我国传统的仔猪断奶时间为56日龄左右,而现代生猪养殖为了追求生产效率,仔猪早期断奶已成为规模化猪场的普遍生产方式,一般在21~35日龄时断奶[1-2]。然而,35日龄前仔猪消化道发育尚未健全,饲粮由液体母乳转为适口性较差的固体饲粮,使肠道内环境受到很大冲击。断奶伴随着转群运输、新猪舍环境适应、仔猪混养等,使仔猪发生断奶应激,引起采食量下降、消化不良、腹泻和生长缓慢等一系列“仔猪早期断奶综合征”[3]。因此,选择合适的断奶时间,平衡母猪流转效率和仔猪健康,对于猪场的综合效益尤为重要。
肠道不仅是动物吸收营养的主要场所,也是体内重要的免疫器官[4]。断奶应激严重损伤仔猪肠道形态和功能,导致肠黏膜屏障受损,引起肠道形态发生变化[5]、肠道通透性增加[6-8],极大地影响其未来的生产性能和免疫功能。基于肠道功能发育规律是选择和优化仔猪断奶时间的关键基础。目前研究多集中在不同断奶日龄(14~35日龄)对仔猪生长性能、消化器官和酶活性的影响[2, 9-10],但有关断奶后仔猪肠道形态和功能发育规律的研究较少。本试验旨在研究21~28日龄仔猪肠道结构、紧密连接蛋白和细胞因子的发育性变化以及21日龄断奶对仔猪肠道形态和功能的损伤,为合理选择早期断奶时间、制订预防断奶应激损伤策略提供新思路。
1 材料与方法 1.1 试验设计与试验饲粮采用2×3双因子完全随机试验设计,组别(哺乳组、断奶组)和日龄(22、24和28日龄)为2个主效应。选取6窝体况相近的健康大白仔猪,每窝为10~12头。21日龄时,每窝选取6头平均体重为(6.1±0.2) kg的仔猪,随机分为2组,分别为断奶组(于保育舍饲喂基础饲粮)和哺乳组(于原产床继续哺食母乳)。断奶仔猪单栏饲养,舍内温度和光照时间与哺乳仔猪保持一致,温度维持在30 ℃左右;自由饮水,采用湿拌料方式饲喂,每天08:00和16:00饲喂玉米-豆粕型基础饲粮。基础饲粮参照NRC(2012)营养标准配制成粉料,营养成分满足5~10 kg仔猪的营养需要量,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
分别于22、24和28日龄时,仔猪禁食12 h后,进行前腔静脉采血,肝素抗凝,静置1 h,4 000 r/min离心后制备血浆,-80 ℃保存。按照窝源一致的原则,每窝选取2头(哺乳组和断奶组各1头,即每组6头仔猪),放血处死后,剖开腹腔分离出空肠和回肠,采集空肠前端和回肠末端肠道,使用预冷的生理盐水轻轻冲洗后,手术刀刮取黏膜,分装于2 mL冻存管,液氮速冻后转移至-80 ℃保存;剪取约1 cm的空肠和回肠肠环,浸入4%多聚甲醛固定。
1.3 测定指标与方法 1.3.1 生长性能分别于22和28日龄时对仔猪称重,计算仔猪断奶后的平均日增重(按照生长至28日龄的12头仔猪计算)。每天观察仔猪的健康和腹泻情况,计算腹泻率。
腹泻率(%)=[试验期内仔猪腹泻头数/(试验天数×同组仔猪头数)]×100。
1.3.2 肠道形态将固定好的空肠和回肠肠环组织石蜡包埋后切片(3~4 μm),采用苏木精-伊红(HE)染色,中性树脂封片,光学显微镜下观察。Leica LAX软件拍照并对空肠、回肠的绒毛高度和隐窝深度作定量分析。
1.3.3 血浆和肠道黏膜的二胺氧化酶(DAO)活性肠道黏膜组织进行10%组织匀浆。血浆和肠道黏膜组织液的DAO活性采用试剂盒(南京建成生物工程研究所)测定。
1.3.4 肠道黏膜相关基因表达空肠和回肠黏膜总RNA的提取采用试剂盒(QIAGEN),并用微量分光光度计(Bio-Drop)测定总RNA的纯度和浓度。按照试剂盒(PrimeScriptTM RT Reagent Kit with gDNA Eraser,TaKaRa)操作步骤反转录合成cDNA。反应体系按照SYBR® Premix Ex TaqTM试剂盒说明书配制。荧光定量PCR反应条件:95 ℃预变性30 s;95 ℃变性10 s;60 ℃退火延伸30 s,共40个循环;熔解曲线的制作参照荧光定量PCR仪(Bio-Rad CFX 96)操作说明书进行。基因的mRNA表达量采用2-ΔΔCt方法计算,以β-肌动蛋白(β-actin)作为内参基因,引物见表 2,由英潍捷基贸易有限公司合成。
|
|
表 2 引物序列信息 Table 2 Primer sequence information |
采用JMP 10.0软件分析数据,生长性能采用t检验统计分析,其余指标采用2×3双因子统计分析,Tukey法进行多重比较。P < 0.05为差异显著,P < 0.01为差异极显著。
2 结果 2.1 21日龄断奶对仔猪生长性能的影响由表 3可知,2组仔猪的初重(断奶时体重)无显著差异(P>0.05)。与哺乳组相比,28日龄(断奶后第7天)时,断奶组仔猪的末重和平均日增重均极显著降低(P < 0.01)。断奶组仔猪断奶后第1~3天的腹泻率高于哺乳组,但无显著差异(P>0.05)。
|
|
表 3 21日龄断奶对仔猪生长性能的影响 Table 3 Effects of weaning at 21 days of age on growth performance of piglets |
由图 1可知,断奶组仔猪的空肠绒毛脱落、隐窝凹陷。由表 4可知,断奶组仔猪的空肠绒毛高度极显著低于哺乳组(P < 0.01)。组别和日龄对空肠隐窝深度和绒毛高度/隐窝深度有极显著交互作用(P < 0.01),断奶组的空肠隐窝深度极显著高于哺乳组(P < 0.01),哺乳组的空肠隐窝深度随着日龄的增加逐渐降低,而断奶组则逐渐增加;22日龄时,断奶组的空肠隐窝深度与哺乳组相比无显著差异(P>0.05);24和28日龄时,断奶组的空肠隐窝深度极显著高于哺乳组(P < 0.01)。断奶组的空肠绒毛高度/隐窝深度极显著低于哺乳组(P < 0.01),哺乳组28日龄时的空肠绒毛高度/隐窝深度极显著高于22和24日龄时(P < 0.01),而断奶组在不同日龄时无显著差异(P>0.05)。
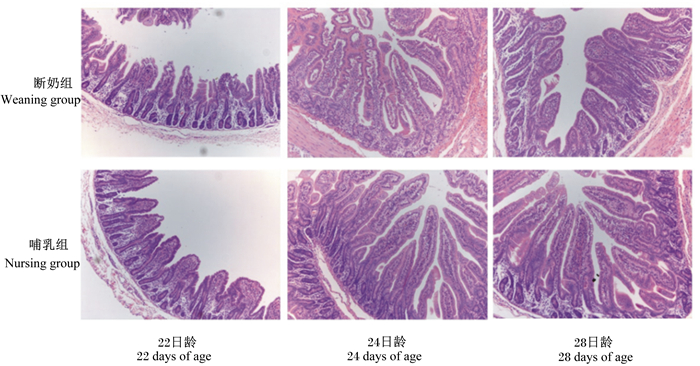
|
图 1 21日龄断奶对仔猪空肠形态的影响 Figure 1 Effects of weaning at 21 days of age on jejunum morphology of piglets (40×) |
|
|
表 4 21日龄断奶对仔猪空肠组织形态的影响 Table 4 Effects of weaning at 21 days of age on jejunum morphology of piglets |
由图 2可知仔猪回肠形态的变化。由表 5可知,与空肠不同,组别和日龄对仔猪的回肠绒毛高度有极显著交互作用(P < 0.01),哺乳组的回肠绒毛高度极显著高于断奶组(P < 0.01),哺乳组24日龄时的回肠绒毛高度极显著高于22日龄时(P < 0.01),而断奶组在不同日龄时无显著差异(P>0.05)。断奶组的回肠隐窝深度极显著高于哺乳组(P < 0.01),回肠隐窝深度随着日龄的增加逐渐增加(P < 0.05)。断奶组的回肠绒毛高度/隐窝深度极显著低于哺乳组(P < 0.01),2组的回肠绒毛高度/隐窝深度在不同日龄时均无显著差异(P>0.05)。
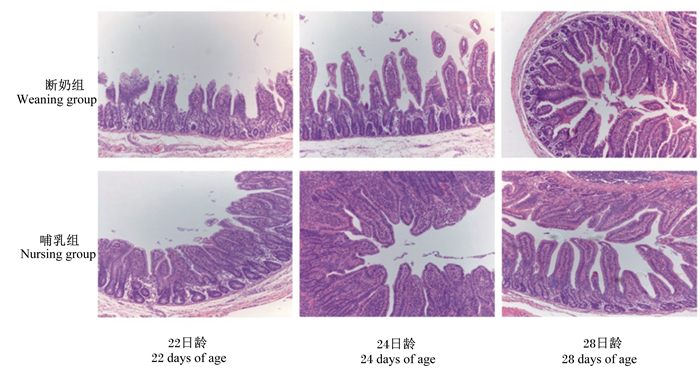
|
图 2 21日龄断奶对仔猪回肠形态的影响 Figure 2 Effects of weaning at 21 days of age on ileum morphology of piglets (40×) |
|
|
表 5 21日龄断奶对仔猪回肠组织形态的影响 Table 5 Effects of weaning at 21 days of age on ileum morphology of piglets |
由表 6可知,组别和日龄对仔猪的空肠黏膜DAO活性有显著交互作用(P < 0.05),断奶组的空肠黏膜DAO活性极显著低于哺乳组(P < 0.01)。2组的血浆DAO活性在不同日龄时无显著差异(P>0.05)。断奶组的回肠黏膜DAO活性显著低于哺乳组(P < 0.05),但2组的回肠黏膜DAO活性在不同日龄时无显著差异(P>0.05)。
|
|
表 6 21日龄断奶对仔猪血浆和肠道黏膜DAO活性的影响 Table 6 Effects of weaning at 21 days of age on the activity of DAO in plasma and intestinal mucosa of piglets |
由图 3可知,组别和日龄对仔猪空肠黏膜闭锁蛋白(occludin)的mRNA表达量有极显著交互作用(P < 0.01),哺乳组空肠黏膜occludin的mRNA表达量随着日龄的增加先升高后降低(P < 0.05),断奶组在不同日龄时无显著差异(P>0.05);与哺乳组相比,断奶组22日龄时空肠黏膜occludin的mRNA表达量有下降趋势(P=0.05),24日龄时显著降低(P < 0.05)。空肠黏膜闭锁小带蛋白1(ZO-1)的mRNA表达量随着日龄的增加先升高后降低(P < 0.05)。断奶组回肠黏膜ZO-1和occludin的mRNA表达量显著低于哺乳组(P < 0.05),24日龄时回肠黏膜ZO-1的mRNA表达量显著高于22日龄时(P < 0.05)。
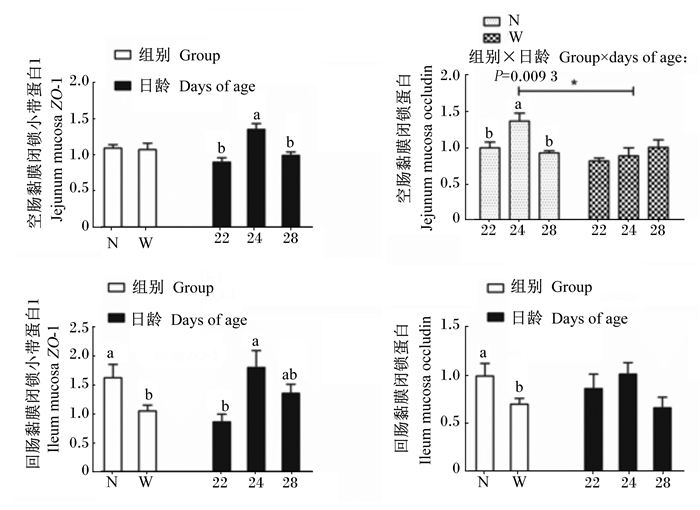
|
同一项目数据柱上标不同小写字母表示差异显著(P < 0.05),相同或无字母表示差异不显著(P>0.05)。*表示2组间差异显著(P < 0.05)。N代表哺乳组,W代表断奶组;22、24、28分别表示22、24、28日龄。下图同。 Value columns in the same item with different small letter superscripts mean significant difference (P < 0.05), while with the same or no letter superscripts mean no significant differences (P>0.05). * means significant difference between two groups. N represents the nursing group, and W represents the weaning group. 22, 24 and 28 mean 22, 24 and 28 days of age, respectively. The same as below. 图 3 21日龄断奶对仔猪肠道黏膜紧密连接蛋白mRNA表达量的影响 Figure 3 Effects of weaning at 21 days of age on mRNA expression of tight junction protein of intestinal mucosa of piglets |
由图 4可知,组别和日龄对仔猪空肠黏膜肿瘤坏死因子α(TNF-α)的mRNA表达量有显著交互作用(P < 0.05),断奶组24和28日龄时空肠黏膜TNF-α的mRNA表达量显著高于哺乳组(P < 0.05)。断奶组空肠黏膜白细胞介素10(IL-10)的mRNA表达量显著低于哺乳组(P < 0.05)。日龄对空肠黏膜细胞因子的mRNA表达量无显著影响(P>0.05)。
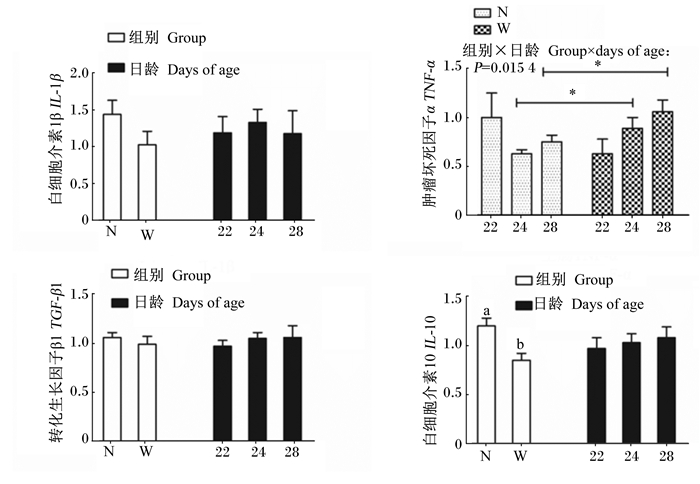
|
图 4 21日龄断奶对仔猪空肠黏膜细胞因子mRNA表达量的影响 Figure 4 Effects of weaning at 21 days of age on mRNA expression of cytokines of jejunum mucosa of piglets |
由图 5可知,与空肠黏膜不同,组别和日龄对仔猪回肠黏膜细胞因子的mRNA表达量无显著交互作用(P>0.05)。回肠黏膜白细胞介素1β(IL-1β)的mRNA表达量随着日龄的增加而降低(P < 0.05)。2组回肠黏膜细胞因子的mRNA表达量无显著差异(P>0.05)。
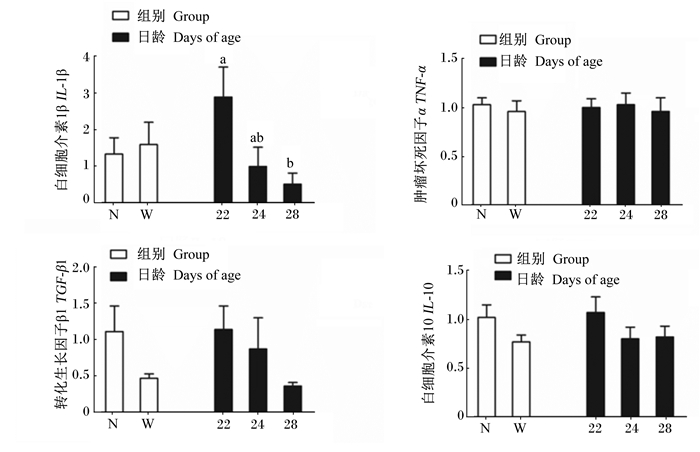
|
图 5 21日龄断奶对仔猪回肠黏膜细胞因子mRNA表达量的影响 Figure 5 Effects of weaning at 21 days of age on mRNA expression of cytokines of ileum mucosa of piglets |
仔猪消化道发育尚未健全,肠道免疫功能低下,微生物区系不稳定,断奶时会遭遇环境、营养、心理等多方面的挑战,造成仔猪断奶后生理机能紊乱。研究表明,肠道疾病往往随着断奶应激而出现[11-12],这表明断奶应激在疾病感染中起到至关重要的作用。本试验中,21日龄断奶诱发了仔猪应激,表现为体重显著下降,平均日增重降低了86.46%,断奶后第1天有16.67%存在腹泻情况,生长性能受到严重影响。肠道是断奶应激的主要损伤部位之一。空肠和回肠是小肠中营养物质吸收的重要肠段,其绒毛形态和功能发育是否良好,直接影响机体对营养物质的吸收。本试验中,仔猪断奶后空肠和回肠绒毛变短,并伴有脱落现象,同时隐窝凹陷,说明21日龄断奶严重影响了仔猪肠道的形态和发育。断奶后绒毛变短,减少了肠道吸收面积,是引起仔猪平均日增重极显著降低的重要原因之一。
肠道绒毛作为机体营养物质吸收的主要部位,小肠绒毛上皮细胞将消化道中的氨基酸、葡萄糖、无机盐等吸收进入血液,若此部位受损,会影响营养物质的吸收。DAO主要存在于哺乳动物肠道黏膜或绒毛上皮细胞中,是组胺等多胺物质进行代谢的重要调节酶,并在细胞增殖中发挥重要作用[13],肠道黏膜DAO活性与肠道黏膜绒毛高度、肠上皮细胞的完整性和成熟度有密切关系[14-16]。本试验结果表明,哺乳组仔猪的空肠黏膜DAO活性随着日龄的增加逐渐降低,断奶组却无显著变化,这表明哺乳期仔猪肠道处于不断发育的过程中,上皮细胞不断发生增殖分化,28日龄时空肠黏膜DAO活性降低说明仔猪肠道上皮细胞发育趋于稳定。而断奶组的仔猪回肠黏膜DAO活性显著低于哺乳组,说明21日龄断奶严重损坏仔猪肠道上皮细胞的完整性和成熟度,造成仔猪肠道绒毛变短。
肠道黏膜中肠上皮细胞、黏蛋白、肠道黏膜相关淋巴组织及其分泌的细胞因子、抗菌肽、微生物区系等形成了天然的屏障[17],维护肠道和机体健康。肠上皮细胞通过紧密连接等细胞连接形成机械屏障,外周血中DAO活性是肠上皮细胞机械屏障损伤的重要标志。正常生理条件下血浆DAO活性较低,当肠道黏膜受损时,DAO可以由肠黏膜进入血液循环导致血浆中DAO活性增加。本试验中,断奶后血浆DAO活性提高了10.67%,但由于动物个体差异大,未达到显著水平。断奶后回肠黏膜ZO-1、occludin的mRNA表达量显著降低,24日龄时空肠和回肠黏膜ZO-1的mRNA表达量显著高于22日龄时,这表明21日龄断奶,肠上皮细胞间紧密连接的结构蛋白表达在转录水平受到抑制,破坏了肠黏膜屏障,增加了肠道通透性。栾兆双[18]研究发现,仔猪断奶后紧密连接蛋白ZO-1、occludin的mRNA表达量显著降低,28日龄后逐渐升高;Weiler等[19]研究发现,在应激条件下,ZO-1、occludin的mRNA相对表达量降低,与本研究结果基本一致。28日龄时断奶仔猪空肠occludin的mRNA表达量基本达到哺乳仔猪水平,说明断奶7 d后,肠道黏膜损伤基本得到修复。
得益于肠上皮细胞形成的机械屏障,水、离子等可溶性小分子物质可以选择性地通过,而毒素、病原体等有害物质被阻止进入[17]。由于21日龄断奶造成了肠道屏障损伤,毒素、病原体等有害物质进入,可能激活肠黏膜中固有层淋巴组织、派伊氏结(PP结)、肠系膜淋巴结[20],产生免疫应答,分泌细胞因子。本试验研究发现,断奶组24和28日龄时空肠黏膜TNF-α的mRNA表达量显著高于哺乳组,而空肠黏膜IL-10的mRNA表达量显著低于哺乳组。Pié等[21]研究也发现,仔猪断奶后肠道促炎性细胞因子IL-1β、白细胞介素6(IL-6)和TNF-α的mRNA表达量迅速升高,肠道出现炎症反应。研究表明,肠黏膜的树突状细胞在黏膜中不断捕获由杯状细胞转运的抗原,分泌IL-10和转化生长因子β(TGF-β)并迁移至肠系膜集合淋巴结,活化初始T细胞为Treg,在免疫耐受中发挥至关重要的作用[22-23]。在人和小鼠中敲除抗炎性因子IL-10或其受体后会表现强烈的炎症反应,还使促炎性因子的分泌增加[24-26]。与空肠黏膜相比,断奶并未引起回肠黏膜细胞因子mRNA表达量的显著变化,表明在断奶应激条件下仔猪回肠的免疫稳定能力优于空肠,推测与回肠上富有黏膜相关淋巴组织相关。PP结作为黏膜淋巴相关组织的重要组成部分,主要集中在回肠[27-28],它是一个完整的执行免疫应答的功能场所,聚集着执行适应性免疫的CD4+T细胞、CD8+T细胞、B细胞和巨噬细胞等,当肠道中的抗原被传递到黏膜相关淋巴组织后,会激活B细胞引起体液免疫应答,同时也可被树突状细胞捕获后活化T细胞,引起更强烈的免疫应答。本试验中回肠黏膜免疫屏障表现出的强耐受力可能与此机制相关。
断奶时间的选择不仅要考虑母猪的流转,还要考虑仔猪的成活率。目前猪场常采用21或28日龄断奶。21~28日龄是仔猪快速发育阶段,断奶的窗口期应基于仔猪发育的生理状况来确定。本研究结果表明,21~28日龄时,哺乳仔猪肠道绒毛持续发育,表现为绒毛高度逐渐增长;肠道屏障功能在24日龄时最高,表现为肠上皮细胞间的紧密连接蛋白的mRNA表达量最高,肠黏膜促炎性因子TNF-α的mRNA表达量在24日龄时下降。综合这些指标,24日龄是仔猪肠道发育的关键点。21日龄断奶后,仔猪生长至24日龄时肠道绒毛损伤最为严重,表现为肠道绒毛最短且脱落现象最严重、血浆DAO活性最高、肠道紧密连接蛋白的mRNA表达量严重下降、促炎性因子的mRNA表达量上升。由此可知,24日龄是断奶应激损伤最严重的时间点。在这个时间节点的严重应激损伤一方面是因为应激反应发展的进程[18, 21],另一方面是因为仔猪肠道发育在24日龄前后的变化显著。尽管21日龄断奶后至28日龄,仔猪肠道形态、屏障功能均有所恢复,但推荐在24日龄时仔猪完成消化道发育进程后断奶。本研究还发现了断奶影响肠道屏障功能的重要黏膜细胞因子。关于饲料添加剂改善仔猪生长性能、维持肠道健康已有许多报道[29-31]。因此,在选择合理的断奶时间的同时,配合新型饲料添加剂促进抗炎性细胞因子的表达、加强肠道屏障功能,将有助于仔猪更好地度过断奶应激。
4 结论① 哺乳仔猪22~28日龄肠道发育趋于成熟。
② 21日龄断奶会破坏仔猪肠道上皮细胞的结构,肠道绒毛变短和脱落,影响营养物质吸收,造成仔猪腹泻,降低平均日增重。
③ 21日龄断奶抑制肠道紧密连接蛋白mRNA的表达,损伤屏障功能,导致肠道通透性增加,引起肠道炎症反应。
④ 24日龄是仔猪肠道发育的关键时间点,以24日龄后断奶为宜。
| [1] |
任慧波, 杜丽飞, 彭英林, 等. 早期断奶仔猪的饲养管理技术[J]. 猪业科学, 2011, 28(11): 110-111. DOI:10.3969/j.issn.1673-5358.2011.11.043 |
| [2] |
鄯来平. 庭院式养猪的仔猪断奶适宜时间的研究[J]. 中国畜牧兽医文摘, 2013(5): 74-75. |
| [3] |
CAMPBELL J M, CRENSHAW J D, POLO J. The biological stress of early weaned piglets[J]. Journal of Animal Science and Biotechnology, 2013, 4(1): 19. DOI:10.1186/2049-1891-4-19 |
| [4] |
LI X, AKHTAR S, CHOUDHRY M A. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury[J]. Biochimica et Biophysica Acta:Molecular Basis of Disease, 2012, 1822(2): 196-203. DOI:10.1016/j.bbadis.2011.09.019 |
| [5] |
HU C H, XIAO K, LUAN Z S, et al. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs[J]. Journal of Animal Science, 2013, 91(3): 1094-1101. DOI:10.2527/jas.2012-5796 |
| [6] |
MOESER A J, KLOK C V, RYAN K A, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2007, 292(1): G173-G181. DOI:10.1152/ajpgi.00197.2006 |
| [7] |
POHL C S, MEDLAND J E, MACKEY E, et al. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity[J]. Neurogastroenterology & Motility the Official Journal of the European Gastrointestinal Motility Society, 2017, 29(11): e13118. |
| [8] |
MOESER A J, RYAN K A, NIGHOT P K, et al. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2007, 293(2): G413-G421. DOI:10.1152/ajpgi.00304.2006 |
| [9] |
TSUKAHARA T, INOUE R, NAKATANI M, et al. Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine[J]. Animal Science Journal, 2016, 87(1): 67-75. DOI:10.1111/asj.12399 |
| [10] |
LELIVELD L M C, RIEMENSPERGER A V, GARDINER G E, et al. Effect of weaning age and postweaning feeding programme on the growth performance of pigs to 10 weeks of age[J]. Livestock Science, 2013, 157(1): 225-233. DOI:10.1016/j.livsci.2013.06.030 |
| [11] |
JONES P H, ROE J M, MILLER B G. Effects of stressors on immune parameters and on the faecal shedding of enterotoxigenic Escherichia coli in piglets following experimental inoculation[J]. Research in Veterinary Science, 2001, 70(1): 9-17. DOI:10.1053/rvsc.2000.0436 |
| [12] |
MELIN L, MATTSSON S, KATOULI M, et al. Development of post-weaning diarrhoea in piglets. Relation to presence of Escherichia coli strains and rotavirus[J]. Zoonoses and Public Health, 2004, 51(1): 12-22. |
| [13] |
WOLVEKAMP M C, DE BRUIN R W. Diamine oxidase:an overview of historical, biochemical and functional aspects[J]. Digestive Diseases, 1994, 12(1): 2-14. DOI:10.1159/000171432 |
| [14] |
DANG S, SHEN Y, YIN K, et al. TREM-1 promotes pancreatitis-associated intestinal barrier dysfunction[J]. Gastroenterology Research and Practice, 2012, 2012: 720865. |
| [15] |
TIAN R, TAN J T, WANG R L, et al. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis[J]. European Review for Medical & Pharmacological Sciences, 2013, 17(3): 349-355. |
| [16] |
徐建雄. 不同断奶日龄仔猪的氧化应激损伤及其机理研究[D]. 博士学位论文. 南京: 南京农业大学, 2014.
|
| [17] |
DE MEDINA F S, ROMERO-CALVO I, MASCARAQUE C, et al. Intestinal inflammation and mucosal barrier function[J]. Inflammatory Bowel Diseases, 2014, 20(12): 2394-2404. DOI:10.1097/MIB.0000000000000204 |
| [18] |
栾兆双. 断奶应激对仔猪肠上皮细胞紧密连接和p38 MAPK的影响[D]. 硕士学位论文. 杭州: 浙江大学, 2013.
|
| [19] |
WEILER F, MARBE T, SCHEPPACH W, et al. Influence of protein kinase C on transcription of the tight junction elements ZO-1 and occludin[J]. Journal of Cellular Physiology, 2005, 204(1): 83-86. DOI:10.1002/(ISSN)1097-4652 |
| [20] |
PEREZ-LOPEZ A, BEHNSEN J, NUCCIO S P, et al. Mucosal immunity to pathogenic intestinal bacteria[J]. Nature Reviews Immunology, 2016, 16(3): 135-148. DOI:10.1038/nri.2015.17 |
| [21] |
PIÉ S, LALLẼS J P, BLAZY F, et al. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets[J]. Journal of Nutrition, 2004, 134(3): 641-647. DOI:10.1093/jn/134.3.641 |
| [22] |
KONKEL J E, CHEN W J. Balancing acts:the role of TGF-beta in the mucosal immune system[J]. Trends in Molecular Medicine, 2011, 17(11): 668-676. DOI:10.1016/j.molmed.2011.07.002 |
| [23] |
SAKAGUCHI S, YAMAGUCHI T, NOMURA T, et al. Regulatory T cells and immune tolerance[J]. Cell, 2008, 133(5): 775-787. DOI:10.1016/j.cell.2008.05.009 |
| [24] |
BEGUE B, VERDIER J, RIEUX-LAUCAT F, et al. Defective IL-10 signaling defining a subgroup of patients with inflammatory bowel disease[J]. The American Journal of Gastroenterology, 2011, 106(8): 1544-1555. DOI:10.1038/ajg.2011.112 |
| [25] |
GLOCKER E O, KOTLARZ D, BOZTUG K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor[J]. New England Journal of Medicine, 2009, 361(21): 2033-2045. DOI:10.1056/NEJMoa0907206 |
| [26] |
KVHN R, LÖHLER J, RENNICK D, et al. Interleukin-10-deficient mice develop chronic enterocolitis[J]. Cell, 1993, 75(2): 263-274. DOI:10.1016/0092-8674(93)80068-P |
| [27] |
JUNG C, HUGOT J P, BARREAU F. Peyer's patches:the immune sensors of the intestine[J]. International Journal of Inflammation, 2010, 2010: 823710. |
| [28] |
VAN KRUININGEN H J, WEST A B, FREDA B J, et al. Distribution of Peyer's patches in the distal ileum[J]. Inflammatory Bowel Diseases, 2002, 8(3): 180-185. DOI:10.1097/00054725-200205000-00004 |
| [29] |
CHEN H, MAO X B, HE J, et al. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets[J]. British Journal of Nutrition, 2013, 110(10): 1837-1848. DOI:10.1017/S0007114513001293 |
| [30] |
ZHENG P, YU B, HE J, et al. Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets[J]. British Journal of Nutrition, 2013, 109(12): 2253-2260. DOI:10.1017/S0007114512004321 |
| [31] |
李玉鹏, 李海花, 王柳懿, 等. 丁酸梭菌对断奶仔猪生长性能、肠道屏障功能和血清细胞因子含量的影响[J]. 动物营养学报, 2017, 29(8): 2961-2968. |




