动物易受到高温环境的影响,肉鸡因其体温高、无汗腺以及代谢旺盛等特点更容易遭受热应激的危害。肠道既是消化吸收营养物质的重要器官,又是保护机体免受食物抗原、病原微生物等损害的先天性屏障。胃肠道对体温的上升高度敏感,大量病理学研究表明热应激引发的黏膜损伤与内毒素(ET)血症、缺氧和炎症反应密切相关[1-4]。肠腔内的单层上皮细胞作为物理屏障参与机体黏膜防御系统的组成[5]。屏障功能由相邻细胞间的连接得以维持,其中,紧密连接通过控制细胞旁通透性在屏障功能完整性上发挥关键作用[6]。已有研究表明热应激破坏小鼠肠道紧密连接和微绒毛结构[7];热应激可引发多灶性淋巴浆细胞性肠炎,并降低肉鸡的采食量和体增重[2]。考虑到肠道屏障性能并非维持现状,而是由隐窝干细胞不断地增殖、分化,并脱落在肠腔表面,对屏障性能进行动态修复[8]。本试验通过探究急性热应激后肠道屏障紧密连接蛋白[闭合蛋白(claudin)-1、闭锁蛋白(occludin)和闭合小环蛋白-1(ZO-1)]、营养物质转运载体[阳离子氨基酸转运蛋白(CAT)-1、寡肽转运蛋白-1(PepT-1)、葡萄糖转运蛋白-2(GLUT-2)]以及能量调控信号通路中肝激酶B1(LKB1)、腺苷酸活化蛋白激酶(AMPK)α1基因表达的变化,从肠道的屏障功能、营养物质转运以及能量调控3个方面分析热应激对肉仔鸡肠道功能的影响。
1 材料与方法 1.1 试验设计试验选取80只体况基本一致的28日龄健康爱拔益加(AA)肉仔鸡,随机分为热应激组(试验组)和对照组,每组40只鸡,分为8个重复,每个重复5只鸡。试验在2间设施条件完全相同的小型鸡舍(10 m2左右)进行,鸡舍整体进行隔热处理,配置加热器、空调和加湿器。所有鸡饲养在同一层鸡笼,利用空调和加热器控制鸡舍内温度,根据温度计监测鸡舍内温度。热应激组08:00—18:00进行10 h急性热应激,温度为(35±1) ℃,对照组全天室温(23±1) ℃。
1.2 饲养管理试验期间肉仔鸡饲养在2间设施条件完全相同的鸡舍中,采用笼养,执行24 h光照程序(白天18 h,晚上6 h),饲喂参照NRC(1994)肉鸡营养需要量标准配制的基础饲粮,其组成及营养水平见表 1。每天记录各重复的耗料量,饲养阶段自由采食和饮水。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of basal diets (air-dry basis) |
肉鸡28日龄开始试验,急性热应激10 h后,所有鸡进行称重,试验组和对照组每重复各选择1只接近平均体重的鸡,同时进行采样。翅静脉采血,离心分离血清,屠宰后迅速打开腹腔,取出空肠,并剪取空肠前段约10 cm的肠段组织,在无菌生理盐水中漂洗后,采集黏膜样本,迅速放于液氮中保存。
1.4 指标测定 1.4.1 生长性能每天记录各重复的耗料量,准确记录28日龄各重复的初始鸡重,10 h热应激结束后统计各重复鸡重、耗料量以及采样鸡重。
体增重=总鸡重+死亡鸡重-初始鸡重;
总采食量=加料重-余料重;
平均采食量=总采食量/总鸡数;
料重比=总采食量/体增重;
存活率=100×存栏鸡数/总鸡数。
1.4.2 血清指标翅静脉采血,血液于常温倾斜静置,待血清析出后,离心机(飞鸽KA-1000型,上海安亭科学仪器厂)以3 000 r/min离心10 min,取血清,-20 ℃保存待测。全自动生化分析仪(7170A,HITACHI, 日本)测定血清葡萄糖(GLU)、总胆固醇(TCHO)和甘油三酯(TG)含量。采用生物素双抗体夹心酶联免疫吸附试验(ELISA)测定血清ET含量,所需试剂盒购自南京建成生物工程研究所,使用ELx808酶标仪(Gene有限公司)测定。
1.4.3 空肠黏膜基因mRNA表达量的测定空肠黏膜总RNA的提取按照动物组织/细胞RNA提取试剂盒(北京康为世纪生物科技有限公司)说明书进行,提取的总RNA用微量紫外分光光度计(DS-11, DeNovix, 美国)在260 nm波长下检测RNA的浓度和纯度。
cDNA的合成采用罗氏第1链cDNA合成试剂盒(Transcriptor First Strand cDNA Synthesis Kit, Roche, 德国),反应体系为20 μL,反转录反应参数:25 ℃ 10 min,55 ℃ 30 min,85 ℃ 5 min。反应结束后于-20 ℃保存待用。
实时荧光定量PCR的反应程序:第1步预变性,95 ℃ 10 s;第2步PCR反应,95 ℃ 5 s、60 ℃ 40 s,40个循环。所有引物均根据FastStart Universal SYBR Green Master (Rox)试剂盒要求,参照GenBank上已发表的序列,采用DNAMAN 5.0设计(跨内含子),引物DNA由上海生工生物工程公司合成,引物序列见表 2。
|
|
表 2 实时荧光定量PCR引物序列 Table 2 Primer sequences for fluorescence-based quantitative real-time PCR |
参照Livak等[9]的方法用2-ΔΔCT法定量目标基因相对表达量,以β-肌动蛋白(β-actin)和TATA盒结合蛋白(TBP)作为内参基因进行校准。
1.5 统计分析基因表达结果采用相对表达量的形式,β-actin和TBP作为内参基因。数据统计采用SPSS 24.0软件进行t检验,置信区间为95%,P < 0.05表示处理效应差异显著。
2 结果与分析 2.1 急性热应激对肉仔鸡生长性能的影响由表 3可知,与对照组相比,急性热应激显著降低了肉仔鸡的平均体增重和平均采食量(P < 0.05),显著提高了料重比(P < 0.05)。
|
|
表 3 急性热应激对肉仔鸡生长性能的影响 Table 3 Effects of acute heat stress on growth performance of broilers |
由表 4可知,与对照组相比,试验组肉仔鸡血清ET含量均显著高于对照组(P < 0.05),急性热应激对肉仔鸡血清GLU、TG和TCHO含量无显著影响(P>0.05)。
|
|
表 4 急性热应激对肉仔鸡血清指标的影响 Table 4 Effects of acute heat stress on serum indices of broilers |
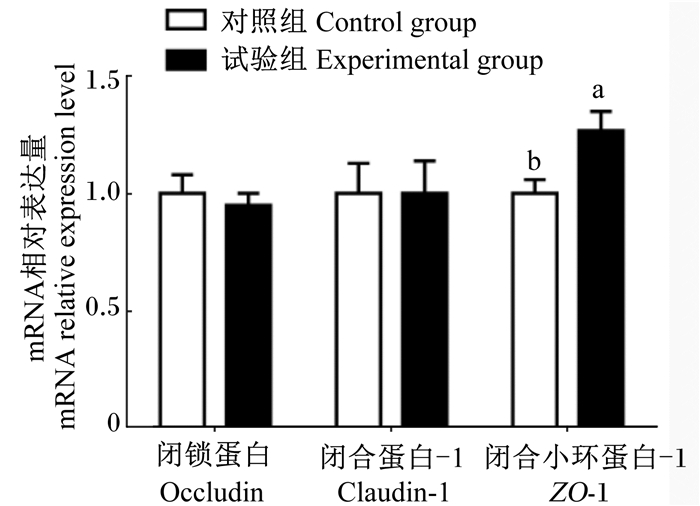
由图 1可知,与对照组相比,急性热应激对肉仔鸡空肠黏膜occludin和claudin-1基因的相对表达量无显著影响(P>0.05),显著提高肉仔鸡空肠黏膜ZO-1基因的相对表达量(P < 0.05)。
|
柱形图标注不同字母表示差异显著(P< 0.05)。下图同。 Column diagrams with different letter superscripts mean significant difference (P< 0.05). The same as below. 图 1 急性热应激对肉仔鸡肠道屏障基因表达的影响 Figure 1 Effects of acute heat stress on intestinal barrier gene expression of broilers |
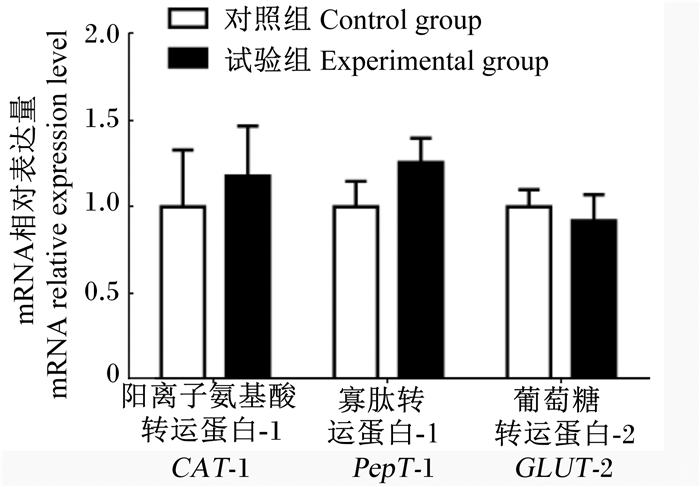
由图 2可知,与对照组相比,急性热应激对肉仔鸡空肠黏膜CAT-1、PepT-1和GLUT-2基因的相对表达量无显著影响(P>0.05)。
|
图 2 急性热应激对肉仔鸡肠道营养物质转运载体基因表达的影响 Figure 2 Effects of acute heat stress on intestinal nutrient transporter gene expression of broilers |
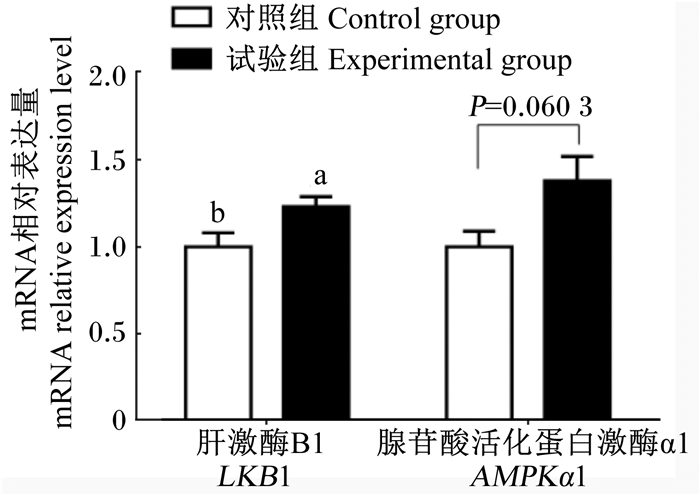
由图 3可知,与对照组相比,试验组肉仔鸡空肠黏膜AMPKα1基因的相对表达量有上升趋势(P=0.060 3),肉仔鸡空肠黏膜LKB1基因的相对表达量显著上升(P < 0.05)。
|
图 3 急性热应激对肉仔鸡肠道LKB1和AMPKα1基因表达的影响 Figure 3 Effects of acute heat stress on gene expression of intestinal LKB1 and AMPKα1 |
热应激一直以来是影响肉鸡生长性能的重要环境因素。本研究结果显示急性热应激显著降低了肉仔鸡平均采食量和平均体增重,显著提高了料重比,总体降低了肉仔鸡生长性能,这与前人研究结果[10-14]一致。已有研究表明,热应激诱导下丘脑-垂体-肾上腺皮质轴(hypothalamic-pituitary-adrenal, HPA)的激活,肾上腺皮质大量释放皮质酮影响采食行为是造成生长性能下降的主要因素[10]。相关研究显示,机体在应激条件下通过促进体内GLU的动员和生产提高血糖水平以维持稳态[15]。急性应激下,HPA轴通过与肾上腺素、胰岛素、胰高血糖素和交感神经系统的互作,提高血糖水平,满足机体重要器官的能量供给[13]。而在长期应激时,HPA轴糖皮质激素的负反馈调节障碍,会持续促进糖皮质激素分泌,使脂肪和肌肉组织的脂肪动员和肝脏糖异生加强,导致游离脂肪酸和血糖水平升高[16]。本研究结果证实,急性热应激造成肉仔鸡生长性能下降,与前人研究结果相一致。
3.2 急性热应激对肉仔鸡肠道屏障完整性的影响肠道上皮细胞(intestinal epithelial cells,IEC)具有物理屏障功能,可以将肠腔内容物所在的严酷外界环境与组织内部环境分离开来,防止外来病原物的入侵,因此IEC可介导急性非特异性免疫反应,参与机体第一道防线,在先天性免疫系统中发挥重要作用[17-19]。IEC的完整性是维持内外环境间这种具有选择性的物理屏障的关键,紧密连接作为重要的细胞间连接环绕在上皮细胞顶端,能够控制物质穿越上皮细胞的转运活动,在维持IEC完整性上至关重要,其通透性决定着整个肠上皮细胞的屏障功能[20-21]。当肠道屏障受损时,细菌移位穿越肠上皮细胞,增加了肠道病原菌感染的机率,细菌代谢过程中产生的ET随之进入血液循环,因此血液ET含量间接反映了肠道屏障的完整性[22-23]。在本研究中,急性热应激显著提高了肉鸡血清ET含量,说明热应激增加了紧密连接的通透性,肠道屏障的完整性受损。
3.3 急性热应激对肉仔鸡肠道紧密连接蛋白基因表达的影响机体对热应激的另一个重要的适应性反应是增加外周血流量,进而导致肠内供血减少和缺氧诱导的氧化应激反应[24]。而肠上皮缺血会导致肠道紧密连接和黏着连接的功能障碍使肠道屏障受损[25]。在猪和大鼠上的研究证实,热应激对肠道紧密连接影响显著,生长猪热应激24 h导致了claudin-1、occludin和ZO-1的mRNA表达上升,occludin和claudin-3的蛋白表达同样有所上升,并且基因水平的变化先于蛋白水平的变化[26-27];大鼠于40 ℃环境下每天热应激2 h,连续应激3 d后发现紧密连接结构损伤,绒毛高度缩短,肠上皮细胞脱落从而暴露固有层,导致细菌移位[28]。本研究中,急性热应激10 h造成肉仔鸡空肠黏膜ZO-1基因的相对表达量显著上升。在罗斯肉鸡上的研究表明,热应激显著提高了空肠claudin-5和ZO-1的基因表达,而并没有影响occludin的基因表达[24],这与本研究结果相似。
3.4 急性热应激对肉仔鸡肠道营养物质转运蛋白基因表达的影响肠道吸收饲粮中的蛋白质是通过蛋白酶、肽酶、氨基酸和小肽转运蛋白共同完成,肠道发育、遗传选择以及饲粮粗蛋白质的含量和质量等都可以调节鸡小肠氨基酸和小肽转运蛋白[29]。早期研究表明热应激可降低肉鸡蛋白质和氨基酸消化率[30],近期有报道进一步证实热应激通过影响氨基酸转运蛋白降低肉鸡生长性能[32]。CAT作为重要的转运氨基酸的蛋白可以介导阳离子氨基酸的双向转运,从而支持蛋白质的合成、一氧化氮合成以及多胺生物合成等重要代谢功能。有研究证明敲除CAT基因后的小鼠不能存活[32],进一步论证了该基因的重要性。
蛋白质通过消化酶降解后除了产生游离氨基酸外,还有一部分小肽(主要是二肽和三肽),通过小肠刷状缘膜PepT-1进入细胞后,水解形成游离氨基酸以进入门静脉血[33]。在对小肽吸收转运相关研究中,小肽转运载体常作为主要的研究对象,其RNA表达数量及蛋白转运活性或数量,也被作为主要的研究指标。在本研究中,急性热应激10 h并未造成营养物质转运载体CAT-1、PepT-1和GLUT-2基因相对表达量的显著变化,其原因可能与热应激的模型有关,短时间急性热应激没有对其基因表达造成影响。
3.5 热应激对肉仔鸡肠道AMPK信号通路基因表达的影响AMPK是一种丝氨酸/苏氨酸激酶,在大多数真核组织中对细胞能量的平衡至关重要。不同的生理应激诱导ATP消耗,使细胞内AMP/ATP值增加,从而导致AMPK活性增加[34]。LKB1是AMPK主要的上游激酶,几乎在所有的组织中表达[35]。大量研究表明热应激可影响AMPK活性,岩蟹在18~30 ℃热应激处理期间,AMPK活性上升9.1倍[36];对C2C12肌管进行1 h轻度热应激可提高AMPK活性[37];热应激条件下奶牛AMPK活性上升[38]。在家禽上,夏季高温应激造成了肉鸡胸肌组织中AMP/ATP值、AMPK活性显著升高[39];LKB1基因已证实在鸡肠道中表达,并且存在与哺乳动物功能相同的LKB1-AMPK信号通路[40]。本研究中,试验组肉仔鸡空肠黏膜AMPKα1基因相对表达量与对照组相比有上升,但并未出现显著差异,而试验组肉仔鸡空肠黏膜LKB1基因的相对表达量显著高于对照组,说明热应激可激活AMPK上游激酶。
4 结论急性热应激显著降低肉仔鸡生长性能,损伤肠道屏障,但对肠道氨基酸、小肽及GLU的转运没有造成影响;急性热应激影响肉仔鸡空肠黏膜LKB1基因的表达。
| [1] |
HALL D M, BUETTNER G R, OBERLEY L W, et al. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia[J]. American Journal of Physiology Heart and Circulatory Physiology, 2001, 280(2): H509-H521. DOI:10.1152/ajpheart.2001.280.2.H509 |
| [2] |
QUINTEIRO-FILHO W M, GOMES A V S, PINHEIRO M L, et al. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis[J]. Avian Pathology, 2012, 41(5): 421-427. DOI:10.1080/03079457.2012.709315 |
| [3] |
DOKLADNY K, MOSELEY P L, MA T Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability[J]. American Journal of Physiology Gastrointestinal and Liver Physiology, 2006, 290(2): G204-G212. DOI:10.1152/ajpgi.00401.2005 |
| [4] |
PEARCE S C, MANI V, BODDICKER R L, et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs[J]. PLoS One, 2013, 8(8): e70215. DOI:10.1371/journal.pone.0070215 |
| [5] |
BERKES J, VISWANATHAN V K, SAVKOVIC S D, et al. Intestinal epithelial responses to enteric pathogens:effects on the tight junction barrier, ion transport, and inflammation[J]. Gut, 2003, 52(3): 439-451. DOI:10.1136/gut.52.3.439 |
| [6] |
BALKOVETZ D F, KATZ J. Bacterial invasion by a paracellular route:divide and conquer[J]. Microbes and Infection, 2003, 5(7): 613-619. DOI:10.1016/S1286-4579(03)00089-3 |
| [7] |
HE S S, LIU F H, XU L, et al. Protective effects of ferulic acid against heat stress-induced intestinal epithelial barrier dysfunction in vitro and in vivo[J]. PLoS One, 2016, 11(2): e0145236. DOI:10.1371/journal.pone.0145236 |
| [8] |
VAN DER FLIER L G, CLEVERS H. Stem cells, self-renewal, and differentiation in the intestinal epithelium[J]. Annual Review of Physiology, 2009, 71: 241-260. DOI:10.1146/annurev.physiol.010908.163145 |
| [9] |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262 |
| [10] |
QUINTEIRO-FILHO W M, RIBEIRO A, FERRAZ-DE-PAULA V, et al. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens[J]. Poultry Science, 2010, 89(9): 1905-1914. DOI:10.3382/ps.2010-00812 |
| [11] |
KAMEL N N, AHMED A M H, MEHAISEN G M K, et al. Depression of leukocyte protein synthesis, immune function and growth performance induced by high environmental temperature in broiler chickens[J]. International Journal of Biometeorology, 2017, 61(9): 1637-1645. DOI:10.1007/s00484-017-1342-0 |
| [12] |
LIN H, BUYSE J, DU R, et al. Response of rectal temperature of broiler chickens to thermal environment factors[J]. Arch Geflugelkd, 2004, 68(3): 126-131. |
| [13] |
LIN H, DECUYPERE E, BUYSE J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus):2.Short-term effect[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2004, 139(4): 745-751. DOI:10.1016/j.cbpc.2004.09.014 |
| [14] |
LIN H, ZHANG H F, JIAO H C, et al. Thermoregulation responses of broiler chickens to humidity at different ambient temperatures.Ⅰ.Four weeks of age[J]. Poultry Science, 2005, 84(8): 1166-1172. DOI:10.1093/ps/84.8.1166 |
| [15] |
VIRDEN W S, KIDD M T. Physiological stress in broilers:ramifications on nutrient digestibility and responses[J]. The Journal of Applied Poultry Research, 2009, 18(2): 338-347. DOI:10.3382/japr.2007-00093 |
| [16] |
LIN H, DECUYPERE E, BUYSE J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus):1.Chronic exposure[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2004, 139(4): 737-744. DOI:10.1016/j.cbpc.2004.09.013 |
| [17] |
GEWIRTZ A T, LIU Y, SITARAMAN S V, et al. Intestinal epithelial pathobiology:past, present and future[J]. Best Practice & Research Clinical Gastroenterology, 2002, 16(6): 851-867. |
| [18] |
DWINELL M B, JOHANESEN P A, SMITH J M. Immunobiology of epithelial chemokines in the intestinal mucosa[J]. Surgery, 2003, 133(6): 601-607. DOI:10.1067/msy.2003.143 |
| [19] |
SHAO L, SERRANO D, MAYER L. The role of epithelial cells in immune regulation in the gut[J]. Seminars in Immunology, 2001, 13(3): 163-176. DOI:10.1006/smim.2000.0311 |
| [20] |
OSWALD I P. Role of intestinal epithelial cells in the innate immune defence of the pig intestine[J]. Veterinary Research, 2006, 37(3): 359-368. DOI:10.1051/vetres:2006006 |
| [21] |
VAN ITALLIE C M, ANDERSON J M. Architecture of tight junctions and principles of molecular composition[J]. Seminars in Cell & Developmental Biology, 2014, 36: 157-166. |
| [22] |
TURNER J R. Molecular basis of epithelial barrier regulation:from basic mechanisms to clinical application[J]. The American Journal of Pathology, 2006, 169(6): 1901-1909. DOI:10.2353/ajpath.2006.060681 |
| [23] |
MENG J J, YU H D, MA J, et al. Morphine Induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner[J]. PLoS One, 2013, 8(1): e54040. DOI:10.1371/journal.pone.0054040 |
| [24] |
VARASTEH S, BRABER S, AKBARI P, et al. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides[J]. PLoS One, 2015, 10(9): e0138975. DOI:10.1371/journal.pone.0138975 |
| [25] |
LEON L R, HELWIG B G. Heat stroke:role of the systemic inflammatory response[J]. Journal of Applied Physiology, 2010, 109(6): 1980-1988. DOI:10.1152/japplphysiol.00301.2010 |
| [26] |
PEARCE S C, MANI V, BODDICKER R L, et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs[J]. PLoS One, 2013, 8(8): e70215. DOI:10.1371/journal.pone.0070215 |
| [27] |
PEARCE S C, MANI V, WEBER T E, et al. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs[J]. Journal of Animal Science, 2013, 91(11): 5183-5193. DOI:10.2527/jas.2013-6759 |
| [28] |
LIU X X, LI H R, LU A, et al. Reduction of intestinal mucosal immune function in heat-stressed rats and bacterial translocation[J]. International Journal of Hyperthermia, 2012, 28(8): 756-765. DOI:10.3109/02656736.2012.729173 |
| [29] |
GILBERT E R, LI H, EMMERSON D A, et al. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers[J]. Poultry Science, 2007, 86(8): 1739-1753. DOI:10.1093/ps/86.8.1739 |
| [30] |
WALLIS IR, BALNAVE D. The influence of environmental temperature, age and sex on the digestibility of amino acids in growing broiler chickens[J]. British Poultry Science, 1984, 25(3): 401-407. DOI:10.1080/00071668408454880 |
| [31] |
HABASHY W S, MILFORT M C, ADOMAKO K, et al. Effect of heat stress on amino acid digestibility and transporters in meat-type chickens[J]. Poultry Science, 2017, 96(7): 2312-2319. DOI:10.3382/ps/pex027 |
| [32] |
HATZOGLOU M, FERNANDEZ J, YAMAN I, et al. Regulation of cationic amino acid transport:the story of the CAT-1 transporter[J]. Annual Review of Nutrition, 2004, 24: 377-399. DOI:10.1146/annurev.nutr.23.011702.073120 |
| [33] |
RADEVA G, BUYSE M, HINDLET P, et al. Regulation of the oligopeptide transporter, PEPT-1, in DSS-induced rat colitis[J]. Digestive Diseases and Sciences, 2007, 52(7): 1653-1661. DOI:10.1007/s10620-006-9667-2 |
| [34] |
HARDIE D G, ROSS F A, HAWLEY S A. AMPK:a nutrient and energy sensor that maintains energy homeostasis[J]. Nature Reviews Molecular Cell Biology, 2012, 13(4): 251-262. DOI:10.1038/nrm3311 |
| [35] |
HARDIE D G. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function[J]. Genes & Development, 2011, 25(18): 1895-1908. |
| [36] |
FREDERICH M, O'ROURKE M R, FUREY N B, et al. AMP-activated protein kinase (AMPK) in the rock crab, cancer irroratus:an early indicator of temperature stress[J]. The Journal of Experimental Biology, 2009, 212: 722-730. DOI:10.1242/jeb.021998 |
| [37] |
LIU C T, BROOKS G A. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes[J]. Journal of Applied Physiology, 2012, 112(3): 354-361. DOI:10.1152/japplphysiol.00989.2011 |
| [38] |
MIN L, CHENG J B, SHI B L, et al. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows[J]. Journal of Zhejiang University:Science B, 2015, 16(6): 541-548. DOI:10.1631/jzus.B1400341 |
| [39] |
XING T, XU X L, ZHOU G H, et al. The effect of transportation of broilers during summer on the expression of heat shock protein 70, postmortem metabolism and meat quality[J]. Journal of Animal Science, 2015, 93(1): 62-70. DOI:10.2527/jas.2014-7831 |
| [40] |
PROSZKOWIEC-WEGLARZ M, RICHARDS M P, RAMACHANDRAN R, et al. Characterization of the AMP-activated protein kinase pathway in chickens[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2006, 143(1): 92-106. DOI:10.1016/j.cbpb.2005.10.009 |




