antioxidant indexes of Jian carp |
大量研究表明,生物发酵能有效提高植物性蛋白质的营养价值。据报道,通过发酵可增加蛋白质和小肽含量来提高饲料营养价值[3, 18]。从表 1中数据可以看出,豆渣发酵后营养价值得到明显改善,养分含量得到提高,抗营养因子含量降低。豆渣作为家畜的蛋白质饲料,已经取得良好的生产效果[10, 19]。本研究结果也证实,建鲤饲料中发酵豆渣的添加水平为10.2%时可以有效改善其生长性能。这说明发酵豆渣作为鱼类饲料蛋白质源是可行的。研究显示,豆粕发酵后添加于饲粮中也可以显著改善猪[20]和肉鸡[21]的生长性能,同样,在大西洋鲑[22]、虹鳟[23]和日本沼虾[24]上也有类似的报道。这可能是因为发酵能够提高豆渣中营养物质含量[18],减少或降低抗营养因子含量[25-26],进而促进动物生长。此外,研究发现,豆粕发酵后添加于饲料中既不导致虹鳟肠道形态学发生变化[23],也不导致大西洋鲑肠道病变[22]。随后的研究进一步证实,饲料添加枯草芽孢杆菌发酵豆粕会显著改善石斑鱼肝脏和后肠的组织形态[27]。这些结果表明饲料发酵有利于动物消化道健康,该技术可应用于陆生家畜和水生动物饲料中。
本试验中,发酵豆渣添加水平达到24%时会显著降低建鲤的生长速度,但摄食率并没有降低,这表明发酵豆渣的适口性不是抑制建鲤生长的因素。一方面,发酵可以提高动物肠道中消化酶的活性,进而提高消化率;另一方面,发酵物中的枯草芽孢杆菌和乳酸菌也被视作水产养殖的益生菌,可以促进鱼类的生长和健康[28]。但豆渣发酵后粗纤维和抗营养因子(胰蛋白酶抑制因子)含量仍然很高,这是否是影响发酵豆渣在建鲤饲料中高剂量应用的限制因素,值得今后进一步研究。
本试验结果显示,发酵豆渣的添加水平会显著影响建鲤的肝体比,在添加水平为12%时肝体比达到最大。从试验鱼来看,建鲤的体重小于50 g,处于肝脏的发育时期。这表明发酵豆渣可以促进建鲤肝脏的健康发育,进而促进其生长。在小鼠[6]和仓鼠[7]上的研究发现,饲粮中添加豆渣能提高肝脏中甾醇14α-去甲基化酶(CYP51)和过氧化物酶体增殖剂激活受体α(PPARα)基因的表达,从而降低肝脏的脂肪沉积。众所周知,大豆蛋白中富含的异黄酮能减少肝脏脂肪的生成[29]。这些结果表明,豆渣或发酵豆渣有利于动物肝脏的健康。本试验中还发现,发酵豆渣可以降低建鲤的脏体比。从本试验结果来看,脏体比降低与肝体比无关,这可能是降低了鱼类肠系膜脂肪所致,值得我们关注和深入研究。
3.2 发酵豆渣对建鲤血浆生化指标和抗氧化能力的影响血浆生化指标可以反映鱼类的生理和营养状况。研究表明,豆渣可以调节糖尿病小鼠的血浆葡萄糖含量[30];同样,发酵桑叶可以改善大口黑鲈的血浆葡萄糖含量[31]。而本试验中发酵豆渣的添加对建鲤血浆葡萄糖含量并未产生显著影响,这可能与试验动物种类和试验材料有关。但发酵豆渣可以显著降低建鲤血浆总胆固醇含量,与在真鲷[32]上的研究结果一致。同样也发现,豆渣可以降低老鼠[33]和叙利亚仓鼠[7]血浆胆固醇含量。据报道,大豆蛋白能改善动物的血脂含量[30]。这些结果表明发酵豆渣参与调节动物的血脂代谢。此外,豆渣在预防高胆固醇血症[34]和高脂血症[7]方面也发挥重要作用,这与豆渣能增强肝脏β-氧化相关基因的表达有关[6]。相反,Lim等[35]发现罗非鱼的血液生化指标不受豆粕发酵过程的影响。因此,关于鱼类营养和临床医学之间的关系需要进一步研究。
研究表明,植物发酵产品可以增强鱼类的非特异性免疫反应,提高鱼类的抗氧化能力[21, 36]。已有研究指出,豆渣是抗氧化成分的潜在来源[2, 37-38]。本研究发现,饲料中添加发酵豆渣显著降低建鲤血浆丙二醛含量,升高血浆超氧化物歧化酶和过氧化氢酶活性。在黑鲷上的研究同样发现发酵豆粕能够提高鱼体的抗氧化能力[38],在对日本沼虾的研究中也有类似的结果[25],这可能与发酵豆渣或发酵豆粕含有异黄酮和抗氧化剂有关。关于鱼类营养与抗氧化能力的研究资料有限[39],豆渣富含抗氧化物质,今后有必要对其抗氧化能力进行深入研究,了解其作用机制。
4 结论① 发酵豆渣作为建鲤饲料的蛋白质源是可行的。
② 建鲤饲料中发酵豆渣的适宜添加水平为10.2%,可以改善建鲤的生长性能,过高的添加水平(24%)会抑制建鲤的生长,但可以提高机体的抗氧化能力。
| [1] |
SCIALABBA E H, JAN O, TOSTIVINT C, et al. Food wastage footprint:impacts on natural resources.Summary report[M]. [S.l.]: [s.n.], 2013.
|
| [2] |
LI B, QIAO M Y, LU F. Composition, nutrition, and utilization of okara (soybean residue)[J]. Food Reviews International, 2012, 28(3): 231-252. DOI:10.1080/87559129.2011.595023 |
| [3] |
WENG C V, YANG K L C A U, LIU S Q. Okara (soybean residue) biotransformation by yeast Yarrowia lipolytica[J]. International Journal of Food Microbiology, 2016, 235: 1-9. DOI:10.1016/j.ijfoodmicro.2016.06.039 |
| [4] |
ZHU Y P, FAN J F, CHENG Y Q, et al. Improvement of the antioxidant activity of Chinese traditional fermented okara (Meitauza) using Bacillus subtilis B2[J]. Food Control, 2008, 19(7): 654-661. DOI:10.1016/j.foodcont.2007.07.009 |
| [5] |
DEVI M K A, GONDI M, SAKTHIVELU G, et al. Functional attributes of soybean seeds and products, with reference to isoflavone content and antioxidant activity[J]. Food Chemistry, 2009, 114(3): 771-776. DOI:10.1016/j.foodchem.2008.10.011 |
| [6] |
KIM H S, YU O K, BYUN M S, et al. Okara, a soybean by-product, prevents high fat diet-induced obesity and improves serum lipid profiles in C57BL/6J mice[J]. Food Science and Biotechnology, 2016, 25(2): 607-613. DOI:10.1007/s10068-016-0085-8 |
| [7] |
VILLANUEVA M J, YOKOYAMA W H, HONG Y J, et al. Effect of high-fat diets supplemented with okara soybean by-product on lipid profiles of plasma, liver and faeces in Syrian hamsters[J]. Food Chemistry, 2011, 124(1): 72-79. DOI:10.1016/j.foodchem.2010.05.106 |
| [8] |
RAHMAN M M, ABDULLAH R B, KHADIJAH W E, et al. Feed intake, digestibility and growth performance of goats offered Napier grass supplemented with molasses protected palm kernel cake and soya waste[J]. Asian Journal of Animal and Veterinary Advances, 2013, 8(8): 527-534. |
| [9] |
KIM S W, VAN H E, JI F, et al. Fermented soybean meal as a vegetable protein source for nursery pigs:Ⅰ.Effects on growth performance of nursery pigs[J]. Journal of Animal Science, 2010, 88(1): 214-224. DOI:10.2527/jas.2009-1993 |
| [10] |
SINHA S, SINHA A, MAHTO D, et al. Study on the growth performance of the broiler after feeding of okara meal containing with or without non-starch polysaccharides degrading enzyme[J]. Veterinary World, 2013, 6(6): 325-328. DOI:10.5455/vetworld. |
| [11] |
WONG M H, TANG L Y, KWOK F S. The use of enzyme-digested soybean residue for feeding common carp[J]. Biomedical and Environmental Sciences, 1996, 9(4): 418-423. |
| [12] |
RASHAD M M, MAHMOUD A E, ABDOU H M, et al. Improvement of nutritional quality and antioxidant activities of yeast fermented soybean curd residue[J]. African Journal of Biotechnology, 2011, 10(28): 5504-5513. |
| [13] |
余永红, 邓泽元, 李静, 等. 面包串珠霉发酵对豆渣成分影响研究[J]. 食品科学, 2005(9): 129-131. |
| [14] |
TANG J W, SUN H, YAO X H, et al. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers[J]. Asian-Australasian Journal of Animal Sciences, 2012, 25(3): 393-400. DOI:10.5713/ajas.2011.11381 |
| [15] |
LI S, SANG Y, DAN Z, et al. Optimization of fermentation conditions for crude polysaccharides by Morchella esculenta, using soybean curd residue[J]. Industrial Crops & Products, 2013, 50(10): 666-672. |
| [16] |
VAINTRAUB I A, LAPTEVA N A. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing[J]. Analytical Biochemistry, 1988, 175(1): 227-230. DOI:10.1016/0003-2697(88)90382-X |
| [17] |
SMITH C, VAN MEGEN W, TWAALFHOVEN L, et al. The determination of trypsin inhibitor levels in foodstuffs[J]. Journal of the Science of Food & Agriculture, 2010, 31(4): 341-350. |
| [18] |
HONG K J, LEE C H, KIM S W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals[J]. Journal of Medicinal Food, 2004, 7(4): 430-435. DOI:10.1089/jmf.2004.7.430 |
| [19] |
HARTHAN L B, CHERNEY D J R. Okara as a protein supplement affects feed intake and milk composition of ewes and growth performance of lambs[J]. Animal Nutrition, 2017, 3(2): 171-174. DOI:10.1016/j.aninu.2017.04.001 |
| [20] |
KIM S S, PHAM M A, KIM K W, et al. Effects of microbial fermentation of soybean on growth performances, phosphorus availability, and antioxidant activity in diets for juvenile olive flounder (Paralichthys olivaceus)[J]. Food Science and Biotechnology, 2010, 19(6): 1605-1610. DOI:10.1007/s10068-010-0227-3 |
| [21] |
FENG J, LIU X, XU Z R, et al. Effects of Aspergillus oryzae 3.042 fermented soybean meal on growth performance and plasma biochemical parameters in broilers[J]. Animal Feed Science and Technology, 2007, 134(3/4): 235-242. |
| [22] |
REFSTIE S, SAHLSTRÖM S, BRÅTHEN E, et al. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar).[J]. Aquaculture, 2005, 246(1/2/3/4): 331-345. |
| [23] |
YAMAMOTO T, IWASHITA Y, MATSUNARI H, et al. Influence of fermentation conditions for soybean meal in a non-fish meal diet on the growth performance and physiological condition of rainbow trout Oncorhynchus mykiss[J]. Aquaculture, 2010, 309(1/2/3/4): 173-180. |
| [24] |
DING Z L, ZHANG Y X, YE J Y, et al. An evaluation of replacing fish meal with fermented soybean meal in the diet of Macrobrachium nipponense:growth, nonspecific immunity, and resistance to Aeromonas hydrophila[J]. Fish & Shellfish Immunology, 2015, 44(1): 295-301. |
| [25] |
WANG Y, LIU X T, WANG H L, et al. Optimization of processing conditions for solid-state fermented soybean meal and its effects on growth performance and nutrient digestibility of weanling pigs[J]. Livestock Science, 2014, 170: 91-99. DOI:10.1016/j.livsci.2014.07.020 |
| [26] |
REDDY N R, PIERSON M D. Reduction in antinutritional and toxic components in plant foods by fermentation[J]. Food Research International, 1994, 27(3): 281-290. DOI:10.1016/0963-9969(94)90096-5 |
| [27] |
SHIU Y L, HSIEH S L, GUEI W C, et al. Using Bacillus subtilis E20-fermented soybean meal as replacement for fish meal in the diet of orange-spotted grouper (Epinephelus coioides, Hamilton)[J]. Aquaculture Research, 2015, 46(6): 1403-1416. DOI:10.1111/are.2015.46.issue-6 |
| [28] |
NAYAK S K. Probiotics and immunity:a fish perspective[J]. Fish & Shellfish Immunology, 2010, 29(1): 2-14. |
| [29] |
TORRES N, TORRE-VILLALVAZO I, TOVAR A R. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders[J]. Journal of Nutritional Biochemistry, 2006, 17(6): 365-373. DOI:10.1016/j.jnutbio.2005.11.005 |
| [30] |
徐虹, 蔺新英, 王芸, 等. 豆渣粉对糖尿病小鼠血糖血脂及肝肾组织形态的影响[J]. 营养学报, 2000, 22(2): 171-174. |
| [31] |
赵鹏飞, 彭祥和, 陈拥军, 等. 高脂或低蛋白日粮中添加发酵桑叶对大口黑鲈生长、代谢与抗氧化能力的影响[J]. 淡水渔业, 2016, 46(6): 86-91. |
| [32] |
AZARM H M, LEE S M. Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli[J]. Aquaculture Research, 2014, 45(6): 994-1003. DOI:10.1111/are.2014.45.issue-6 |
| [33] |
MATSUMOTO K, WATANABE Y S, YOKOYAMA S I. Okara, soybean residue, prevents obesity in a diet-induced murine obesity model[J]. Bioscience, Biotechnology, and Biochemistry, 2007, 71(3): 720-727. DOI:10.1271/bbb.60563 |
| [34] |
ROSSI E A, CAVALLINI D C U, CARLOS I Z, et al. Intake of isoflavone-supplemented soy yogurt fermented with Enterococcus faecium lowers serum total cholesterol and non-HDL cholesterol of hypercholesterolemic rats[J]. European Food Research and Technology, 2008, 228(2): 275-282. DOI:10.1007/s00217-008-0932-9 |
| [35] |
LIM S J, LEE K J. A microbial fermentation of soybean and cottonseed meal increases antioxidant activity and gossypol detoxification in diets for Nile tilapia, Oreochromis niloticus[J]. Journal of the World Aquaculture Society, 2011, 42(4): 494-503. DOI:10.1111/j.1749-7345.2011.00491.x |
| [36] |
ASHIDA T, TAKEL Y, TAKAGAKI M, et al. The dietary effects of a fermented vegetable product on glutathione peroxidase activity and lipid peroxidation of Japanese flounder Paralichthys olivaceus[J]. Fisheries Science, 2006, 72(1): 179-184. DOI:10.1111/fis.2006.72.issue-1 |
| [37] |
AMIN I, MUKHRIZAH O. Antioxidant capacity of methanolic and water extracts prepared from food-processing by-products[J]. Journal of the Science of Food and Agriculture, 2010, 86(5): 778-784. |
| [38] |
JANKOWIAK L, TRIFUNOVIC O, BOOM R M, et al. The potential of crude okara for isoflavone production[J]. Journal of Food Engineering, 2014, 124: 166-172. DOI:10.1016/j.jfoodeng.2013.10.011 |
| [39] |
MOURENTE G, DÍAZ-SALVAGO E, BELL J G, et al. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata, L.) fed dietary oxidised oil:attenuation by dietary vitamin E[J]. Aquaculture, 2002, 214(1/2/3/4): 343-361. |




