2. 华南农业大学群体微生物研究中心, 广州 510642
2. Integrative Microbiology Research Centre, South China Agricultural University, Guangzhou 510642, China
仔猪早期断奶是养猪业最重要的饲养技术之一,可以大幅度提高生产效率。然而,仔猪肠道发育不够完善,被动免疫力不足,过早断奶使其肠道易遭受外来病原体的侵袭,引发各种肠道疾病。腹泻便是其中最为常见的肠道疾病,也是初生仔猪死亡的主要原因。农业部数据显示,2016年我国新生活仔猪8.79亿头,其中有1.52亿头因腹泻等疾病在断奶前后死亡或淘汰,直接导致经济损失高达240亿元。即使是畜牧业发达国家,仔猪腹泻问题同样突出[1]。
产肠毒素大肠杆菌(enterotoxigenic Escherichia coli,ETEC)是引起初生幼畜腹泻的主要病原体之一[2]。ETEC可通过新生儿或幼畜口腔进入肠道,随之大量繁殖,分泌肠毒素使机体致病。ETEC可降低肠道黏膜的天然屏障防御力,破坏黏膜结构,从而损伤肠道黏膜功能[2-4]。当畜禽感染ETEC时,表现出食欲不振、精神萎靡、日渐消瘦、腹泻等症状,严重者休克死亡。针对ETEC感染导致的腹泻问题,通常采用抗生素来治疗。但是长期使用抗生素暴露出越来越多的问题,例如抗生素残留、环境污染和耐药性等[5]。因此,ETEC对畜牧业的影响及其预防治疗措施逐渐被重视。
为缓解ETEC的毒害作用,许多学者在功能性物质领域进行了诸多尝试。研究发现,某些功能性氨基酸、益生菌、微量金属元素和植物提取物等对ETEC诱导损伤的仔猪肠道均具有较好的缓解效果。本文总结了近年来ETEC对仔猪肠道黏膜屏障功能的影响及其损伤修复研究进展,以期为仔猪腹泻防控提供思路。
1 ETEC的致病机理 1.1 病原大肠杆菌(Escherichia coli)是大肠埃希菌的俗称,属于肠杆菌科(Enterobacteriaceae),埃希氏菌属(Escherichia)[2]。大部分大肠杆菌不致病,是人和动物肠道中的常居菌。20世纪中期,人们发现了病原性大肠杆菌的存在,其主要的作用靶标是肠道。病原性大肠杆菌与非致病性大肠杆菌的主要区别在于抗原结构不同,因而免疫特性也不同[6]。目前根据病原性大肠杆菌的致病特点,可将其分为ETEC、肠道致病性大肠杆菌(EPEC)、肠道组织侵入性大肠杆菌(EIEC)、肠道出血性大肠杆菌(EHEC)、肠黏附性大肠杆菌(EAEC)、集聚性黏附大肠杆菌(EaggEC)、产志贺样毒素且具侵袭力的大肠杆菌(ESIEC)、产Vero细胞毒素性大肠杆菌(VTEC)等,其中ETEC是引起幼畜急性腹泻的主要病原体[7]。ETEC侵入肠道后,定植、扩繁并分泌毒素,引起肠道上皮细胞代谢紊乱甚至死亡,从而破坏肠道黏膜结构,引起肠道功能紊乱,进而导致急性腹泻[2]。
1.2 ETEC的致病因子ETEC的致病因子主要包括具有宿主特异性的黏附素和肠毒素。ETEC极易通过粪口途径传播,当ETEC侵袭肠道后便通过黏附素与肠道上皮细胞上的受体牢牢结合,使其得以抵抗肠道蠕动的冲刷作用而定植下来,随后ETEC大量繁殖并分泌肠毒素。肠毒素与肠道上皮细胞的受体结合后,引发肠道上皮细胞渗透压失衡,大量液体分泌到肠道,导致动物腹泻[2]。综上所述,ETEC引发动物腹泻主要包括定植和分泌肠毒素2个阶段[8-9]。ETEC侵袭肠道的过程如图 1所示[10]。
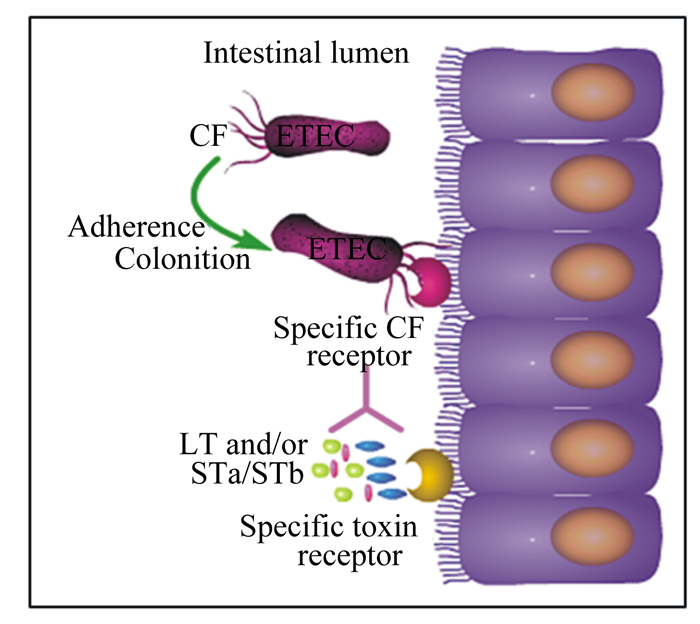
|
Intestinal lumen:肠腔;Adherence:黏附;CF:黏附因子;Specific CF receptor:特异性黏附因子受体;Colonition:定植;Specific toxin receptor:特异性毒素受体。LT and/or STa/STb:不耐热肠毒素和/或耐热肠毒素a或耐热肠毒素b。 图 1 ETEC侵袭肠道的过程 Fig. 1 ETEC invasion of intestinal tract[10] |
黏附素是一类表达于细菌细胞膜表面呈丝状的蛋白质,通常由100个结构相同的亚单位和各种不同的微小辅助蛋白质构成,是ETEC的重要毒力因子之一[8]。ETEC感染肠道的第1步就是依靠黏附素结合到宿主的肠道上皮细胞上。动物源ETEC黏附素主要包括K88、K99、987P、F18、F17和F41等。ETEC菌毛黏附素的研究始于20世纪60年代,Stirm等[11]首次从动物体分离出大肠杆菌黏附素抗原K88,并证实了腹泻仔猪的肠道菌株中表达了这种抗原。随后,一系列黏附素抗原也被陆续发现。
1.2.2 肠毒素肠毒素是ETEC定植后,在肠道繁殖过程中产生的代谢产物,也是ETEC导致腹泻的直接因子。肠毒素有不耐热肠毒素(heat-labile enterotoxin,LT)和耐热肠毒素(heat-stable enterotoxin,ST)2种,任意一种存在便能引发动物腹泻[2, 6, 10]。LT对热不稳定,60 ℃即可灭活。LT由1个A亚基和5个相同的B亚基组成,B亚基围绕着A亚基组成1个五边形结构,蛋白质分子质量较大,有免疫原性。LT与宿主细胞膜结合后传递给肠道上皮细胞膜上的神经节苷脂,毒素被吞噬转运至细胞质;A亚基激活细胞质中的腺苷酸环化酶,引起环腺苷酸(cyclic adenosine monophosphate,cAMP)浓度升高;cAMP浓度升高激活cAMP依赖性蛋白激酶,进而诱导氯离子通道被激活;氯离子的过量分泌打破肠道上皮细胞的渗透压稳态,引起水分和电解质进入肠腔,最终导致水样腹泻[2]。与LT相比,ST具有热稳定性,即使在100 ℃高温中放置30 min依然能够保持活性。ST的分子质量较LT小,一般为46~97 ku,免疫原性较弱,有STa和STb 2个亚型。ST的致病机理与LT有所不同,STa可激活肠道上皮细胞中的鸟苷酸环化酶(guanylate cyclase,GC),使得细胞内环鸟苷酸(cyclic guanosine monophosphate,cGMP)浓度升高,最终引起细胞中电解质分泌失衡而导致腹泻[2];而STb激活G蛋白引起的钙离子浓度升高可上调钙调蛋白依赖性蛋白激酶Ⅱ(calmodulin dependent protein kinase Ⅱ,CAMKⅡ)蛋白表达,进而激活囊性纤维跨膜调控子(cystic fibrosis transmembrane conductance regulator,CFTR),而CFTR是一种氯离子通道,控制氯离子的外排,当其被激活时,氯离子会向肠腔排出,最终导致水样腹泻。此外,钙离子浓度升高可激活蛋白激酶C(protein kinase C,PKC),PKC也可使CFTR磷酸化,同时抑制钠氢交换因子3(Na/H exchange 3,NHE3)的活性,致使钠离子无法向细胞内转运,最终导致细胞内外渗透压失衡,细胞代谢紊乱[10]。
1.2.3 脂多糖(lipopolysaccharide,LPS)ETEC属于革兰氏阴性菌,LPS是革兰氏阴性菌外膜的主要成分[12]。相较黏附素和肠毒素,LPS的毒性作用较弱,但高剂量LPS可增加促炎症因子白细胞介素(IL)-6、IL-8和IL-1β的基因表达水平和自噬相关蛋白质的表达水平,上调炎症信号通路核因子-κB(NF-κB)的表达水平,诱导肠道炎症[13-15];同时降低Claudin-1等紧密连接蛋白的表达水平,增加上皮通透性,破坏其结构和功能[13-17]。大量LPS穿过肠道黏膜屏障进入血液循环[18-19],降低血液中超氧化物歧化酶(superoxide dismutase,SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)活性,增加空肠中丙二醛(malondialdehyde,MDA)含量,降低机体的抗氧化水平[17],最终引起全身性炎症。
2 ETEC对肠道黏膜屏障功能的影响肠道黏膜屏障是指肠道黏膜能够防止外来有害物质(如细菌和毒素等)穿过肠黏膜进入机体内其他组织、器官和血液循环的结构和功能的总和[20]。它由机械屏障、生物屏障、免疫屏障和化学屏障共同组成,担负着抵御外界病原体入侵的第1道防线[21-23]。因而肠道黏膜上皮分布着多种先天性防御外来病原体入侵的机制,包括上皮细胞快速周转、被感染细胞的外排和自噬等[24-28]。肠道黏膜屏障功能一旦被破坏,肠道上皮的通透性会随之增加,上皮内组织暴露于病原体下,导致病原体快速迁移和易位[27-29]。因此,完整的肠道黏膜上皮以及良好的生理状态是动物健康生长的重要保障。
2.1 ETEC对肠道黏膜机械屏障的影响肠道黏膜机械屏障功能主要由完整的肠道上皮细胞及细胞间的紧密连接作用来体现。跨膜电阻值(TEER)是反映肠道上皮通透性的一个重要指标[30-31]。Nakashima等[32]研究表明,4×10-6~1×10-5 mol/L ETEC毒素在2 h内就能够降低T84单层细胞的TEER值,TEER值降低说明肠道上皮通透性增加,屏障功能受损。紧密连接蛋白可连接相邻2个细胞骨架,封闭细胞间隙,因此其表达量高低也可部分代表黏膜机械屏障功能的正常与否。紧密连接蛋白主要包括ZO-1、Claudins和Occludins等[33]。研究表明,ETEC可降低仔猪回肠组织和人结肠癌上皮细胞中ZO-1、Occludins和Claudin-1的蛋白表达水平[34-38],提示ETEC可在一定程度上破坏肠道上皮的完整性。
2.2 ETEC对肠道黏膜生物屏障的影响肠道常驻菌群是肠道黏膜天然的生物屏障,它们通过相互竞争排斥和相互依赖共生的作用,使得外来病原体难以定植并侵袭肠道黏膜[20-22]。肠道内正常菌群能够激活机体的免疫系统,促进免疫功能成熟和完善,同时还能够产生非特异性免疫调节因子,抑制或杀死外来细菌。另外,这些菌群能分泌一些酶参与营养物质消化,为肠道上皮细胞或其他菌群提供营养物质,维持肠道微生物菌群的平衡和多样性[39]。研究表明,ETEC可显著降低肠道微生物多样性,同时还会影响肠道免疫功能[40-43]。
2.3 ETEC对肠道黏膜免疫屏障的影响肠道黏膜免疫系统由固有层淋巴细胞、肠上皮内淋巴细胞等相关淋巴组织分泌的免疫球蛋白A(immunoglobulin A,IgA)及其他免疫因子组成。当病原体入侵时,肠道黏膜免疫系统会启动炎症反应,分泌炎症因子,进而刺激黏膜免疫系统分泌IgA和免疫球蛋白M(IgM)等免疫调节因子抵御病原体,防止病原体迁移[21]。研究表明,当ETEC感染肠道上皮细胞时,可导致促炎症因子IL-8水平升高[29]。Setty等[44]和Daudelin等[45]研究发现,ETEC可提高动物肠道组织中IL-8和肿瘤坏死因子(TNF)-α的蛋白表达水平。NF-κB信号通路可调节先天性免疫,研究认为IL-8和TNF-α蛋白表达水平上调是由于ETEC阻止了核因子抑制蛋白(IκBα)泛素化,从而激活NF-κB信号通路引起的[46-47]。
另外,ETEC感染还会降低肠道组织Toll样受体4(Toll-like receptor 4,TLR4)的基因表达水平[48]。TLR4为参与非特异性免疫的一类重要蛋白质,也是连接非特异性免疫和特异性免疫的桥梁。TLR4基因表达水平降低,意味着肠道免疫监督能力下降。肠道黏膜的先天性免疫力下降,使得肠道黏膜免疫系统对外来病原体的侵袭无法进行有效应答[49-51]。
3 ETEC对肠道黏膜吸收功能的影响完整的肠道黏膜结构是营养物质消化和吸收的基础。肠道上皮吸收细胞负责吸收营养物质,毛细血管和毛细淋巴管则负责将吸收的营养物质输送至机体各组织中。当肠绒毛受到破坏时,肠道上皮吸收细胞和分布于肠黏膜中的毛细淋巴管、毛细血管的数量随之减少,肠道对营养物质的吸收能力也随之降低。研究表明,ETEC可降低肠道绒毛高度,缩小绒毛体积,增加隐窝深度[52-53]。肠道黏膜对营养物质的吸收效率不仅与肠道绒毛的体积和表面积有关,还与营养转运蛋白活性密切相关。小肠刷状缘和基底膜内有着特定的营养转运蛋白。研究表明,ETEC感染的Caco-2细胞中硫胺素转运蛋白-1和硫胺素转运蛋白-2表达量减少,导致硫胺素的吸收显著受到抑制[54-56]。硫胺素被称为精神性维生素,能够改善精神状态,消除疲劳,使得皮毛滋润有光泽。感染了ETEC的仔猪会出现精神萎靡、皮毛无光泽,可能跟硫胺素吸收被抑制有关。
4 ETEC对肠道干细胞的影响肠上皮更新或再生的过程是肠道干细胞不断发育的结果。在适宜培养条件下,肠道干细胞可扩增为类似肠上皮结构的类肠团(图 2)[57]。类肠团是由单层细胞组成的海胆样空腔组织,肠道干细胞位于隐窝状出芽顶端,其子代细胞——短暂扩增细胞(TA cell)延伸汇入由成熟肠道上皮细胞组成的类似肠绒毛上皮的中央大空腔中[58]。肠道干细胞不仅是维持肠道上皮正常更新的动力之源,在损伤刺激条件下,也是肠上皮自我修复的驱动力所在[59]。
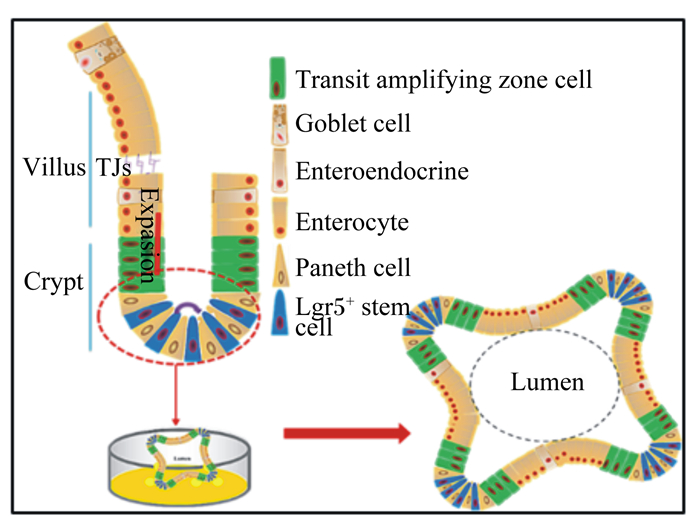
|
Transit amplifying zone cell:短暂扩增区域细胞;Goblet cell:杯状细胞;Enteroendocrine:肠内分泌细胞;Enterocyte:肠上皮细胞;Paneth cell:潘氏细胞;Lgr5+ stem cell:Lgr5+干细胞;Lumen:腔;Villus:绒毛;Crypt:隐窝; TJs:紧密连接;Expasion:扩增。 图 2 肠道干细胞模型 Fig. 2 Model of intestinal stem cells[57] |
Pattison等[60]用ST处理人和小鼠的类肠团,均发现ST可激活GC,使得cGMP浓度升高,激活PKC,进而激活CFTR,引起电解质分泌失调。此外,本课题组已成功建立了猪类肠团的培养体系,用ST处理猪类肠团发现,ST可降低干细胞活性,抑制类肠团的出芽,促进其凋亡,抑制β-连环蛋白(β-catenin)、+4位置肠道干细胞标志蛋白Bmi1、紧密连接蛋白ZO-1和Claudin-1的蛋白表达。而β-catenin是Wnt/β-catenin信号通路的主要效应因子,负责调控肠道干细胞活性和肠道稳态;Bmi1是肠道沉默型干细胞的标志。ST可能通过抑制Wnt/β-catenin信号通路,降低干细胞活性,进而导致其分化过程发生紊乱,类肠团出芽受阻;ST也可降低类肠团微肠道结构的屏障功能。
5 修复ETEC诱导的肠道黏膜损伤ETEC破坏肠道黏膜的结构和功能,危害机体健康,因此预防和治疗ETEC造成的肠道损伤具有重要意义。研究表明,精氨酸可通过5-氟尿嘧啶(5FU)降低肠道黏膜炎症[61]。谷氨酰胺可通过激活肠道黏膜的先天性免疫来调节微生物稳态,抵御外来病原体的入侵[62]。Liu等[63]研究发现,在ETEC感染肠道之前,口服精氨酸和谷氨酰胺可以削弱ETEC在肠道中的定植能力,并激活先天性免疫蛋白受体(pIgR),同时肠道黏膜中分泌型免疫球蛋白A(sIgA)、黏蛋白以及抗菌肽的表达上调。Tang等[64]研究发现,蛋氨酸可降低ETEC对肠道上皮细胞的毒力作用,加速坏死的肠上皮细胞脱落,促进肠上皮更新。
除功能性氨基酸外,某些益生菌同样具有此修复作用,Li等[65]研究表明,与ETEC相比,添加嗜酸乳杆菌可降低仔猪肠道黏膜中IL-1β、IL-8和TNF-α的蛋白表达水平,提高Toll样受体2(TLR2)和TLR4的蛋白表达水平。嗜酸乳杆菌能够促进细胞因子IL-17的分泌,主要是其分泌的γ-氨基丁酸发挥作用。γ-氨基丁酸通过激活雷帕霉素复合物1-核糖体蛋白激酶1(mTORC1-S6K1)信号,促进IL-17分泌[45]。嗜酸乳杆菌还可增加ZO-1和Occludin的蛋白表达水平[64-66],增强肠道黏膜屏障功能;同时可激活蛋白激酶B(Akt)信号通路,促进肠道上皮细胞增殖[67]。乳杆菌对于维持肠道菌群平衡、降低肠道炎症反应和腹泻指数也有正向调控作用[68]。因此,人们在治疗腹泻疾病时采取一些新型的营养干预措施。例如,在感染ETEC的仔猪饲粮中添加微生态制剂(含有1×108 CFU/g嗜酸乳杆菌、干酪乳杆菌、嗜热双歧杆菌和屎肠球菌)可增加仔猪空肠绒毛高度,改善生长性能[69]。饲粮中添加凝结芽孢杆菌、苯甲酸和牛至油复合添加剂可降低ETEC感染仔猪空肠黏膜中MDA含量,提高空肠黏膜的总抗氧化能力(T-AOC)及钠葡萄糖共转运载体1(SGLT1)、寡肽转运蛋白1(PepT1)的基因表达水平[70]。
此外,研究人员发现其他一些物质,包括微量元素、植物提取物等,可在不同程度上缓解ETEC诱导的损伤。Skrovanek等[71]研究表明,锌(Zn)可抑制ETEC毒力基因cfa1、cexE、sta2和degP的表达,从而降低ETEC的毒力。Heim等[72]研究发现,海藻提取物可降低肠道黏膜的炎症因子水平,维持肠道黏膜结构完整,提高ETEC感染断奶仔猪的生长性能。Xiao等[66]研究表明,壳聚糖可通过增加仔猪空肠黏膜中IL-6的基因表达水平,增强免疫应答,以缓解ETEC损伤。
6 小结ETEC破坏仔猪肠道上皮黏膜的完整性,损伤屏障功能,导致其防御能力下降,致使仔猪肠道黏膜易遭受外源病原体的侵袭而发生腹泻。肠道上皮黏膜完整性的改变可能与肠道干细胞活性降低有关。然而关于ETEC影响肠道干细胞的研究严重匮乏,更不用说对其影响机制的解析。虽然目前ETEC引起的仔猪腹泻防控工作,尤其是营养调控方向,取得了一定的进展,但研究层次较浅,并未深入剖析其内在的调控机理。一些功能性物质如何修复ETEC诱导的肠道黏膜损伤和影响肠道干细胞值得重点关注。总之,深入研究ETEC的致病机理及挖掘功能性物质的潜在价值可为预防和治疗仔猪病原性腹泻难题提供理论依据和切实可行的营养干预策略,从而解决畜牧生产面临的问题,促进产业健康发展。
| [1] |
HANKE D, POHLMANN A, SAUTER-LOUIS C, et al. Porcine epidemic diarrhea in europe:in-detail analyses of disease dynamics and molecular epidemiology[J]. Viruses, 2017, 9(7): 177. DOI:10.3390/v9070177 |
| [2] |
DUBREUIL J D, ISAACSON R E, SCHIFFERLI D M. Animal enterotoxigenic Escherichia coli[J]. Ecosal Plus, 2016, 7(1): 1-17. |
| [3] |
QADRI F, SVENNERHOLM A M, FARUQUE A S G, et al. Enterotoxigenic Escherichia coli in developing countries:epidemiology, microbiology, clinical features, treatment, and prevention[J]. Clinical Microbiology Reviews, 2005, 18(3): 465-483. DOI:10.1128/CMR.18.3.465-483.2005 |
| [4] |
ZHANG H H, XU Y P, ZHANG Z J, et al. Protective immunity of a multivalent vaccine candidate against piglet diarrhea caused by enterotoxigenic Escherichia coli (ETEC) in a pig model[J]. Vaccine, 2018, 36(5): 723-728. DOI:10.1016/j.vaccine.2017.12.026 |
| [5] |
SPELLBERG B, BARTLETT J G, GILBERT D N. The future of antibiotics and resistance[J]. The New England Journal of Medicine, 2013, 368(4): 299-302. DOI:10.1056/NEJMp1215093 |
| [6] |
ZHANG W P, SACK D A. Current progress in developing subunit vaccines against enterotoxigenic Escherichia coli-associated diarrhea[J]. Clinical and Vaccine Immunology, 2015, 22(9): 983-991. DOI:10.1128/CVI.00224-15 |
| [7] |
FLECKENSTEIN J M, RASKO D A.Overcoming enterotoxigenic Escherichia coli pathogen diversity: translational molecular approaches to inform vaccine design[M]//THOMAS S.Vaccine Design.New York: Humana Press, 2016: 363-383.
|
| [8] |
CODDENS A, VALIS E, BENKTANDER J, et al. Erythrocyte and porcine intestinal glycosphingolipids recognized by F4 fimbriae of enterotoxigenic Escherichia coli[J]. PLoS One, 2011, 6(9): e23309. DOI:10.1371/journal.pone.0023309 |
| [9] |
REN J, YAN X M, AI H S, et al. Susceptibility towards enterotoxigenic Escherichia coli F4ac diarrhea is governed by the MUC13 gene in pigs[J]. PLoS One, 2012, 7(9): e44573. DOI:10.1371/journal.pone.0044573 |
| [10] |
DUBREUIL J D.Escherichia coli STb enterotoxin: a multifaceted molecule[M]//GOPALAKRISHNAKONE P, STILES B, ALAPE-GIRÍN A, et al.Microbial Toxins.Dordrecht: Springer Press, 2016: 1-18.
|
| [11] |
STIRM S, ØRSKOV I, ØRSKOV F. K88, an episome-determined protein antigen of Escherichia coli[J]. Nature, 1966, 209(5022): 507-508. DOI:10.1038/209507a0 |
| [12] |
刘绍琼.mTOR信号通路在LPS诱导肉鸡肠道黏膜免疫应激中的作用[D].博士学位论文.泰安: 山东农业大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10434-1015306630.htm
|
| [13] |
GUO S H, AL-SADI R, SAID H M, et al. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14[J]. The American Journal of Pathology, 2013, 182(2): 375-387. DOI:10.1016/j.ajpath.2012.10.014 |
| [14] |
GUO S H, AL-SADI R, NIGHOT P K, et al. 75 Lipopolysaccharide induced increase in intestinal tight junction permeability is regulated by FAK dependent activation of MYD88 signaling pathway[J]. Gastroenterology, 2014, 146(5): S-21. |
| [15] |
GUO S H, NIGHOT M, AL-SADI R, et al. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88[J]. The Journal of Immunology, 2015, 195(10): 4999-5010. DOI:10.4049/jimmunol.1402598 |
| [16] |
STEWART R K, DANGI A, HUANG C, et al. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion-and endotoxin-induced acute liver injury[J]. Journal of Hepatology, 2014, 60(2): 298-305. DOI:10.1016/j.jhep.2013.09.013 |
| [17] |
CARCHMAN E H, WHELAN S, LOUGHRAN P, et al. Experimental sepsis-induced mitochondrial biogenesis is dependent on autophagy, TLR4, and TLR9 signaling in liver[J]. The FASEB Journal, 2013, 27(12): 4703-4711. DOI:10.1096/fj.13-229476 |
| [18] |
CAO S T, ZHANG Q H, WANG C C, et al. LPS challenge increased intestinal permeability, disrupted mitochondrial function and triggered mitophagy of piglets[J]. Innate Immunity, 2018, 24(4): 221-230. DOI:10.1177/1753425918769372 |
| [19] |
ZHU H L, PI D A, LENG W, et al. Asparagine preserves intestinal barrier function from LPS-induced injury and regulates CRF/CRFR signaling pathway[J]. Innate Immunity, 2017, 23(6): 546-556. DOI:10.1177/1753425917721631 |
| [20] |
PARK C M, PARK J Y, NOH K H, et al. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells[J]. Journal of Ethnopharmacology, 2011, 133(2): 834-842. DOI:10.1016/j.jep.2010.11.015 |
| [21] |
ALONSO C, VICARIO M, PIGRAU M, et al.Intestinal barrier function and the brain-gut axis[M]//LYTE M, CRYAN J F.Microbial Endocrinology: the Microbiota-Gut-Brain Axis in Health And Disease.New York: Springer Press, 2014: 73-113.
|
| [22] |
BRZOZOWSKI B, MAZUR-BIALY A, PAJDO R, et al. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD):role of brain-gut axis[J]. Current Neuropharmacology, 2016, 14(8): 892-900. DOI:10.2174/1570159X14666160404124127 |
| [23] |
BAUMGART D C, DIGNASS A U. Intestinal barrier function[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2002, 5(6): 685-694. DOI:10.1097/00075197-200211000-00012 |
| [24] |
CHOY A, ROY C R. Autophagy and bacterial infection:an evolving arms race[J]. Trends in Microbiology, 2013, 21(9): 451-456. DOI:10.1016/j.tim.2013.06.009 |
| [25] |
CASTREJÍN-JIMÉNEZ N S, LEYVA-PAREDES K, HERNÁNDEZ-GONZÁLEZ J C, et al. The role of autophagy in bacterial infections[J]. Bioscience Trends, 2015, 9(3): 149-159. DOI:10.5582/bst.2015.01035 |
| [26] |
CEMMA M, BRUMELL J H. Interactions of pathogenic bacteria with autophagy systems[J]. Current Biology, 2012, 22(13): R540-R545. DOI:10.1016/j.cub.2012.06.001 |
| [27] |
ILAN Y. Leaky gut and the liver:a role for bacterial translocation in nonalcoholic steatohepatitis[J]. World Journal of Gastroenterology, 2012, 18(21): 2609-2618. DOI:10.3748/wjg.v18.i21.2609 |
| [28] |
LIU X X, LI H R, LU A, et al. Reduction of intestinal mucosal immune function in heat-stressed rats and bacterial translocation[J]. International Journal of Hyperthermia, 2012, 28(8): 756-765. DOI:10.3109/02656736.2012.729173 |
| [29] |
CRAPSER J, RITZEL R, VERMA R, et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice[J]. Aging, 2016, 8(5): 1049-1060. |
| [30] |
AKBARI P, BRABER S, Varasteh S, et al. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins[J]. Archives of Toxicology, 2017, 91(3): 1007-1029. DOI:10.1007/s00204-016-1794-8 |
| [31] |
BISCHOFF S C, BARBARA G, BUURMAN W, et al. Intestinal permeability-a new target for disease prevention and therapy[J]. BMC Gastroenterology, 2014, 14: 189. DOI:10.1186/s12876-014-0189-7 |
| [32] |
NAKASHIMA R, KAMATA Y, NISHIKAWA Y. Effects of Escherichia coli heat-stable enterotoxin and guanylin on the barrier integrity of intestinal epithelial T84 cells[J]. Veterinary Immunology and Immunopathology, 2013, 152(1/2): 78-81. |
| [33] |
DEVRIENDT B, STUYVEN E, VERDONCK F, et al. Enterotoxigenic Escherichia coli (K88) induce proinflammatory responses in porcine intestinal epithelial cells[J]. Developmental & Comparative Immunology, 2010, 34(11): 1175-1182. |
| [34] |
CAMPBELL H K, MAIERS J L, DEMALI K A. Interplay between tight junctions & adherens junctions[J]. Experimental Cell Research, 2017, 358(1): 39-44. DOI:10.1016/j.yexcr.2017.03.061 |
| [35] |
RALEIGH D R, BOE D M, YU D, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function[J]. Journal of Cell Biology, 2011, 193(3): 565-582. DOI:10.1083/jcb.201010065 |
| [36] |
MUKIZA C N, DUBREUIL J D. Escherichia coli heat-stable toxin b impairs intestinal epithelial barrier function by altering tight junction proteins[J]. Infection and Immunity, 2013, 81(8): 2819-2827. DOI:10.1128/IAI.00455-13 |
| [37] |
NASSOUR H, DUBREUIL J D. Escherichia coli STb enterotoxin dislodges claudin-1 from epithelial tight junctions[J]. PLoS One, 2014, 9(11): e113273. DOI:10.1371/journal.pone.0113273 |
| [38] |
STAFF PO. Correction:escherichia coli STb enterotoxin dislodges claudin-1 from epithelial tight junctions[J]. PLoS One, 2015, 10(3): e0118983. DOI:10.1371/journal.pone.0118983 |
| [39] |
WU X, SU D. Enterotoxigenic Escherichia coli infection induces tight junction proteins expression in mice[J]. Iranian Journal of Veterinary Research, 2018, 19(1): 35-40. |
| [40] |
HILL D R, HUANG S, NAGY M S, et al. Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium[J]. eLife, 2017, 6: e29132. DOI:10.7554/eLife.29132 |
| [41] |
XU C L, WANG Y M, SUN R, et al. Modulatory effects of vasoactive intestinal peptide on intestinal mucosal immunity and microbial community of weaned piglets challenged by an enterotoxigenic Escherichia coli (K88)[J]. PLoS One, 2014, 9(8): e104183. DOI:10.1371/journal.pone.0104183 |
| [42] |
MESSORI S, TREVISI P, SIMONGIOVANNI A, et al. Effect of susceptibility to enterotoxigenic Escherichia coli F4 and of dietary tryptophan on gut microbiota diversity observed in healthy young pigs[J]. Veterinary Microbiology, 2013, 162(1): 173-179. DOI:10.1016/j.vetmic.2012.09.001 |
| [43] |
POP M, PAULSON J N, CHAKRABORTY S, et al. Individual-specific changes in the human gut microbiota after challenge with enterotoxigenic Escherichia coli and subsequent ciprofloxacin treatment[J]. BMC Genomics, 2016, 17: 440. DOI:10.1186/s12864-016-2777-0 |
| [44] |
SETTY P, FOUTZ T, BOEDEKER E C. A new mouse model for human enterotoxigenic E. coli (ETEC) infection:effect of the gut microbiota on intestinal secretion[J]. Gastroenterology, 2017, 152(5): S815-S816. |
| [45] |
DAUDELIN J F, LESSARD M, BEAUDOIN F, et al. Administration of probiotics influences F4(K88)-positive enterotoxigenic Escherichia coli attachment and intestinal cytokine expression in weaned pigs[J]. Veterinary Research, 2011, 42: 69. DOI:10.1186/1297-9716-42-69 |
| [46] |
REN W K, YIN J, XIAO H, et al. Intestinal microbiota-derived GABA mediates interleukin-17 expression during enterotoxigenic Escherichia coli infection[J]. Frontiers in Immunology, 2016, 7(9): 685-700. |
| [47] |
WANG X G, HARDWIDGE P R. Enterotoxigenic Escherichia coli prevents host NF-κB activation by targeting IκBα polyubiquitination[J]. Infection and Immunity, 2012, 80(12): 4417-4425. DOI:10.1128/IAI.00809-12 |
| [48] |
WANG G C, GEISBRECHT B V, RUETER C, et al. Enterotoxigenic Escherichia coli flagellin inhibits TNF-induced NF-κB activation in intestinal Epithelial cells[J]. Pathogens, 2017, 6(2): 18. DOI:10.3390/pathogens6020018 |
| [49] |
BAO W B, YE L, PAN Z Y, et al. Analysis of polymorphism in the porcine TLR4 gene and its expression related to Escherichia coli F18 infection[J]. Czech Journal of Animal Science, 2011, 56(11): 475-482. DOI:10.17221/CJAS |
| [50] |
LOOS M, GEENS M, SCHAUVLIEGE S, et al. Role of heat-stable enterotoxins in the induction of early immune responses in piglets after infection with enterotoxigenic Escherichia coli[J]. PLoS One, 2012, 7(7): e41041. DOI:10.1371/journal.pone.0041041 |
| [51] |
REN W K, YIN J, DUAN J L, et al. Mouse intestinal innate immune responses altered by enterotoxigenic Escherichia coli (ETEC) infection[J]. Microbes and Infection, 2014, 16(11): 954-961. DOI:10.1016/j.micinf.2014.09.005 |
| [52] |
SUGIHARTO S, POULSEN A S R, CANIBE N, et al. Effect of bovine colostrum feeding in comparison with milk replacer and natural feeding on the immune responses and colonisation of enterotoxigenic Escherichia coli in the intestinal tissue of piglets[J]. British Journal of Nutrition, 2015, 113(6): 923-934. DOI:10.1017/S0007114514003201 |
| [53] |
KIM S J, KWON C H, PARK B C, et al. Effects of a lipid-encapsulated zinc oxide dietarysupplement, on growth parameters and intestinal morphology in weanling pigs artificially infected with enterotoxigenic Escherichia coli[J]. Journal of Animal Science and Technology, 2015, 57: 4. DOI:10.1186/s40781-014-0038-9 |
| [54] |
LV Y, LI X N, ZHANG L, et al. Injury and mechanism of recombinant E. coli expressing STa on piglets colon[J]. Journal of Veterinary Medical Science, 2018, 80(2): 205-212. DOI:10.1292/jvms.17-0528 |
| [55] |
GHOSAL A, CHATTERJEE N S, CHOU T, et al. Enterotoxigenic Escherichia coli infection and intestinal thiamin uptake:studies with intestinal epithelial Caco-2 monolayers[J]. American Journal of Physiology-Cell Physiology, 2013, 305(11): C1185-C1191. DOI:10.1152/ajpcell.00276.2013 |
| [56] |
ASHOKKUMAR B, KUMAR J S, HECHT G A, et al. Enteropathogenic Escherichia coli inhibits intestinal vitamin B1 (thiamin) uptake:studies with human-derived intestinal epithelial Caco-2 cells[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2009, 297(4): G825-G833. DOI:10.1152/ajpgi.00250.2009 |
| [57] |
ZACHOS N C, KOVBASNJUK O, FOULKE-ABEL J, et al. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology[J]. Journal of Biological Chemistry, 2015, 291(8): 3759-3766. |
| [58] |
SATO T, VRIES R G, SNIPPERT H J, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche[J]. Nature, 2009, 459(7244): 262-265. DOI:10.1038/nature07935 |
| [59] |
ANDERSSON-ROLF A, ZILBAUER M, KOO B K, et al. Stem cells in repair of gastrointestinal epithelia[J]. Physiology, 2017, 32(4): 278-289. DOI:10.1152/physiol.00005.2017 |
| [60] |
PATTISON A M, BLOMAIN E S, MERLINO D J, et al. Intestinal enteroids model guanylate cyclase c-dependent secretion induced by heat-stable enterotoxins[J]. Infection and Immunity, 2016, 84(10): 3083-3091. DOI:10.1128/IAI.00639-16 |
| [61] |
LEOCÁDIO P C L, ANTUNES M M, TEIXEIRA L G, et al. L-Arginine pretreatment reduces intestinal mucositis as induced by 5-FU in mice[J]. Nutrition and Cancer, 2015, 67(3): 486-493. DOI:10.1080/01635581.2015.1004730 |
| [62] |
REN W K, DUAN J L, YIN J, et al. Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine[J]. Amino Acids, 2014, 46(10): 2403-2413. DOI:10.1007/s00726-014-1793-0 |
| [63] |
LIU G, REN W K, FANG J, et al. L-glutamine and L-arginine protect against enterotoxigenic Escherichia coli infection via intestinal innate immunity in mice[J]. Amino Acids, 2017, 49(12): 1945-1954. DOI:10.1007/s00726-017-2410-9 |
| [64] |
TANG Y L, TAN B, XIONG X, et al. Methionine deficiency reduces autophagy and accelerates death in intestinal epithelial cells infected with enterotoxigenic Escherichia coli[J]. Amino Acids, 2015, 47(10): 2199-2204. DOI:10.1007/s00726-014-1781-4 |
| [65] |
LI H H, ZHANG L, CHEN L B, et al. Lactobacillus acidophilus alleviates the inflammatory response to enterotoxigenic Escherichia coli K88 via inhibition of the NF-κB and p38 mitogen-activated protein kinase signaling pathways in piglets[J]. BMC Microbiology, 2016, 16: 273. DOI:10.1186/s12866-016-0862-9 |
| [66] |
XIAO D F, WANG Y F, LIU G, et al. Effects of chitosan on intestinal inflammation in weaned pigs challenged by enterotoxigenic Escherichia coli[J]. PLoS One, 2014, 9(8): e104192. DOI:10.1371/journal.pone.0104192 |
| [67] |
ZHANG W, ZHU Y H, YANG J C, et al. A selected Lactobacillus rhamnosus strain promotes egfr-independent akt activation in an enterotoxigenic Escherichia coli K88-infected IPEC-J2 cell model[J]. PLoS One, 2015, 10(4): e0125717. DOI:10.1371/journal.pone.0125717 |
| [68] |
谷巍, 王丽荣, 孙明杰, 等. 嗜酸乳杆菌发酵中药对产肠毒素大肠杆菌K88所致腹泻的防治作用[J]. 中国畜牧兽医, 2018, 45(3): 798-806. |
| [69] |
李亚雄, 仇正兴, 何闪, 等. 日粮中添加微生态制剂对断奶仔猪生长性能、肠道健康和由F18+大肠杆菌引起的仔猪腹泻的影响[J]. 国外畜牧学(猪与禽), 2016(4): 61-66. |
| [70] |
蒲俊宁, 陈代文, 田刚, 等. 苯甲酸、凝结芽孢杆菌和牛至油复合添加剂对大肠杆菌攻毒仔猪生长性能、抗氧化能力和空肠消化吸收功能的影响[J]. 动物营养学报, 2018, 30(9): 3652-3661. DOI:10.3969/j.issn.1006-267x.2018.09.036 |
| [71] |
SKROVANEK S, DIGUILIO K, BAILEY R, et al. Zinc and gastrointestinal disease[J]. World Journal of Gastrointestinal Pathophysiology, 2014, 5(4): 496-513. DOI:10.4291/wjgp.v5.i4.496 |
| [72] |
HEIM G, SWEENEY T, O'SHEA C J, et al. Effect of maternal supplementation with seaweed extracts on growth performance and aspects of gastrointestinal health of newly weaned piglets after challenge with enterotoxigenic Escherichia coli K88[J]. British Journal of Nutrition, 2014, 112(12): 1955-1965. DOI:10.1017/S0007114514003171 |




