菊粉是植物中储备性多糖,主要来源于植物,如大丽花块茎、菊苣和菊芋的根部[1-2],但也可以从某些细菌或真菌中获得。大量的研究表明菊粉在平衡肠道微生物、调节免疫、调控机体脂质代谢和氧化还原系统等方面有着重要的作用,可作为脂肪、糖的替代品以及益生元[3],但目前其主要应用于食品和制药行业[4-5]。在Web of Science中检索“Inulin”后分析发现,动物学研究方向文献仅占所有文献(10 650篇)的1.36%,因此菊粉在畜牧生产中的研究尚处于起步阶段。随着抗生素滥用问题的日益严重及畜产品安全意识的不断增强,“无抗”养殖将是必然的趋势,而菊粉因其天然、无毒副、具有提高免疫力及抗氧化等重要作用,因而具备作为绿色植物源性添加剂的发展潜力。但目前菊粉在畜禽生产中的添加量及其作用机理尚不明确。因此,本文就菊粉的国内外研究现状,总结菊粉的主要生理功能及其在畜禽生产中的应用,以期为其进一步开发和应用提供理论参考。
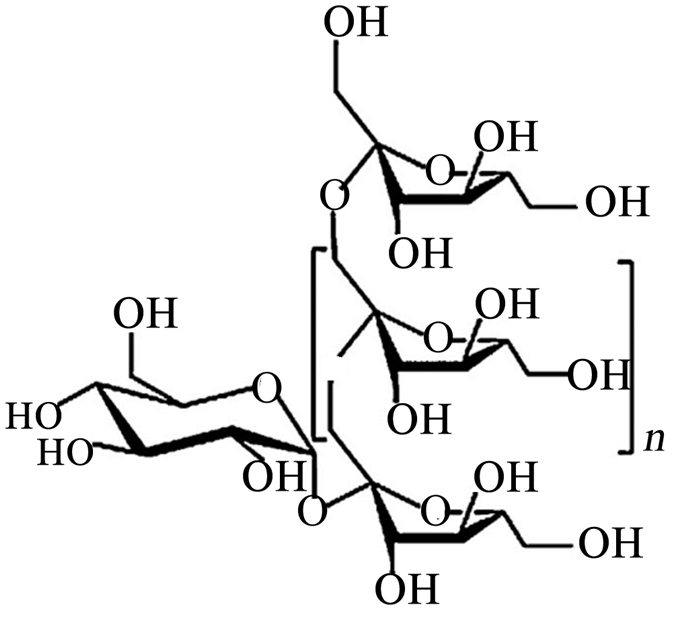
1 菊粉的理化性质及其提取工艺 1.1 理化性质菊粉是一类天然果聚糖的混合物。果聚糖是果糖单体通过β-(2, 1)-糖苷键连接而成并以葡萄糖单体终止的碳水化合物。菊粉分子约由31个β-D-呋喃果糖和1~2个吡喃菊糖残基聚合而成,其聚合度(degree of polymerization, DP)为2~100。β-构型使得菊糖型果聚糖不能被具有α-糖苷键特异性的单胃动物的消化酶水解,但是可被微生物降解[6]。菊粉可以被内切菊粉酶和外切菊粉酶水解。外切菊粉酶从链的非还原端去除末端果糖残基,而内切菊粉酶作用于内部链接[7-8]。菊粉水解后生成果糖和少量葡萄糖,DP少于10的菊粉称为果寡糖(FOS)[9]。菊粉的DP影响其重要的物理性质,如溶解性、热稳定性、甜味度和益生元活性等。菊粉的DP不仅取决于植物的来源、收获时间及其收获后贮存的条件,其提取工艺对DP也有很大的影响[8]。菊粉的分子式表示为GFn[3],其中G为终端葡萄糖单位,F代表果糖分子,n代表果糖单位数。菊粉的化学结构如图 1所示。菊苣来源的菊粉是一种白色粉末,呈透明状,无味,长链菊粉和FOS的甜度分别为蔗糖的10%[10]和30%~50%[11]。通常情况下,菊粉的溶解度随着DP的增加而降低。标准菊粉在室温下的溶解度仅为10%,而FOS在室温下的溶解度约为80%[12]。Wada等[13]研究报道了3种不同DP的菊粉在各温度下的溶解度变化,其中各温度下酶合成的菊粉溶解度最高,其次是DP=10~12的菊粉,最后是DP=23~25的菊粉。
|
图 1 菊粉的化学结构 Figure 1 The chemical structure of inulin[3] |
目前工业生产中,菊粉主要从菊芋和菊苣中提取。菊芋具有许多理想的生长性状,如耐寒耐旱、抗风沙、耐盐碱、繁殖力强、抗病虫害高等,可以在沙地和海滩耕种,近年来被认为是工业生产果糖和菊粉原料的重要来源之一[14]。从菊苣根部提取菊粉的工艺主要有传统水浸提法和超声辅助提取法,包括提取水溶性组分、纯化和干燥3个步骤。在提取水溶性组分时通常是将切割或磨碎的菊苣根在水中煮沸,而水的pH、固液比以及沸腾的时间等条件都会影响提取菊粉的DP[8, 15]。菊粉的纯化则是利用提取物中不同DP的溶解度差异来完成的,最终从菊苣根中提取的菊粉含量可以达到10%[16]。肖仔君等[17]得出用水浸提法从菊芋中提取菊粉的最佳条件为:提取时间100 min,浸提温度85 ℃,料液比1:15。除传统水浸提法外,超声辅助提取法也已经用于高产菊粉的提取,该方法的主要影响因素包括超声振幅、温度和时间。从牛蒡根提取菊粉时,振幅及振幅时间的提高均会提高菊粉产量,但是温度对其影响不大,最佳提取条件为:超声处理时间25 min,超声振幅83.22%,温度36.76 ℃[18]。需要注意的是,超声波会使菊粉分子碎片化,使其化学组成发生变化。后又有学者提出了一种从菊芋茎块中提取菊粉的新方法,确保菊粉提取量(每100 g块茎提取16.39 g菊粉)比传统水浸提法高出约14%[19]。该方法是将茎块用加压水洗涤以去油,自然烘干,在80 ℃下用热水处理10 min以使多酚氧化酶失活,然后将其悬浮于蒸馏水中,经过3次切块和萃取,最后对产生的过滤液进行澄清[19]。
2 菊粉的生理功能 2.1 作为益生元的益生效应通常情况下,肠道微生物中乳酸杆菌和双歧杆菌等有益菌对保护机体健康有着重要的作用。菊粉能够增加结肠中双歧杆菌和乳酸杆菌的数量和提高其活性等[20],从而保护肠道健康,这种作用被称为益生效应。文献中最普遍的思路是通过形成短链脂肪酸(SCFAs)的间接机制来解释菊粉型益生元的作用。菊粉和FOS在后肠道发酵产生SCFAs(如乙酸、丁酸和丙酸),使得肠道pH降低,从而刺激有益菌的增殖[21-22]。SCFAs不仅为宿主提供能量来源,还具有多种作用,如调节肠上皮细胞的增殖、分化和迁移,影响脂质代谢和糖代谢等。此外,SCFAs对维持肠道内环境稳定以及影响肠黏膜屏障起到关键作用。Rebolé等[23]在肉鸡饲粮中添加10 g/kg菊粉时,观察到盲肠中乳杆菌的数量增加;在提高菊粉添加水平达到20 g/kg时,小肠远端部分和盲肠中双歧杆菌和乳杆菌的数量增加。菊粉调节肠道微生物菌群的作用受菊粉自身的DP影响。Zhu等[24]发现FOS干预治疗小鼠肠道微生物效果好于菊粉,可能的原因是由于FOS和菊粉在被肠道微生物利用之前都要水解成单糖,而具有低聚合度的果聚糖经历相对快速的微生物发酵,而长链果聚糖更耐发酵并且在胃肠道经历的时间更长[25]。Patterson等[26]研究报道,短链和长链的菊粉能够不同程度地影响肠道菌群的生长和活性。研究报道,短链和长链菊粉以50:50的比例混合在发挥益生效应方面具有很好的优势,减少了有害气体的产生,同时增强了益生效应[27]。
2.2 免疫调节作用目前对于菊粉发挥免疫作用机制的研究多集中在2个方面:一方面,菊粉作为配体与Toll样受体2(TLR2)和Toll样受体4(TLR4)结合,刺激巨噬细胞、单核细胞等免疫细胞发挥作用,从而实现免疫调节的作用;另一方面,其发酵产物(SCFAs、H2)可作为信号传导分子,影响腺苷酸活化蛋白激酶(AMPK)活性和核因子-κB(NF-κB)信号传导途径。Vogt等[28]证明菊粉型果聚糖可通过TLR2调节人外周血单核细胞(HPBM)的活性,较低DP菊粉型果聚糖增加HPBM中白细胞介素-10(IL-10)与白细胞介素-12(IL-12)的分泌。有研究报道,在大鼠肠上皮细胞中,低聚糖通过激活TLR4并参与NF-κB信号传导来增强免疫应答,从而产生非益生效应[29]。Capitán-Cañadas等[30]也指出,在大鼠单核细胞中,益生元低聚糖通过激活TLR4直接调节促炎细胞因子的产生。Huang等[31]研究表明,在肉鸡饲粮中添加5~10 g/kg的菊粉会显著改善肉鸡21日龄的免疫功能,但是在后期(42日龄)改善效果不如前期,可能的原因是前期肉鸡肠道及免疫功能发育不完善,此时添加菊粉效果更加显著。
2.3 调节脂质代谢作用菊粉在肠道中的发酵产物SCFAs中,除部分丁酸盐作为能量来源被结肠细胞代谢利用外,其余的被运送到肝脏,丙酸盐主要作为糖原异生的前体[32],而醋酸盐和丁酸盐主要参与脂质的生物合成[33-34]。除了作为底物之外,SCFAs还可以作为信号分子被特定的G蛋白偶联受体(GPRs)感知并参与调节脂质和葡萄糖代谢[35]。大鼠饲粮中添加低聚果糖导致血清磷脂、甘油三酯和极低密度脂蛋白(VLDL)含量的显著降低。这主要是通过降低肝脏中脂肪生成酶、苹果酸酶、ATP柠檬酸裂合酶、乙酰辅酶A羧化酶、葡萄糖-6-磷酸1-脱氢酶等脂肪酸合成酶的活性来介导的[36-37]。Beylot等[38]认为,大鼠试验的研究结果已经清楚地证明了菊粉会降低甘油三酯的含量,较合理的解释是:菊粉型果聚糖可减少脂肪生成酶在肝脏表达并降低其活性,从而减少脂肪酸和甘油三酯的合成。Kim等[39]研究报道,菊粉通过增加胆汁酸的分泌和抑制3-羟基-3-甲基戊二酰辅酶A(HMG-CoA)还原酶(与胆固醇合成相关的限制酶)的活性来降低大鼠血液中胆固醇的含量。Yusrizal等[40]在肉鸡饲粮中添加菊粉(10 g/kg),发现血清胆固醇含量显著降低。Sang-Oh等[41]试验发现2个试验组(微囊化菊粉添加量分别为250、300 mg/kg)蛋鸡血液总胆固醇和甘油三酯的含量比对照组显著降低。但Velasco等[42]报道指出,菊粉的添加不会导致血清胆固醇、低密度脂蛋白胆固醇(LDL-C)和高密度脂蛋白胆固醇(HDL-C)含量的降低。Dewulf等[43]在喂食高脂肪饮食的小鼠中发现,与皮下脂肪组织中GPR43表达强相关的体脂肪增加,而补充菊粉可以减少GPR43的表达和脂肪的增加。不过也有人认为菊粉型果聚糖导致甘油三酯代谢的差异性改变,这取决于饲粮的类型[44]。而菊粉对血清胆固醇含量的降低作用则存在很多争议性,这仍待进一步研究。
2.4 促进矿物质吸收作用研究表明,菊粉添加到畜禽饲料中对钙、磷、锌、铜和铁的吸收代谢有积极影响。Chen等[45]研究报道,在蛋鸡饲粮中添加低聚果糖和菊粉增加了血清钙含量,并显著增加了胫骨中总灰分、钙和磷含量。Ortiz等[46]也同样证明,在肉仔鸡饲粮中添加菊粉后钙、锌和铜的保留率分别提高了18.4%、35.5%和46.6%,而对镁和铁的保留率无显著影响。目前关于菊粉对提高矿物质吸收的机制有较多种说法:菊粉在肠道中产生SCFAs和有机酸,造成肠道pH降低从而增加了矿物质的溶解度,SCFAs还可能通过离子交换系统影响钙的吸收[47]。此外,菊粉通过肠道微生物发酵产物对肠细胞起到增殖作用,从而增加肠道吸收表面积,增加钙结合蛋白的表达,释放骨调节因子[48]。Yasuda等[49]和Tako等[50]研究均表明,菊粉通过影响肠上皮细胞中铁转运蛋白、相关酶和铁蛋白编码基因的表达以及其对炎症相关基因的抑制,进而对铁代谢产生积极影响。研究表明,菊粉对机体矿物质的吸收利用受多种因素的影响,如动物的年龄、生理阶段等。当动物处于快速增长发育阶段或者雌激素分泌不足、动物对钙的需求更高时,菊粉的添加效果更明显。在发育期雄性大鼠试验中,5%~10%菊苣菊粉添加到饲粮中显著增强了大鼠全身骨矿物质含量(BMC)和骨密度(BMD)[51]。青春期女性给予FOS和菊粉3周,发现钙吸收增加了18%[52]。另有研究报道,饲粮中添加适量的菊粉可显著增加猪红细胞中血红蛋白含量(MCH)和红细胞比容(PCV)(MCH是机体中铁含量的有效评估指标),但随着添加量的增加,对MCH和PCV出现负面效应[20]。
3 菊粉在畜禽生产中的应用仔猪断奶应激往往会导致肠道形态结构以及肠道微生态的平衡被打破,易造成仔猪腹泻等疾病的发生。菊粉在肠道中能够被双歧杆菌和乳酸菌利用,产生SCFAs,降低肠道pH,刺激免疫球蛋白的产生,有助于竞争性排除病原体[53],从而保护肠道健康。Tako等[50]发现在仔猪饲粮中添加4%的菊粉,盲肠内容物中双歧杆菌和乳杆菌的数量以及十二指肠中黏蛋白基因的表达显著增加。Spencer等[54]研究表明,菊粉型果聚糖显著增加断奶仔猪肠绒毛高度和绒毛高度/隐窝深度。Pierce等[55]也发现在断奶仔猪饲粮中添加15 g/kg菊粉时,仔猪回肠pH显著下降,空肠绒毛高度显著增加。另外,Hansen等[56]在育肥猪饲粮中添加150 g/kg菊粉,发现可以减少氨排放量33%。Petkevičius等[57]研究发现,菊粉能显著减少猪粪便虫卵数,具有非常好的驱虫效果。
以上研究表明,菊粉型果聚糖对猪的肠道健康具有多种有益效果,而菊粉在家禽生产中也已得到广泛关注。在肉鸡饲粮中添加菊粉可以激活涉及免疫过程的基因和途径,从而调节肉鸡的免疫能力[58]。Sang-Oh等[59]在肉鸡饲粮中添加微胶囊化菊粉和抗生素进行对比,喂食微胶囊化菊粉组血清中免疫球蛋白G(IgG)、免疫球蛋白M(IgM)和免疫球蛋白A(IgA)的含量显著增加,0.20和0.25 g/kg微胶囊化菊粉组肉鸡血清中IgG含量分别比对照组提高155.6%和168.5%,分别比0.08 g/kg阿维霉素组提高125.1%和135.5%。此外,Nabizadeh等[60]发现肉鸡饲粮中添加5~10 g/kg菊粉导致总抗绵羊红细胞(SRBC)和IgG含量显著增加。有学者还指出,菊粉还可能通过刺激丁酸盐和黏蛋白的产生而潜在地改善肠道健康[24]。Huang等[31]的研究表明,菊粉添加量为5和10 g/kg时增加了21和42日龄肉鸡盲肠内容物的IgA含量;菊粉添加量为10和15 g/kg时增强了21和42日龄肉鸡空肠中黏蛋白mRNA的表达。然而,关于菊粉对家禽生长性能影响的文献报道结果不一。Ortiz等[46]在肉仔鸡饲粮中添加5~20 g/kg菊粉,结果发现,与对照组相比,肉仔鸡的生长性能和肠道(十二指肠、空肠、回肠和盲肠)的形态学数据均无显著变化。同样,也有其他学者发现菊粉添加到禽类饲粮中对生长性能没有产生显著影响[61-62]。相反,Rebolé等[23]试验发现,在第1阶段(7~21 d),添加10和20 g/kg菊粉组肉仔鸡的体重增加显著高于对照组,添加10 g/kg菊粉组肉仔鸡试验全期(7~35 d)总体重也显著增加。菊粉对家禽生长性能影响方面的报道不尽相同,可能是多种因素造成的,如菊粉产品质量、添加水平、家禽的品种以及饲养环境等。
4 小结综上所述,菊粉具有多种生理功能,有助于平衡畜禽肠道微生态、抑制病原微生物、调节机体免疫力等。但目前关于菊粉对畜禽生长性能的作用出现不同的研究结果,这可能与菊粉作为一种益生元,其有效性依赖于试验动物、添加量以及添加持续的时间等因素有关,故在全球“无抗养殖”的步伐走得越来越快的今天,迫切需要进一步研究菊粉在各种畜禽生产中的适宜添加量、对不同畜禽品种的作用效果和作用机制,以为菊粉在畜牧业生产中的应用及畜禽的绿色养殖提供理论基础。
| [1] |
PETKOVA N T, OGNYANOV M, TODOROVA M, et al. Ultrasound-assisted extraction and characterisation of inulin-type fructan from roots of elecampane (Inula helenium L.)[J]. Acta Scientifica Naturalis, 2015, 1(2): 225-235. |
| [2] |
HU Y X, ZHANG J, YU C W, et al. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group[J]. International Journal of Biological Macromolecules, 2014, 70: 44-49. DOI:10.1016/j.ijbiomac.2014.06.024 |
| [3] |
SHOAIB M, SHEHZAD A, OMAR M, et al. Inulin:properties, health benefits and food applications[J]. Carbohydrate Polymers, 2016, 147: 444-454. DOI:10.1016/j.carbpol.2016.04.020 |
| [4] |
VASSILEV D, PETKOVA N, KOLEVA M, et al. Ultrasound-assisted synthesis of sucrose and fructooligosaccharides esters as bio-plasticizers[J]. Journal of Renewable Materials, 2016, 4(1): 24-30. DOI:10.7569/JRM.2015.634125 |
| [5] |
LIU J, LU J F, WEN X Y, et al. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury[J]. International Journal of Biological Macromolecules, 2015, 72: 1479-1484. DOI:10.1016/j.ijbiomac.2014.09.066 |
| [6] |
MUDANNAYAKE D C, WIMALASIRI K M S, SILVA K F S T, et al. Comparison of properties of new sources of partially purified inulin to those of commercially pure chicory inulin[J]. Journal of Food Science, 2015, 80(5): C950-C960. DOI:10.1111/jfds.2015.80.issue-5 |
| [7] |
FLAMM G, GLINSMANN W, KRITCHEVSKY D, et al. Inulin and oligofructose as dietary fiber:a review of the evidence[J]. Critical Reviews in Food Science and Nutrition, 2001, 41(5): 353-362. DOI:10.1080/20014091091841 |
| [8] |
DE OLIVEIRA A J B, GONÇALVES R A C, CHIERRITO T P, et al. Structure and degree of polymerisation of fructooligosaccharides present in roots and leaves of Stevia rebaudiana (Bert.) Bertoni[J]. Food Chemistry, 2011, 129(2): 305-311. DOI:10.1016/j.foodchem.2011.04.057 |
| [9] |
RONKART S N, BLECKER C, FOURMANOIR H, et al. Isolation and identification of inulooligosaccharides resulting from inulin hydrolysis[J]. Analytica Chimica Acta, 2007, 604(1): 81-87. DOI:10.1016/j.aca.2007.07.073 |
| [10] |
VALLURU R, VAN DER ENDE W. Plant fructans in stress environments:emerging concepts and future prospects[J]. Journal of Experimental Botany, 2008, 59(11): 2905-2916. DOI:10.1093/jxb/ern164 |
| [11] |
KAUR N, GUPTA A K. Applications of inulin and oligofructose in health and nutrition.[J]. Journal of Biosciences, 2002, 27(7): 703-714. DOI:10.1007/BF02708379 |
| [12] |
FRANCK A. Technological functionality of inulin and oligofructose[J]. British Journal of Nutrition, 2002, 87(Suppl.2): S287-S291. |
| [13] |
WADA T, SUGATANI J, TERADA E, et al. Physicochemical characterization and biological effects of inulin enzymatically synthesized from sucrose[J]. Journal of Agricultural and Food Chemistry, 2005, 53(4): 1246-1253. DOI:10.1021/jf048711u |
| [14] |
LI S Z, CHAN-HALBRENDT C. Ethanol production in (the) People's republic of China:potential and technologies[J]. Applied Energy, 2009, 86(Suppl.1): S162-S169. |
| [15] |
PANCHEV I, DELCHEV N, KOVACHEVA D, et al. Physicochemical characteristics of inulins obtained from Jerusalem artichoke (Helianthus tuberosus L.)[J]. European Food Research and Technology, 2011, 233(5): 889-896. DOI:10.1007/s00217-011-1584-8 |
| [16] |
COUSSEMENT P A A. Inulin and oligofructose:safe intakes and legal status[J]. The Journal of Nutrition, 1999, 129(7): 1412S-1417S. DOI:10.1093/jn/129.7.1412S |
| [17] |
肖仔君, 朱定和, 王小红, 等. 菊芋中菊粉提取工艺的研究[J]. 现代食品科技, 2013(2): 315-318. |
| [18] |
MILANI E, KOOCHEKI A, GOLIMOVAHHED Q A. Extraction of inulin from burdock root (Arctium lappa) using high intensity ultrasound[J]. International Journal of Food Science & Technology, 2011, 46(8): 1699-1704. |
| [19] |
LI B, MENG X J, SUN L W. Isolation, chemical characterization and in vitro antioxidant activities of polysaccharides from Aconitum coreanum[J]. Journal of Medicinal Plants Research, 2012(7): 1353-1360. |
| [20] |
SAMANTA A K, SENANI S, KOLTE A P, et al. Effect of prebiotic on digestibility of total mixed ration[J]. The Indian Veterinary Journal, 2012, 89(1): 41-42. |
| [21] |
TARINI J, WOLEVER T M S. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects[J]. Applied Physiology, Nutrition, and Metabolism, 2010, 35(1): 9-16. DOI:10.1139/H09-119 |
| [22] |
RÍOS-COVIÁN D, RUAS-MADIEDO P, MARGOLLES A, et al. Intestinal short chain fatty acids and their link with diet and human health[J]. Frontiers in Microbiology, 2016, 7: 185. |
| [23] |
REBOLÉ A, ORTIZ L T, RODRÍGUEZ M L, et al. Effects of inulin and enzyme complex, individually or in combination, on growth performance, intestinal microflora, cecal fermentation characteristics, and jejunal histomorphology in broiler chickens fed a wheat-and barley-based diet[J]. Poultry Science, 2010, 89(2): 276-286. DOI:10.3382/ps.2009-00336 |
| [24] |
ZHU L M, QIN S, ZHAI S X, et al. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice[J]. FEMS Microbiology Letters, 2017, 364(10). DOI:10.1093/femsle/fnx075 |
| [25] |
VAN DE WIELE T, BOON N, POSSEMIERS S, et al. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects[J]. Journal of Applied Microbiology, 2007, 102(2): 452-460. |
| [26] |
PATTERSON J K, YASUDA K, WELCH R M, et al. Supplemental dietary inulin of variable chain lengths alters intestinal bacterial populations in young pigs[J]. The Journal of Nutrition, 2010, 140(12): 2158-2161. DOI:10.3945/jn.110.130302 |
| [27] |
ARCIA P L, COSTELL E, TÁRREGA A, et al. Inulin blend as prebiotic and fat replacer in dairy desserts:optimization by response surface methodology[J]. Journal of Dairy Science, 2011, 94(5): 2192-2200. DOI:10.3168/jds.2010-3873 |
| [28] |
VOGT L, RAMASAMY U, MEYER D, et al. Immune modulation by different types of β2→1-fructans is Toll-like receptor dependent[J]. PLoS One, 2013, 8(7): e68367. DOI:10.1371/journal.pone.0068367 |
| [29] |
ORTEGA-GONZÁLEZ M, OCÓN B, ROMERO-CALVO I, et al. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB[J]. Molecular Nutrition & Food Research, 2014, 58(2): 384-393. |
| [30] |
CAPITÁN-CAÑADAS F, ORTEGA-GONZÁLEZ M, GUADIX E M, et al. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4[J]. Molecular Nutrition & Food Research, 2014, 58(5): 1098-1110. |
| [31] |
HUANG Q Q, WEI Y N, LV Y J, et al. Effect of dietary inulin supplements on growth performance and intestinal immunological parameters of broiler chickens[J]. Livestock Science, 2015, 180: 172-176. DOI:10.1016/j.livsci.2015.07.015 |
| [32] |
ROY C C, KIEN C L, BOUTHILLIER L, et al. Short-chain fatty acids:ready for prime time?[J]. Nutrition in Clinical Practice, 2006, 21(6): 351-366. |
| [33] |
DEN BESTEN G, LANGE K, HAVINGA R, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2013, 305(12): G900-G910. DOI:10.1152/ajpgi.00265.2013 |
| [34] |
RÍOS-COVIÁN D, RUAS-MADIEDO P, MARGOLLES A, et al. Intestinal short chain fatty acids and their link with diet and human health[J]. Frontiers in Microbiology, 2016, 7: 185. |
| [35] |
DE BESTEN G, VAN EUNEN K, GROEN A K, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism[J]. Journal of Lipid Research, 2013, 54(9): 2325-2340. DOI:10.1194/jlr.R036012 |
| [36] |
FIORDALISO M, KOK N, DESAGER J P, et al. Dietary oligofructose lowers triglycerides, phospholipids and cholesterol in serum and very low density lipoproteins of rats[J]. Lipids, 1995, 30(2): 163-167. |
| [37] |
KOK N, ROBERFROID M, ROBERT A, et al. Involvement of lipogenesis in the lower VLDL secretion induced by oligofructose in rats[J]. British Journal of Nutrition, 1996, 76(6): 881-890. DOI:10.1079/BJN19960094 |
| [38] |
BEYLOT M. Effects of inulin-type fructans on lipid metabolism in man and in animal models[J]. British Journal of Nutrition, 2005, 93(Suppl.1): S163-S168. |
| [39] |
KIM M, SHIN H K. The water-soluble extract of chicory influences serum and liver lipid concentrations, cecal short-chain fatty acid concentrations and fecal lipid excretion in rats[J]. The Journal of Nutrition, 1998, 128(10): 1731-1736. DOI:10.1093/jn/128.10.1731 |
| [40] |
YUSRIZAL, CHEN T C. Effect of adding chicory fructans in feed on broiler growth performance, serum cholesterol and intestinal length[J]. International Journal of Poultry Science, 2003, 2(3): 214-219. DOI:10.3923/ijps.2003.214.219 |
| [41] |
SANG-OH P, BYUNG-SUNG P. Effect of feeding inulin oligosaccharides on cecum bacteria, egg quality and egg production in laying hens[J]. African Journal of Biotechnology, 2012, 11(39): 9516-9521. DOI:10.5897/AJB |
| [42] |
VELASCO S, ORTIZ L T, ALZUETA C, et al. Effect of inulin supplementation and dietary fat source on performance, blood serum metabolites, liver lipids, abdominal fat deposition, and tissue fatty acid composition in broiler chickens[J]. Poultry Science, 2010, 89(8): 1651-1662. DOI:10.3382/ps.2010-00687 |
| [43] |
DEWULF E M, CANI P D, NEYRINCK A M, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice[J]. The Journal of Nutritional Biochemistry, 2011, 22(8): 712-722. DOI:10.1016/j.jnutbio.2010.05.009 |
| [44] |
DELZENNE N M, DAUBIOUL C, NEYRINCK A, et al. Inulin and oligofructose modulate lipid metabolism in animals:review of biochemical events and future prospects[J]. British Journal of Nutrition, 2002, 87(Suppl.2): S255-S259. |
| [45] |
CHEN Y C, CHEN T C. Mineral utilization in layers as influenced by dietary oligofructose and inulin[J]. International Journal of Poultry Science, 2004, 3(7): 442-445. DOI:10.3923/ijps.2004.442.445 |
| [46] |
ORTIZ L T, RODRÍGUEZ M L, ALZUETA C, et al. Effect of inulin on growth performance, intestinal tract sizes, mineral retention and tibial bone mineralisation in broiler chickens[J]. British Poultry Science, 2009, 50(3): 325-332. DOI:10.1080/00071660902806962 |
| [47] |
SCHOLZ-AHRENS K E, SCHREZENMEIR J. Inulin and oligofructose and mineral metabolism:the evidence from animal trials[J]. The Journal of Nutrition, 2007, 137(Suppl.11): 2513S-2523S. |
| [48] |
ŚWIATKIEWICZ S, KORELESKI J, ARCZEWSKA-WŁOSEK A. Effect of prebiotic fructans and organic acids on mineral retention in laying hens[J]. Acta Agriculturae Scandinavica, Section A:Animal Science, 2010, 60(2): 125-128. DOI:10.1080/09064702.2010.482593 |
| [49] |
YASUDA K, DAWSON H D, WASMUTH E V, et al. Supplemental dietary inulin influences expression of iron and inflammation related genes in young pigs[J]. The Journal of Nutrition, 2009, 139(11): 2018-2023. DOI:10.3945/jn.109.110528 |
| [50] |
TAKO E, GLAHN R P, WELCH R M, et al. Dietary inulin affects the expression of intestinal enterocyte iron transporters, receptors and storage protein and alters the microbiota in the pig intestine[J]. British Journal of Nutrition, 2008, 99(3): 472-480. |
| [51] |
ROBERFROID M B. Functional foods:concepts and application to inulin and oligofructose[J]. British Journal of Nutrition, 2002, 87(Suppl.2): S139-S143. |
| [52] |
GRIFFIN I J, DAVILA P M, ABRAMS S A. Non-digestible oligosaccharides and calcium absorption in girls with adequate calcium intakes[J]. British Journal of Nutrition, 2002, 87(Suppl.2): S187-S191. |
| [53] |
SAMOLIŃ SKA W, GRELA E R. Comparative effects of inulin with different polymerization degrees on growth performance, blood trace minerals, and erythrocyte indices in growing-finishing pigs[J]. Biological Trace Element Research, 2017, 176(1): 130-142. |
| [54] |
SPENCER J D, TOUCHETTE K J, LIU H, et al. Effect of spray-dried plasma and fructooligosaccharide on nursery performance and small intestinal morphology of weaned pigs[J]. Journal of Animal Science, 1997, 75(Suppl.1): 199. |
| [55] |
PIERCE K M, SWEENEY T, BROPHY P O, et al. The effect of lactose and inulin on intestinal morphology, selected microbial populations and volatile fatty acid concentrations in the gastro-intestinal tract of the weanling pig[J]. Animal Science, 2006, 82(3): 311-318. |
| [56] |
HANSEN C F, SØRENSEN G, LYNGBYE M. Reduced diet crude protein level, benzoic acid and inulin reduced ammonia, but failed to influence odour emission from finishing pigs[J]. Livestock Science, 2007, 109(1/2/3): 228-231. |
| [57] |
PETKEVIČ IUS S, KNUDSEN K E B, MURRELL K D, et al. The effect of inulin and sugar beet fibre on Oesophagostomum dentatum infection in pigs[J]. Parasitology, 2003, 127(1): 61-68. |
| [58] |
SEVANE N, BIALADE F, VELASCO S, et al. Dietary inulin supplementation modifies significantly the liver transcriptomic profile of broiler chickens[J]. PLoS One, 2014, 9(6): e98942. DOI:10.1371/journal.pone.0098942 |
| [59] |
SANG-OH P, BYUNG-SUNG P. Effect of dietary microencapsulated-inulin on carcass characteristics and growth performance in broiler chickens[J]. Journal of Animal and Veterinary Advances, 2011, 10(10): 1342-1349. DOI:10.3923/javaa.2011.1342.1349 |
| [60] |
NABIZADEH A, GEVORKYAN O, GOLIAN A. Effect of inulin on some hematological, immunological parameters and broiler chickens performance[J]. Journal of Animal and Veterinary Advances, 2012, 11(18): 3304-3311. DOI:10.3923/javaa.2012.3304.3311 |
| [61] |
BIGGS P, PARSONS C M, FAHEY G C. The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks[J]. Poultry Science, 2007, 86(11): 2327-2336. DOI:10.3382/ps.2007-00427 |
| [62] |
REHMAN H, HELLWEG P, TARAS D, et al. Effects of dietary inulin on the intestinal short chain fatty acids and microbial ecology in broiler chickens as revealed by denaturing gradient gel electrophoresis[J]. Poultry Science, 2008, 87(4): 783-789. DOI:10.3382/ps.2007-00271 |




