2. 重庆市肉牛工程技术研究中心, 重庆 402460;
3. 重庆市畜牧技术推广总站, 重庆 401121
2. Beef Cattle Engineering and Technology Research Center of Chongqing, Chongqing 402460, China;
3. Chongqing Animal Husbandry Technology Extension Station, Chongqing 401121, China
2017年,我国奶产量年单产达9 t以上的奶牛超过130万头,约占我国成母牛数量的15%。部分牧场的年单产已经达到12 t,进入世界领先水平。随着奶产量的提高,奶牛的被动淘汰率也随之升高,特别是在围产期(从产前21 d至产后21 d)。一些奶牛场的成母牛淘汰率高达30%。在围产后期,奶牛通过饲料获得的能量远不能满足泌乳量快速增加的需要,导致体脂动员量快速增加,并诱发脂肪肝、酮病等一系列由脂肪代谢障碍导致的营养代谢病[1-3]。围产后期奶牛的脂肪动员受体况、代谢产物、激素、相关酶和脂肪因子等因素的综合调控。奶牛的产前体况评分(BCS)和产后BCS变化量(△BCS)可用来估测围产期奶牛的能量平衡状态[4]。研究发现,产前BCS>3.75或产后△BCS>0.75的奶牛脂肪动员量显著增加,奶产量较高,且产后患病风险增加,首配受胎率降低,淘汰率增加[5-6]。而低体况奶牛患跛行的风险更高,产奶量较低,肝脏损害程度更大[7-8]。本文综述了产前体况对围产期奶牛脂肪代谢产物、脂肪酶活性和脂肪因子表达等方面影响的研究,为进一步开展体况对奶牛产后脂肪代谢的调控作用和调控机制研究供理论依据,为生产上调控奶牛的体况提供实践指导。
1 围产期奶牛的脂肪代谢 1.1 能量供需平衡围产期奶牛要经历妊娠、分娩和泌乳的巨大生理变化。妊娠后期奶牛的营养需求量会增加30%~50%,一部分是由于胎儿营养需求量的增加,一部分由于一系列的机体代谢适应[9]。产前1周的干物质采食量(DMI)比干奶初期下降15%~30%,此时机体能量供给为需求量的87%~125%[10-11]。围产期奶牛的葡萄糖需要量和供给量的关系见图 1,从产前第3天开始奶牛将长时间处于能量负平衡状态,这与黄文明[12]研究结果一致。奶牛产后1周乳腺对葡萄糖、氨基酸和脂肪酸的需要量分别是产前3周的3倍、2倍和5倍,产后1周的泌乳净能需要量约是产前1周的2倍,而能量供给只能满足72%~75%,且其泌乳高峰先于干物质摄入高峰出现,从而使机体长期处于能量负平衡状态[11, 13-14]。
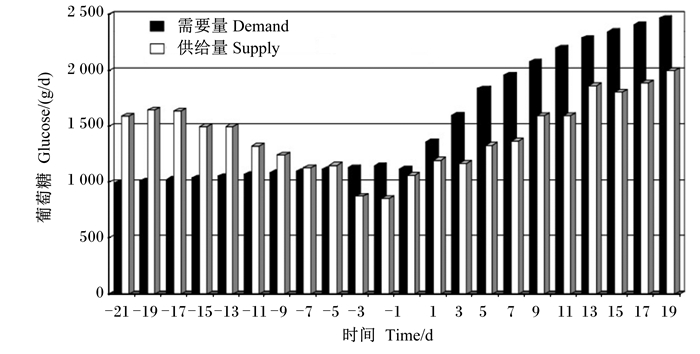
|
图 1 围产期奶牛的葡萄糖需要量和供给量 Fig. 1 Glucose demand and supply of cows during perinatal period[9] |
奶牛在围产期涉及到分娩和泌乳启动的激素变化(包括孕酮的减少和催乳素、生长激素的增加)会直接促进脂肪分解,而高强度的能量需求(特别是葡萄糖)又会加剧脂肪分解速率[15]。当奶牛处于能量负平衡时,机体肾上腺素、胰高血糖素等激素分泌增加,激活腺苷酸环化酶(AC),促进环腺苷酸(cAMP)合成,激活cAMP-蛋白激酶,使脂滴蛋白磷酸化、激素敏感酯酶(HSL)活性升高,促进甘油三酯(TG)水解成甘油和非酯化脂肪酸(NEFA)。一方面,甘油可促进肝脏糖异生,以弥补能量缺口;另一方面,NEFA进入血液循环,再被肝脏摄取利用并被降解供能[16]。研究发现,奶牛产后12周内的脂肪动员量可达90 kg,当脂肪动员量达到3.2 kg/d时,甘油可提供奶牛葡萄糖需求的15%~20%[17-18]。近年来已经确定脂肪组织在调控奶牛采食、能量消耗、脂质代谢和炎症等方面具有重要作用,可通过分泌大量与中枢和外周器官(如脑、肝脏、胰腺和骨骼肌)相互作用的脂肪因子来维持机体能量平衡。
1.3 脂肪代谢障碍NEFA进入肝脏后有3条代谢途径,一是彻底氧化供能;二是部分氧化生成酮体,被外周组织利用;三是再酯化形成TG,以极低密度脂蛋白(very low density lipoprotein, VLDL)的形式运出肝脏[19]。NEFA经β-氧化生成大量的乙酰辅酶A(CoA),同时体蛋白降解所得生酮氨基酸因缺乏草酰乙酸,最后生成β-羟基丁酸(β-hydroxybutyric acid,BHBA)、乙酰乙酸等酮体,血浆酮体浓度高于1.2 mmol/L有患亚临床酮病的风险[20]。Hammon等[21]研究发现,奶牛分娩时机体脂肪动员增加,血浆NEFA浓度急剧上升,并伴随着肝脏TG的聚积,从而导致肝脏糖代谢异常。高浓度的NEFA和BHBA可显著抑制生长激素受体1A(GHR1A)mRNA的表达,损害肝脏内生长激素介导的调控肝脏葡萄糖生成途径[22]。泌乳早期血浆NEFA、BHBA、钙离子和葡萄糖浓度与产后奶牛皱胃变位、酮病和淘汰风险有关[2]。研究证明,产后2周内血浆NEFA浓度大于0.8 mmol/L的经产母牛淘汰风险增加2~4倍[23]。以上研究表明,围产期奶牛普遍存在脂肪代谢障碍以及由此导致的营养代谢病。
2 产前体况对围产期奶牛脂肪动员的调控作用及机制BCS是一种评估奶牛体脂储备的评价指标,可用来监测奶牛的体脂储备量和能量平衡状态。BCS体系主要有美国的5分制(1~5)、新西兰的10分制(1~10)等体况评分系统,国内最常用的是美国的5分制。目前已经可以运用数字图像处理技术、热成像仪等技术自动进行体况评分,其预测值比专业人员评定分数值的效率、准确度更高,且更具客观性[24-26]。在奶牛生产的不同阶段,适时进行体况评分,可及时掌握奶牛体脂储备情况。研究证实,围产期奶牛适宜的BCS有利于提高产后的泌乳性能和繁殖能力,但在生产中普遍存在由于繁殖障碍和饲养管理不当等因素造成的异常体况[27]。
2.1 产前体况对奶牛产后生产性能和健康的影响产前体况是泌乳早期奶牛DMI、产奶量和患病率的重要影响因素。高体况(BCS>3.5)奶牛泌乳期产奶量更高,但产后△BCS更大,肝脏脂肪沉积更多,患代谢疾病的风险更大;低体况(BCS < 3.0)奶牛繁殖性能下降,易出现健康障碍[27-29]。研究发现,产后低体况的奶牛更易患乳房炎;BCS<2.0和BCS>3.0的奶牛患跛足的风险较高,患病率分别为26.6%和24.0%[7, 30]。产前饲喂高能量水平(基础需求量125%)饲粮的奶牛产后△BCS比饲喂低能量水平(基础需求量75%)饲粮的奶牛多1分左右[27],且干奶期BCS的增加会降低产后DMI,加剧产后能量负平衡,增加机体脂肪动员,从而导致酮病发生率增加[31]。这是由于高体况奶牛的DMI比低体况奶牛低,血浆NEFA浓度更高,产后能量失衡更严重。以上研究表明,产前体况对奶牛产后泌乳性能、繁殖性能和患病风险等方面均有影响。
2.2 产前体况对脂肪代谢血液生化指标的影响由于围产期能量需求增加,机体出现能量失衡、脂肪动员量增加,导致脂解代谢产物如NEFA和BHBA浓度的增加[32]。血浆NEFA和BHBA浓度是评估奶牛能量平衡状态的有效指标[33]。产前BCS平均值为4.17(5分制)的高体况奶牛与BCS平均值为2.33的低体况奶牛相比,产后7周内能量负平衡更严重,体脂动员量更多,血浆NEFA和BHBA浓度更高[23-34]。高BHBA浓度的奶牛脂肪组织胰岛素抵抗(insulin resistance, IR)程度更强,导致产后脂肪组织动员比例更高[35-36]。最新研究发现,尽管BCS为3.75的奶牛在分娩前后血浆NEFA浓度较高,但其对脂肪组织中NEFA的分解和甘油的利用更好[37]。这表明了高BCS奶牛能更好地利用这些脂肪代谢产物。
近几年越来越多的研究发现,除了NEFA和BHBA外,胰岛素(insulin, INS)、葡萄糖和胰岛素样生长因子-1(IGF-1)也可以成为衡量能量平衡的指标[34-35]。理想体况(BCS为3.25~3.75)的奶牛产前30 d至产后60 d血液葡萄糖浓度无显著变化;高体况(BCS≥4.00)奶牛产前血液葡萄糖浓度高于理想体况奶牛,而产后血液葡萄糖浓度显著下降[38]。这表明高体况奶牛产后呈现的能量负平衡程度更高。Pires等[34]研究结果表明,血浆IGF-1浓度与产前BCS呈显著正相关。而近年来有研究结果与之相反,产前BCS与产后血浆IGF-1浓度呈显著负相关,与血浆NEFA浓度呈显著正相关[35]。相对于产前BCS<3.0的奶牛来说,肥胖奶牛(BCS>4.0)的血液葡萄糖、INS浓度更高,INS敏感性较低[39]。与之相反,Mann等[40]研究发现,通过脂肪组织中蛋白激酶B(AKT)活性、胰岛素受体(insulin receptor, INSR)数量和过氧化物酶体增殖物激活受体γ(PPARγ)传递出的INS信号在不同BCS奶牛中均没有显著性差异。脂肪组织中INSR和葡萄糖转运蛋白4(GLUT4)具有调控葡萄糖摄取能力的作用。近期的一项研究发现,低BCS(BCS≤3.00)奶牛产后脂肪组织中INSR的mRNA表达丰度高于中BCS(BCS为3.25~3.50)和高BCS(BCS≥3.75)奶牛,且在高BCS奶牛中表达最少;产前高BCS奶牛的GLUT4蛋白表达水平极显著低于中、低BCS奶牛[41]。这表明围产前期高BCS奶牛的葡萄糖摄取能力较低。综上,脂肪组织IR的发生与脂肪细胞表面INSR数量减少和结合力降低有关,产前过肥的奶牛更易发生IR,而INS信号转导与BCS的相关性还需进一步确认。
2.3 产前体况对脂肪酶活性的影响脂肪代谢主要是受内分泌系统和交感神经系统控制,脂肪组织的应答元件主要包括β-肾上腺素能受体(AR)亚型、脂肪甘油三酯脂酶(adipose triglyceride lipase, ATGL)、HSL和单甘油酯脂肪酶(monoglyceride lipase, MGL)的表达与活化,而△BCS也会引起脂肪代谢分子调控机制变化。Akbar等[42]就BCS变化对奶牛代谢和分子变化的影响进行了研究,结果发现,与低体况组(BCS=3.5)奶牛相比,高体况(BCS=5.5)和中等体况组(BCS=4.5)奶牛产后的血浆NEFA、BHBA浓度和肝脏TG沉积更多,且脂肪酸氧化基因[肉碱棕榈酰转移酶1A(CPT1A)、酯酰辅酶A氧化酶1(ACOX1)、极长链酰基辅酶A脱氢酶(ACADVL)]、酮体生成基因[线粒体羟甲基戊二酸单酰辅酶A合酶2(HMGCS2)]、肝细胞因子[成纤维细胞生长因子21(FGF21)]和葡萄糖异生等相关基因表达量显著增加,而生长激素/胰岛素样生长因子-1(GH/IGF-1)相关基因表达更少。这些基因表达有助于机体的糖异生作用,提高奶牛的产奶量。Alharthi等[37]还发现与高BCS奶牛(BCS≥3.75)相比,低BCS奶牛(BCS≤3.25)具有更高的ATGL、脂联素(adiponectin, ADIPOQ)和ATGL的激活蛋白自水解酶结构域5(ABHD5)的表达,表明其更强的基础脂解活性和ADIPOQ分泌能力,NEFA氧化作用较低;而高BCS奶牛则通过脂肪组织内线粒体或过氧化物酶β氧化增加NEFA作为能量的利用率。可能是由于即使血浆NEFA浓度与CPT1A基因表达的增加趋势一致,但NEFA的再酯化基因[磷酸烯醇式丙酮酸羧激酶1(PCK1)]和甘油排出基因[水-甘油通道蛋白7(AQP7)]的表达逐渐减少,甘油激酶(GK)表达增加,由此对脂肪组织内NEFA的利用有一定的刺激作用[37]。
胎球蛋白A(fetuin-A,FetA)是一种具有促进游离脂肪酸转运、增强细胞脂质摄取和脂肪生成功能的急性期蛋白,其编码基因α2-HS-糖蛋白(alpha 2-heremans-schmid glycolprotein, AHSG)在脂肪组织中的表达类似于脂肪生成标志物ABHD5、脂肪酸结合蛋白4(FABP4)、PPARγ的表达模式,对于围产期奶牛适应能量负平衡状态有促进作用。研究发现,产前BCS对血清FetA浓度无影响,而高BCS(BCS≥3.75)奶牛进入围产期时该编码基因AHSG的表达量减少[43]。由此表明,高BCS奶牛适应能量负平衡的能力更弱。此外,关于脂肪酸合成酶基因(FASN)的研究发现,高BCS奶牛FASN mRNA表达量比低BCS奶牛高,中等BCS奶牛产前FASN mRNA表达量最高,产后FASN mRNA表达量均开始下降;且在产前低能饲喂条件下,BCS为5(10分制)的奶牛FASN mRNA表达量仍然高于BCS为4的奶牛,但产后FASN的活性并无显著差异[40, 44]。这表明,产前高、中等BCS奶牛机体脂肪库中脂肪沉积能力更强,过多的脂质会触动胰腺β细胞分泌FetA,但产后各BCS奶牛中脂肪合成能力无明显差异。
2.4 产前体况对脂肪因子动态表达的影响脂肪因子通过旁分泌或自分泌作用调节体脂储备,维持葡萄糖、脂质和能量平衡之间的稳定。一些脂肪细胞因子是由脂肪细胞直接分泌而来,如瘦素(leptin, LP)、ADIPOQ、抵抗素(resistin);另一些则是由脂肪组织中的巨噬细胞分泌以调控脂肪细胞代谢,如趋化因子配体5(CCL5)、白细胞介素-6(IL-6)和肿瘤坏死因子(TNF)等。围产期奶牛体脂过度动员会造成脂肪细胞因子分泌模式的改变,有利于利用脂肪酸作为能量来源以维持胎儿生长和泌乳[45]。LP浓度在生理范围内时抑制脂肪合成,当体内LP和INS浓度明显增高时则提高脂肪细胞摄入脂肪酸的速率,促进脂肪形成[46-47]。研究发现,产前BCS≥3.75的高体况奶牛在产前LP浓度高于BCS < 3.75的奶牛,分娩后急剧下降,可能是由于INS浓度下降和葡萄糖消耗的影响,但总体上高BCS组高于BCS≤2.5的低体况奶牛[34]。ADIPOQ也可促进脂肪细胞的脂肪生成作用,提高肝脏INS敏感性,促进脂肪酸氧化,防止异位脂质积聚,其血浆浓度在干奶末期下降,与奶牛的BCS呈负相关,在葡萄糖代谢水平上与INS敏感性呈正相关[48]。在分娩前后处于能量负平衡的状态下,奶牛的高体况触发了脂肪组织上调ADIPOQ基因表达的反应,且体内CCL5、IL-6和TNF的表达水平均增加[37]。Lange等[49]研究发现,产前饲喂高能饲粮的奶牛产后脂肪因子表达会增加,特别是LP和ADIPOQ。综上所述,ADIPOQ在高BCS奶牛中的过度表达会增加脂肪组织中脂肪质量,提高INS敏感性。
脂肪因子可通过自分泌局部作用于脂肪细胞,维持机体葡萄糖稳态。De Koster等[50]从细胞层面研究不同BCS奶牛的脂肪细胞分解的特征,利用体外组织块培养脂肪细胞,结果显示奶牛BCS与脂肪细胞大小呈正相关,与脂肪细胞数量呈负相关;且肥大脂肪细胞的基质脂解活性更强。这也许是由于肥大细胞中第一限速酶——ATGL活性增加,促进脂肪分解。Depreester等[51]发现,在奶牛干奶末期促炎症脂肪因子LP、IL-6和TNF基因表达与脂肪细胞大小呈正相关,作为抗炎症因子的ADIPOQ在不同BCS奶牛中的表达无显著差异,而血清ADIPOQ循环浓度与BCS间呈显著负相关[52]。ADIPOQ可局部作用于脂肪细胞,增加葡萄糖摄入和血清葡萄糖浓度,促进脂肪组织扩张。然而,Vailati-Riboni等[44]报道,奶牛体况会影响脂肪细胞分化和肥大过程中PPARγ和ADIPOQ的表达,且在BCS为5(10分制)的奶牛中表达量更多,这些细胞因子直接作用于靶组织或内分泌系统调节机体脂肪代谢。
3 小结近年来,关于奶牛体况和产前饲粮营养水平调节围产期奶牛脂肪代谢的研究已有巨大进步。异常体况造成奶牛产后脂肪代谢紊乱,而高体况与低体况奶牛的代谢规律亦不一致,高BCS奶牛在产后主要依赖体脂动员供能,而低BCS奶牛体脂储备缺乏则更多地依赖于体蛋白质降解作用。奶牛产前体况可调控脂肪细胞因子分泌、脂肪酶表达来影响奶牛产后脂肪动员情况,以满足能量供给的代谢需求,但具体的调控机理还有待进一步研究。
| [1] |
GONZÁLEZ F D, MUIÑO R, PEREIRA V, et al. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows[J]. Journal of Veterinary Science, 2011, 12(3): 251-255. DOI:10.4142/jvs.2011.12.3.251 |
| [2] |
SEIFI H A, LEBLANC S J, LESLIE K E, et al. Metabolic predictors of post-partum disease and culling risk in dairy cattle[J]. The Veterinary Journal, 2011, 188(2): 216-220. DOI:10.1016/j.tvjl.2010.04.007 |
| [3] |
DU X L, ZHU Y W, PENG Z C, et al. High concentrations of fatty acids and β-hydroxybutyrate impair the growth hormone-mediated hepatic JAK2-STAT5 pathway in clinically ketotic cows[J]. Journal of Dairy Science, 2018, 101(4): 3476-3487. DOI:10.3168/jds.2017-13234 |
| [4] |
ROCHE J R, KAY J K, FRIGGENS N C, et al. Assessing and managing body condition score for the prevention of metabolic disease in dairy cows[J]. Veterinary Clinics of North America:Food Animal Practice, 2013, 29(2): 323-336. DOI:10.1016/j.cvfa.2013.03.003 |
| [5] |
SMITH R F, OULTRAM J, DOBSON H. Herd monitoring to optimise fertility in the dairy cow:making the most of herd records, metabolic profiling and ultrasonography (research into practice)[J]. Animal, 2014, 8(Suppl.1): 185-198. |
| [6] |
KIM I H, SUH G H. Effect of the amount of body condition loss from the dry to near calving periods on the subsequent body condition change, occurrence of postpartum diseases, metabolic parameters and reproductive performance in Holstein dairy cows[J]. Theriogenology, 2003, 60(8): 1445-1456. DOI:10.1016/S0093-691X(03)00135-3 |
| [7] |
RANDALL L V, GREEN M J, CHAGUNDA M G G, et al. Low body condition predisposes cattle to lameness:An 8-year study of one dairy herd[J]. Journal of Dairy Science, 2015, 98(6): 3766-3777. DOI:10.3168/jds.2014-8863 |
| [8] |
VAILATI-RIBONI M, MEIER S, BURKE C R, et al. Prepartum body condition score and plane of nutrition affect the hepatic transcriptome during the transition period in grazing dairy cows[J]. BMC Genomics, 2016, 17: 854. DOI:10.1186/s12864-016-3191-3 |
| [9] |
BELL A W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation[J]. Journal of Animal Science, 1995, 73(9): 2804-2819. DOI:10.2527/1995.7392804x |
| [10] |
BELL A W, BAUMAN D E. Adaptations of glucose metabolism during pregnancy and lactation[J]. Journal of Mammary Gland Biology and Neoplasia, 1997, 2(3): 265-278. DOI:10.1023/A:1026336505343 |
| [11] |
HUANG W M, TIAN Y J, WANG Y J, et al. Effect of reduced energy density of close-up diets on dry matter intake, lactation performance and energy balance in multiparous Holstein cows[J]. Journal of Animal Science and Biotechnology, 2014, 5: 30. DOI:10.1186/2049-1891-5-30 |
| [12] |
黄文明.围产前期日粮能量水平对奶牛能量代谢和瘤胃适应性影响的研究[D].博士学位论文.北京: 中国农业大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10019-1014225963.htm
|
| [13] |
LEROY J L, VANHOLDER T, VAN KNEGSEL A T, et al. Nutrient prioritization in dairy cows early postpartum:mismatch between metabolism and fertility?[J]. Reproduction in Domestic Animals, 2008, 43(Suppl.2): 96-103. |
| [14] |
苏华维, 曹志军, 李胜利. 围产期奶牛的代谢特点及其营养调控[J]. 中国畜牧杂志, 2011, 46(16): 44-48. |
| [15] |
SORDILLO L M, RAPHAEL W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders[J]. Veterinary Clinics of North America:Food Animal Practice, 2013, 29(2): 267-278. DOI:10.1016/j.cvfa.2013.03.002 |
| [16] |
孙菲菲, 曹阳春, 李生祥, 等. 胆碱对奶牛围产期代谢的调控[J]. 动物营养学报, 2014, 26(1): 26-33. DOI:10.3969/j.issn.1006-267x.2014.01.004 |
| [17] |
DOEPEL L, LOBLEY G E, BERNIER J F, et al. Differences in splanchnic metabolism between late gestation and early lactation dairy cows[J]. Journal of Dairy Science, 2009, 92(7): 3233-3243. DOI:10.3168/jds.2008-1595 |
| [18] |
CHIBISA G E, GOZHO G N, VAN KESSEL A G, et al. Effects of peripartum propylene glycol supplementation on nitrogen metabolism, body composition, and gene expression for the major protein degradation pathways in skeletal muscle in dairy cows[J]. Journal of Dairy Science, 2008, 91(9): 3512-3527. DOI:10.3168/jds.2007-0920 |
| [19] |
WILLIAMS C C, CALMES K J, FERNANDEZ J M, et al. Glucose metabolism and insulin sensitivity in gulf coast native and suffolk ewes during late gestation and early lactation[J]. Small Ruminant Research, 2004, 54(3): 167-171. DOI:10.1016/j.smallrumres.2003.11.007 |
| [20] |
ESPOSITO G, IRONS P C, WEBB E C, et al. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows[J]. Animal Reproduction Science, 2014, 144(3/4): 60-71. |
| [21] |
HAMMON H M, STVRMER G, SCHNEIDER F, et al. Performance and metabolic and endocrine changes with emphasis on glucose metabolism in high-yielding dairy cows with high and low fat content in liver after calving[J]. Journal of Dairy Science, 2009, 92(4): 1554-1566. DOI:10.3168/jds.2008-1634 |
| [22] |
OSPINA P A, NYDAM D V, STOKOL T, et al. Evaluation of nonesterified fatty acids and β-hydroxybutyrate in transition dairy cattle in the northeastern United States:critical thresholds for prediction of clinical diseases[J]. Journal of Dairy Science, 2010, 93(2): 546-554. DOI:10.3168/jds.2009-2277 |
| [23] |
ROBERTS T, CHAPINAL N, LEBLANC S J, et al. Metabolic parameters in transition cows as indicators for early-lactation culling risk[J]. Journal of Dairy Science, 2012, 95(6): 3057-3063. DOI:10.3168/jds.2011-4937 |
| [24] |
FISCHER A, LUGINBVHL T, DELATTRE L, et al. Rear shape in 3 dimensions summarized by principal component analysis is a good predictor of body condition score in Holstein dairy cows[J]. Journal of Dairy Science, 2015, 98(7): 4465-4476. DOI:10.3168/jds.2014-8969 |
| [25] |
HALACHMI I, KLOPČIČ I M, POLAK P, et al. Automatic assessment of dairy cattle body condition score using thermal imaging[J]. Computers and Electronics in Agriculture, 2013, 99: 35-40. DOI:10.1016/j.compag.2013.08.012 |
| [26] |
SPOLIANSKY R, EDAN Y, PARMET Y, et al. Development of automatic body condition scoring using a low-cost 3-dimensional Kinect camera[J]. Journal of Dairy Science, 2016, 99(9): 7714-7725. DOI:10.3168/jds.2015-10607 |
| [27] |
ROCHE J R, MEIER S, HEISER A, et al. Effects of precalving body condition score and prepartum feeding level on production, reproduction, and health parameters in pasture-based transition dairy cows[J]. Journal of Dairy Science, 2015, 98(10): 7164-7182. DOI:10.3168/jds.2014-9269 |
| [28] |
ROCHE J R, FRIGGENS N C, KAY J K, et al. Invited review:body condition score and its association with dairy cow productivity, health, and welfare[J]. Journal of Dairy Science, 2009, 92(12): 5769-5801. DOI:10.3168/jds.2009-2431 |
| [29] |
CHAGAS L M, BASS J J, BLACHE D, et al. Invited review:new perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows[J]. Journal of Dairy Science, 2007, 90(9): 4022-4032. DOI:10.3168/jds.2006-852 |
| [30] |
LOKER S, MIGLIOR F, KOECK A, et al. Relationship between body condition score and health traits in first-lactation Canadian Holsteins[J]. Journal of Dairy Science, 2012, 95(11): 6770-6780. DOI:10.3168/jds.2012-5612 |
| [31] |
WATHES D C, CHENG Z, BOURNE N, et al. Differences between primiparous and multiparous dairy cows in the inter-relationships between metabolic traits, milk yield and body condition score in the periparturient period[J]. Domestic Animal Endocrinology, 2007, 33(2): 203-225. DOI:10.1016/j.domaniend.2006.05.004 |
| [32] |
DJOKOVIC R, CINCOVIC M, KURCUBIC V, et al. Endocrine and metabolic status of dairy cows during transition period[J]. Thai Journal of Veterinary Medicine, 2014, 59-66. |
| [33] |
MOUFFOK C E, MADANI T, SEMARA L, et al. Correlation between body condition score, blood biochemical metabolites, milk yield and quality in algerian montbéliarde cattle[J]. Pakistan Veterinary Journal, 2013, 33(2): 191-194. |
| [34] |
PIRES J A A, DELAVAUD C, FAULCONNIER Y, et al. Effects of body condition score at calving on indicators of fat and protein mobilization of periparturient Holstein-Friesian cows[J]. Journal of Dairy Science, 2013, 96(10): 6423-6439. DOI:10.3168/jds.2013-6801 |
| [35] |
STEFAŃ SKA B, NOWAK W, PRUSZY Ń SKA-OSZMAŁEK E, et al. The effect of body condition score on the biochemical blood indices and reproductive performance of dairy cows[J]. Annals of Animal Science, 2016, 16(1): 129-143. DOI:10.1515/aoas-2015-0064 |
| [36] |
LANGE J, MCCARTHY A, KAY J, et al. Prepartum feeding level and body condition score affect immunological performance in grazing dairy cows during the transition period[J]. Journal of Dairy Science, 2016, 99(3): 2329-2338. DOI:10.3168/jds.2015-10135 |
| [37] |
ALHARTHI A, ZHOU Z, LOPREIATO V, et al. Body condition score prior to parturition is associated with plasma and adipose tissue biomarkers of lipid metabolism and inflammation in Holstein cows[J]. Journal of Animal Science and Biotechnology, 2018, 9: 12. DOI:10.1186/s40104-017-0221-1 |
| [38] |
FOLNOŽIĆ I, TURK R, DURIČIĆ D, et al. Influence of body condition on serum metabolic indicators of lipid mobilization and oxidative stress in dairy cows during the transition period[J]. Reproduction in Domestic Animals, 2015, 50(6): 910-917. DOI:10.1111/rda.2015.50.issue-6 |
| [39] |
RICO J E, BANDARU V V R, DORSKIND J M, et al. Plasma ceramides are elevated in overweight Holstein dairy cows experiencing greater lipolysis and insulin resistance during the transition from late pregnancy to early lactation[J]. Journal of Dairy Science, 2015, 98(11): 7757-7770. DOI:10.3168/jds.2015-9519 |
| [40] |
MANN S, NYDAM D V, ABUELO A, et al. Insulin signaling, inflammation, and lipolysis in subcutaneous adipose tissue of transition dairy cows either overfed energy during the prepartum period or fed a controlled-energy diet[J]. Journal of Dairy Science, 2016, 99(8): 6737-6752. DOI:10.3168/jds.2016-10969 |
| [41] |
JAAKSON H, KARIS P, LING K, et al. Adipose tissue insulin receptor and glucose transporter 4 expression, and blood glucose and insulin responses during glucose tolerance tests in transition Holstein cows with different body condition[J]. Journal of Dairy Science, 2018, 101(1): 752-766. DOI:10.3168/jds.2017-12877 |
| [42] |
AKBAR H, GRALA T M, RIBONI M V, et al. Body condition score at calving affects systemic and hepatic transcriptome indicators of inflammation and nutrient metabolism in grazing dairy cows[J]. Journal of Dairy Science, 2015, 98(2): 1019-1032. DOI:10.3168/jds.2014-8584 |
| [43] |
STRIEDER-BARBOZA C, DE SOUZA J, RAPHAEL W, et al. Fetuin-a:a negative acute-phase protein linked to adipose tissue function in periparturient dairy cows[J]. Journal of Dairy Science, 2018, 101(3): 2602-2616. DOI:10.3168/jds.2017-13644 |
| [44] |
VAILATI-RIBONI M, KANWAL M, BULGARI O, et al. Body condition score and plane of nutrition prepartum affect adipose tissue transcriptome regulators of metabolism and inflammation in grazing dairy cows during the transition period[J]. Journal of Dairy Science, 2016, 99(1): 758-770. DOI:10.3168/jds.2015-10046 |
| [45] |
STERN J H, RUTKOWSKI J M, SCHERER P E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk[J]. Cell Metabolism, 2016, 23(5): 770-784. DOI:10.1016/j.cmet.2016.04.011 |
| [46] |
HO M, FOXALL S, HIGGINBOTTOM M, et al. Leptin-mediated inhibition of the insulin-stimulated increase in fatty acid uptake in differentiated 3T3-L1 adipocytes[J]. Metabolism, 2006, 55(1): 8-12. DOI:10.1016/j.metabol.2005.06.013 |
| [47] |
PALOU A, PICÍ C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life[J]. Appetite, 2009, 52(1): 249-252. DOI:10.1016/j.appet.2008.09.013 |
| [48] |
DE KOSTER J, URH C, HOSTENS M, et al. Relationship between serum adiponectin concentration, body condition score, and peripheral tissue insulin response of dairy cows during the dry period[J]. Domestic Animal Endocrinology, 2017, 59: 100-104. DOI:10.1016/j.domaniend.2016.12.004 |
| [49] |
LANGE J, MCCARTHY A, KAY J, et al. Prepartum feeding level and body condition score affect immunological performance in grazing dairy cows during the transition period[J]. Journal of Dairy Science, 2016, 99(3): 2329-2338. DOI:10.3168/jds.2015-10135 |
| [50] |
DE KOSTER J, VAN DEN BROECK W, HULPIO L, et al. Influence of adipocyte size and adipose depot on the in vitro lipolytic activity and insulin sensitivity of adipose tissue in dairy cows at the end of the dry period[J]. Journal of Dairy Science, 2016, 99(3): 2319-2328. DOI:10.3168/jds.2015-10440 |
| [51] |
DEPREESTER E, DE KOSTER J, VAN POUCKE M, et al. Influence of adipocyte size and adipose depot on the number of adipose tissue macrophages and the expression of adipokines in dairy cows at the end of pregnancy[J]. Journal of Dairy Science, 2018, 101(7): 6542-6555. DOI:10.3168/jds.2017-13777 |
| [52] |
SINGH S P, HÄUSSLER S, HEINZ J F L, et al. Lactation driven dynamics of adiponectin supply from different fat depots to circulation in cows[J]. Domestic Animal Endocrinology, 2014, 47: 35-46. DOI:10.1016/j.domaniend.2013.12.001 |




