骨骼肌是机体蛋白质合成效率最高的组织之一,而蛋白质合成的本质是肌细胞对氨基酸的利用过程[1-2]。有研究发现,机体摄入食物5~7 h后赖氨酸(lysine,Lys)方可被转运到肌肉组织中[3]。与其他必需氨基酸相比,肌细胞中有更多游离Lys聚集,表明肌肉可作为Lys的体内储库。在生长猪和肥育猪上的研究表明,饲粮补充Lys可提高动物机体肌肉蛋白质合成能力和肌肉生长[4-5]。因此,探明Lys等碱性氨基酸转运载体(cationic amino acid transporters, CATs)的功能、表达规律及其调控机制,可为动物体高效吸收利用氨基酸提供理论参考[6-7]。
1 骨骼肌中的CATs以Lys和精氨酸(argine,Arg)等必需氨基酸为主的CATs类型有4种,按其对钠离子(Na+)的依赖性可分为Na+依赖性B0, +转运载体系统和Na+非依赖性b0, +、y+和y+L转运载体系统。且每个系统包括几种不同亚型的转运载体蛋白。其中,b0, +、y+和y+L型转运载体的基因均属于氨基酸溶质转运载体家族(solute carrier family,SLC)7基因。CATs系统分类见表 1。
B0, +由SLC6A14基因编码,它是一种仅在肺脏和唾液腺等少数组织中发现的氨基酸转运载体,能够累积中性和阳离子氨基酸[10]。b0, +由SLC7A9基因编码,是穿过小肠顶膜的主要碱性氨基酸转运蛋白系统[11],在骨骼肌中的mRNA相对表达量较低[12]。y+L由SLC7A7基因编码,在肠和肾脏组织中的mRNA相对表达量较高,在骨骼肌和心脏中mRNA相对表达量均较低[12-13]。
碱性氨基酸转运载体1(CAT-1)、碱性氨基酸转运载体2B(CAT-2B)和碱性氨基酸转运载体3(CAT-3)分别由SLC7A1-3基因编码,它们构成了经典的碱性氨基酸转运蛋白系统y+,y+转运蛋白系统被认为是碱性氨基酸进入细胞的主要吸收途径。其中,CAT-1是骨骼肌中最主要的CATs,几乎在除肝脏外的所有组织中表达。CAT-2有2种由SLC7A2基因编码的剪接变体,分别为CAT-2A和CAT-2B;尽管CAT-2A与CAT-2B的区别只在于一段42个氨基酸的片段,但CAT-2A表现出比CAT-2B低约1/10的底物亲和力且对反式刺激相对不敏感[14]。CAT-2A在肝脏中的mRNA相对表达量较高,在肌肉中的mRNA相对表达量较低[15]。CAT-3在胚胎发育中发挥重要作用,主要在中胚层组织中表达[16]。在非洲爪蟾卵母细胞和哺乳动物细胞中过表达碱性氨基酸转运载体4(CAT-4),未见对氨基酸发挥转运功能,其功能尚不明确[17]。CATs主要负责转运L-Arg、Lys和鸟氨酸等碱性氨基酸,其中CAT-1、CAT-2B和CAT-3是高亲和力的CATs,具有显著的反向刺激效应[18]。在低pH条件下,CAT-1和CAT-2也可转运少量质子化的组氨酸。CATs转运系统的效率取决于2个因素:氨基酸浓度和膜电位。其中,CATs存在底物浓度依赖性转运[19]。此外,CAT-1的底物转运还具有电压依赖性,超极化能够增加氨基酸最大通量并降低电流速率[20-21]。
2 CATs在骨骼肌中的表达规律及其适应性调节 2.1 CATs在骨骼肌中的表达规律Ishida等[12]研究表明,猪背最长肌、股二头肌和菱形肌中主要的CATs为CAT-1和CAT-2,且CAT-1 mRNA的相对表达量随日龄增加而下降,CAT-2 mRNA的相对表达量随日龄增加而增加。张艳[22]研究也表明,长白猪半膜肌和半腱肌中CAT-1 mRNA的相对表达量从出生起持续下降,背最长肌CAT-1 mRNA的相对表达量在断奶后迅速下降,CAT-2 mRNA的相对表达量在半膜肌、半腱肌和背最长肌中均随日龄增加而增加。
CAT-1是骨骼肌中主要的CATs[12],在胚胎期敲除可致死,其mRNA表达丰度随日龄降低表明其在胚胎发育期间发挥着重要作用。一氧化氮(NO)是一种在多种生理过程中起关键作用的信号分子,CAT-2可以为一氧化氮合酶(nitric oxide synthase, NOS)提供Arg,然后合成NO[23]。目前已经确定了3种NOS同工酶(nNOS、iNOS和eNOS),骨骼肌中主要表达的NOS同功酶是nNOS[24],有研究发现[25],大鼠骨骼肌中的nNOS活性随日龄显著增加,表明CAT-2随日龄增加可以为骨骼肌中的nNOS提供更多的Arg,从而合成所需的NO。
2.2 Lys对骨骼肌CATs的调控饲粮中不同Lys水平对不同组织中CAT-1 mRNA相对表达量的影响不同。与饲喂低Lys水平(0.36%)饲粮相比,给猪饲喂含1.00% Lys的饲粮,其半腱肌和背最长肌中CAT-1 mRNA的相对表达量显著增加,但空肠中CAT-1 mRNA的相对表达量却显著降低[26]。此外,饲粮Lys水平为1.35%时显著增加背最长肌中b0, +AT mRNA的表达丰度,对背最长肌中CAT-1 mRNA的相对表达量无显著影响,但半腱肌中b0, +AT和CAT-1 mRNA的相对表达量显著降低[27]。与饲喂0.6%和0.8% Lys组相比,饲喂1.0% Lys组十二指肠、空肠和回肠CAT-1 mRNA的相对表达量均显著下调[28];在肉仔鸡上的研究表明,饲粮高水平Lys可降低肠道CAT-1 mRNA的相对表达量[29]。可见,高水平Lys降低肠道和半腱肌的CAT-1 mRNA的相对表达量,而不同水平Lys对背最长肌中CAT-1 mRNA的相对表达量的影响可能存在一个限度。半腱肌的耐受性比背最长肌差,可能与半腱肌肌纤维收缩性比背最长肌强、消耗更多的能量有关[30-31]。
研究表明,经糖皮质激素地塞米松处理可显著下调心脏微血管内皮细胞CATs mRNA的相对表达量[32],而鸡饲粮中Lys缺乏会增加血浆糖皮质激素皮质酮浓度,降低胸大肌中CATs mRNA的相对表达量[33],表明皮质酮升高可能是导致Lys缺乏期间CATs mRNA相对表达量下降的重要因素。由于Lys水平和糖皮质激素可调节CATs mRNA的相对表达量,所以饲粮氨基酸水平的改变和增加的皮质酮可能对鸡胸大肌CATs mRNA的表达具有协同调控效应[34-35]。
2.3 影响CATs mRNA表达的其他因素蛋白激酶C(protein kinase C, PKC)是具有组织特异性表达并调节细胞生长发育的丝氨酸/苏氨酸蛋白激酶家族[36]。PKC参与不同的细胞过程,例如增殖、分化和凋亡等。PKC的激活对不同细胞类型中的CATs介导的转运具有调控作用,PKC活化降低了3种y+载体CAT-1、CAT-2B和CAT-3的活性。哺乳动物细胞或非洲爪蟾卵母细胞中PKC的激活导致了CAT-1和CAT-3 mRNA的相对表达量下降[37-38]。在肺动脉内皮细胞中也发现了类似规律[39]。
在B淋巴细胞[40]和EA.hy926内皮细胞[41]中观察到经佛波醇-12-肉豆蔻酸酯-13-乙酸酯(phorbol 12-myristate 13-acetate, PMA)处理后,CAT-1 mRNA的相对表达量显著上调。PMA处理也可诱导增加人脐静脉内皮细胞[42]和人Caco-2细胞中CATs介导的氨基酸的转运[43-44]。
2.4 CATs的适应性调节体外研究表明,CATs可根据底物浓度进行适应性调节(图 1)[45-46]:暴露于含较低浓度底物的环境中,CATs转运效率增加称为适应性去阻遏;而当底物浓度较高时,CATs转运效率降低称为适应性阻遏。在去阻遏期间,增加底物氨基酸浓度,高活性的CATs可使氨基酸快速转入,进而促进细胞增殖、迁移和分化等功能恢复。CAT-1的适应性调节可通过任一碱性氨基酸浓度的改变而发生,不受非必需氨基酸的影响[47]。CAT-1基因的第1个外显子包含天冬酰胺合酶启动子中的氨基酸应答元件(amino acid response elements, AARE)和CCAAT增强子结合蛋白(CCAAT enhancer binding protein, C/EBP)同源蛋白基因的结合位点[48],氨基酸缺失可通过诱导这2个基因的转录,激活CAT-1基因发挥转录功能[47, 49-50]。哺乳动物细胞中CAT-1的适应性调节是通过激活真核起始因子2α激酶(general control nonderepressible 2, GCN2)和磷酸化真核起始因子2α(eukaryotic initiation factor 2α, eIF2α)来实现,进而诱导CAT-1 mRNA的转录[51-52],并同时增加CAT-1 mRNA的相对表达量及其稳定性;通过适应性调节,CAT-1 mRNA的相对表达量增加10~15倍,而CAT-1蛋白表达水平增加50倍,表明CAT-1蛋白在Lys缺乏期间被大量翻译,使CAT-1介导的转运协同调控增加[45, 53]。相反,补充相应碱性氨基酸Lys,可导致CAT-1 mRNA相对表达量下调[34-45]。但对CATs转运系统在动物体内的适应性反应及其调控机制鲜见报道,尤其在饲粮氨基酸不足或不平衡状态下机体CATs如何调节尚不清楚。
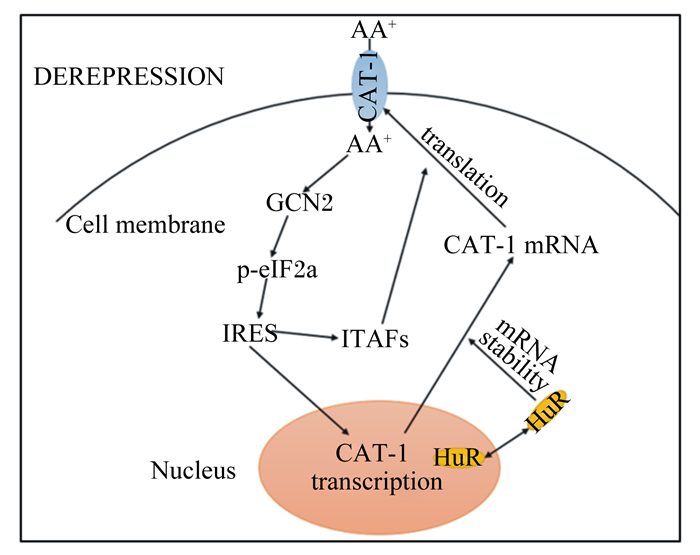
|
AA:氨基酸amino acid;HuR:核质蛋白nucleocytoplasmic protein;IRES:内部核糖体进入序列internal ribosome entry sequence;ITAFs:IRES反式作用因子IRES trans acting factors;eIF2α:真核起始因子2α eukaryotic initiation factor 2α;GCN2:真核生物起始因子2α激酶general control nonderepressible 2;CAT-1:碱性氨基酸转运载体1 cationic amino acid transporters 1;DEREPRESSION:去阻遏;Cell membrane:细胞膜;Nucleus:细胞核;transcription:转录;mRNA stability:mRNA稳定性;translation:翻译。 图 1 CAT-1适应性调节模型 Fig. 1 CAT-1 adaptive adjustment model[46] |
L-Arg、L-Lys与L-鸟氨酸的米氏常数(Km)值相似,这3种底物对于CATs来说具有相似的亲和力。而L-组氨酸只有在pH为5.5被质子化时才能被转运[54]。不同碱性氨基酸之间存在转运载体竞争机制,其中以Lys与Arg之间的拮抗作用最为显著。饲粮中高Lys含量影响Arg的吸收、降解、合成和重吸收,这主要是因为Lys与Arg均为碱性氨基酸,在机体内共用相同的转运系统,因此在吸收过程中存在拮抗关系。过量的Lys会提高机体内Arg分解酶的活性,从而影响Arg在尿素循环中的正常作用。另外,由于两者的重吸收途径也相同,所以饲粮中Lys含量过高,会使肾小管重吸收Arg受阻,尿中Arg排出增加。禽类对Arg的需求量相对较高,而过量Lys能通过提高肾脏Arg酶活性来促进Arg的代谢。在猪的研究中发现两者的拮抗作用相对较弱,可能与猪有较强的排泄Arg的能力有关。
图 2是骨骼肌肌纤维中碱性氨基酸通过CATs进行转运的过程[9],CATs将碱性氨基酸转运到肌细胞中进行蛋白质的合成,并通过Arg酶将Arg转化为鸟氨酸和多胺。有研究表明,Lys过量添加会提高雏鸡对Arg的需要量[55]。Tews等[56]研究表明,饲喂过量Arg会导致大鼠生长受到抑制,脑内Lys含量下降,其他组织中Arg和鸟氨酸含量增加。并且,Lys或Arg缺乏增加了CAT-1转运蛋白的合成和氨基酸转运。Lys、Arg与CAT-1的表达调控受营养物质、激素和生长因子等多方面因素的影响[46],碱性氨基酸之间的拮抗关系可能与氨基酸失衡诱导的CATs适应性调节有关。
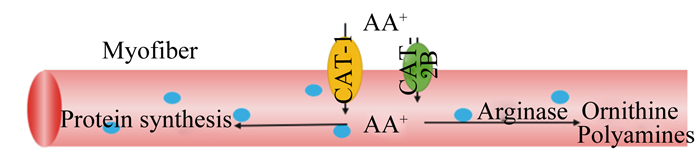
|
AA:氨基酸amino acid;Myofiber:肌纤维;Protein synthesis:蛋白质合成;Arginase:精氨酸酶;Ornithine:鸟氨酸;Polyamines:多胺;CAT-1:碱性氨基酸转运载体1 cationic amino acid transporters 1;CAT-2B:碱性氨基酸转运载体2B cationic amino acid transporters 2B。 图 2 骨骼肌肌纤维CAT-1和CAT-2B的转运作用 Fig. 2 Transport role of CAT-1 and CAT-2B in skeletal myofiber[9] |
Lys与亮氨酸(leucine,Leu)之间也存在转运竞争关系。氨基酸可促进肌肉蛋白质的合成,且Leu比其他氨基酸促进蛋白质合成的能力更强[57]。Stathopulos等[23]研究表明,满足Lys需要而配制的饲粮中含有过量的Leu,这可能会抑制Lys的吸收,导致猪生产性能下降。同时,饲粮Leu与Lys的添加比例影响空肠和肌肉中CATs的表达,过量添加Leu降低了半肌腱和背最长肌CAT-1 mRNA的相对表达量。低蛋白质饲粮可极显著提高猪背最长肌和半腱肌中CAT-1 mRNA的相对表达量。García等[58]研究表明,给猪饲喂Leu与Lys比例为0.88的饲粮其背最长肌中CAT-1 mRNA的相对表达量显著高于Leu与Lys比例为1.20和1.60组。Morales等[27]研究发现,在NRC(2002)营养需要基础上,添加1.35%过量Lys和1.16%过量Leu导致猪半腱肌CAT-1 mRNA的相对表达量降低,对背最长肌的CAT-1 mRNA的相对表达量没有明显影响。总之,Leu添加量过高会降低CAT-1 mRNA的相对表达量,影响Lys等的转运吸收。
4 小结与展望Lys等碱性氨基酸在提高肌肉产量和质量方面发挥重要作用,因此,揭示肌细胞中CATs转运Lys等氨基酸的过程及调控机制,在动物生产上具有理论和现实意义。目前关于CATs的感应转运机制的研究较少,仍有许多问题尚需解决:1)CAT-1是如何将Lys从细胞外转运至细胞内的;2)CAT-1在从胞外到胞内转运Lys的过程是否同时具备感应和转运功能,即CAT-1是否为Lys的感应-转运体;3)是否还有其他与CAT-1协同发挥转运功能的配体。期望未来可更深入的研究肌肉中碱性氨基酸的吸收利用及其与转运载体之间的关系,进而提高动物机体对氨基酸的利用效率,提高动物生长性能和改善畜产品质量。
| [1] |
HUNDAL H S, TAYLOR P M. Amino acid transceptors:gate keepers of nutrient exchange and regulators of nutrient signaling[J]. American Journal of Physiology Endocrinology and Metabolism, 2009, 296(4): E603-E613. DOI:10.1152/ajpendo.91002.2008 |
| [2] |
DENNIS P B, JAESCHKE A, SAITOH M, et al. Mammalian TOR:a homeostatic ATP sensor[J]. Science, 2001, 294(5544): 1102-1105. DOI:10.1126/science.1063518 |
| [3] |
UHE A M, COLLIER G R, O'DEA K. A comparison of the effects of beef, chicken and fish protein on satiety and amino acid profiles in lean male subjects[J]. The Journal of Nutrition, 1992, 122(3): 467-472. DOI:10.1093/jn/122.3.467 |
| [4] |
ROY N, LAPIERRE H, BERNIER J F. Whole-body protein metabolism and plasma profiles of amino acids and hormones in growing barrows fed diets adequate or deficient in lysine[J]. Canadian Journal of Animal Science, 2000, 80(4): 585-595. DOI:10.4141/A98-057 |
| [5] |
SHELTON N W, TOKACH M D, DRITZ S S, et al. Effects of increasing dietary standardized ileal digestible lysine for gilts grown in a commercial finishing environment[J]. Journal of Animal Science, 2011, 89(11): 3587-3595. DOI:10.2527/jas.2010-3030 |
| [6] |
WU G Y. Recent advances in swine amino acid nutrition[J]. Journal of Animal Science and Biotechnology, 2010, 1(2): 118-130. |
| [7] |
REZAEI R, WANG W W, WU Z L, et al. Biochemical and physiological bases for utilization of dietary amino acids by young pigs[J]. Journal of Animal Science and Biotechnology, 2013, 4(2): 90-101. |
| [8] |
姜廷波, 王莹, 赵艳飞, 等. 肠道中赖氨酸转运载体的研究进展[J]. 中国畜牧杂志, 2017, 53(1): 7-11. |
| [9] |
FOTIADIS D, KANAI Y, PALACÍN M. The SLC3 and SLC7 families of amino acid transporters[J]. Molecular Aspects of Medicine, 2013, 34(2/3): 139-158. |
| [10] |
SLOAN J L, MAGER S. Cloning and functional expression of a human Na+ and Cl--dependent neutral and cationic amino acid transporter B0+[J]. Journal of Biological Chemistry, 1999, 274(34): 23740-23745. DOI:10.1074/jbc.274.34.23740 |
| [11] |
BAUCH C, FORSTER N, LOFFING-CUENI D, et al. Functional cooperation of epithelial heteromeric amino acid transporters expressed in madin-darby canine kidney cells[J]. Journal of Biological Chemistry, 2003, 278(2): 1316-1322. DOI:10.1074/jbc.M210449200 |
| [12] |
ISHIDA A, ASHIHARA A, NAKASHIMA K, et al. Expression of cationic amino acid transporters in pig skeletal muscles during postnatal development[J]. Amino Acids, 2017, 49(11): 1805-1814. DOI:10.1007/s00726-017-2478-2 |
| [13] |
PFEIFFER R, ROSSIER G, SPINDLER B, et al. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family[J]. The EMBO Journal, 1999, 18(1): 49-57. DOI:10.1093/emboj/18.1.49 |
| [14] |
CLOSS E I, BOISSEL J P, HABERMEIER A, et al. Structure and function of cationic amino acid transporters (CATs)[J]. The Journal of Membrane Biology, 2006, 213(2): 67-77. |
| [15] |
MANN G E, YUDILEVICH D L, SOBREVIA L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells[J]. Physiological Reviews, 2003, 83(1): 183-252. DOI:10.1152/physrev.00022.2002 |
| [16] |
ZUO J J, XIA W G, XU M, et al. Molecular cloning, tissue distribution and expression of the porcine cationic amino acid transporter CAT3[J]. Journal of Animal and Veterinary Advances, 2013, 12(11): 1070-1077. |
| [17] |
WOLF S, JANZEN A, VÉKONY N, et al. Expression of solute carrier 7A4 (SLC7A4) in the plasma membrane is not sufficient to mediate amino acid transport activity[J]. Biochemical Journal, 2002, 364(3): 767-775. DOI:10.1042/bj20020084 |
| [18] |
CHILLARÓN J, ROCA R, VALENCIA A, et al. Heteromeric amino acid transporters:biochemistry, genetics, and physiology[J]. American Journal of Physiology Renal Physiology, 2001, 281(6): F995-F1018. DOI:10.1152/ajprenal.2001.281.6.F995 |
| [19] |
DEVÉS R, BOYD C A R. Transporters for cationic amino acids in animal cells:discovery, structure, and function[J]. Physiological Reviews, 1998, 78(2): 487-545. DOI:10.1152/physrev.1998.78.2.487 |
| [20] |
KAVANAUGH M P. Voltage dependence of facilitated arginine flux mediated by the system y+ basic amino acid transporter[J]. Biochemistry, 1993, 32(22): 5781-5785. DOI:10.1021/bi00073a009 |
| [21] |
ROTMANN A, CLOSS E I, LIEWALD J F, et al. Intracellular accumulation of L-Arg, kinetics of transport, and potassium leak conductance in oocytes from Xenopus laevis expressing hCAT-1, hCAT-2A, and hCAT-2B[J]. Biochim Biophys Acta:Biomembranes, 2004, 1660(1/2): 138-143. |
| [22] |
张艳.猪CAT-2分子克隆及其mRNA在骨骼肌中的发育性表达与调控[D].硕士学位论文.广州: 华南农业大学, 2007. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1277927
|
| [23] |
STATHOPULOS P B, LU X R, SHEN J, et al. Increased L-arginine uptake and inducible nitric oxide synthase activity in aortas of rats with heart failure[J]. American Journal of Physiology:Heart and Circulatory Physiology, 2001, 280(2): H859-H867. DOI:10.1152/ajpheart.2001.280.2.H859 |
| [24] |
MCCONELL G K, BRADLEY S J, STEPHENS T J, et al. Skeletal muscle nNOS protein content is increased by exercise training in humans[J]. American Journal of Physiology:Regulatory Integrative and Comparative Physiology, 2007, 293(2): R821-R828. DOI:10.1152/ajpregu.00796.2006 |
| [25] |
CAPANNI C, SQUARZONI S, PETRINI S, et al. Increase of neuronal nitric oxide synthase in rat skeletal muscle during ageing[J]. Biochemical and Biophysical Research Communications, 1998, 245(1): 216-219. DOI:10.1006/bbrc.1998.8404 |
| [26] |
GARCÍA-VILLALOBOS H, MORALES-TREJO A, ARAIZA-PIÑA B A, et al. Effects of dietary protein and amino acid levels on the expression of selected cationic amino acid transporters and serum amino acid concentration in growing pigs[J]. Archives of Animal Nutrition, 2012, 66(4): 257-270. DOI:10.1080/1745039X.2012.697351 |
| [27] |
MORALES A, BARRERA M A, ARAIZA A B, et al. Effect of excess levels of lysine and leucine in wheat-based, amino acid-fortified diets on the mRNA expression of two selected cationic amino acid transporters in pigs[J]. Journal of Animal Physiology and Animal Nutrition, 2013, 97(2): 263-270. DOI:10.1111/jpn.2013.97.issue-2 |
| [28] |
WANG X Q, ZENG P L, FENG Y, et al. Effects of dietary lysine levels on apparent nutrient digestibility and cationic amino acid transporter mRNA abundance in the small intestine of finishing pigs, Sus scrofa[J]. Animal Science Journal, 2012, 83(2): 148-155. DOI:10.1111/asj.2012.83.issue-2 |
| [29] |
OSMANYAN A K, HARSINI S G, MAHDAVI R, et al. Intestinal amino acid and peptide transporters in broiler are modulated by dietary amino acids and protein[J]. Amino Acids, 2018, 50(2): 353-357. DOI:10.1007/s00726-017-2510-6 |
| [30] |
KARLSSON A H, KLONT R E, FERNANDEZ X. Skeletal muscle fibres as factors for pork quality[J]. Livestock Production Science, 1999, 60(2/3): 255-269. |
| [31] |
徐子伟, 门小明, 齐珂珂.猪肌肉纤维类型及其代谢特征与肉质形成的关系及机理探讨[C]//中国畜牧兽医学会动物营养学分会第十一次全国动物营养学术研讨会论文集.长沙: 中国畜牧兽医学会, 2012. http://cpfd.cnki.com.cn/Article/CPFDTOTAL-SEKM201210002010.htm
|
| [32] |
SIMMONS W W, UNGUREANU-LONGROIS D, SMITH G K, et al. Glucocorticoids Regulate inducible nitric oxide synthase by inhibiting tetrahydrobiopterin synthesis and L-arginine transport[J]. Journal of Biological Chemistry, 1996, 271(39): 23928-23937. DOI:10.1074/jbc.271.39.23928 |
| [33] |
HUMPHREY B D, STEPHENSEN C B, CALVERT C C, et al. Lysine deficiency and feed restriction independently alter cationic amino acid transporter expression in chickens (Gallus gallus domesticus)[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2006, 143(2): 218-227. |
| [34] |
HYATT S L, AULAK K S, MALANDRO M, et al. Adaptive regulation of the cationic amino acid transporter-1 (CAT-1) in FAO cells[J]. Journal of Biological Chemistry, 1997, 272(32): 19951-19957. DOI:10.1074/jbc.272.32.19951 |
| [35] |
LIU J, HATZOGLOU M. Control of expression of the gene for the arginine transporter Cat-1 in rat liver cells by glucocorticoids and insulin[J]. Amino Acids, 1998, 15(4): 321-337. DOI:10.1007/BF01320897 |
| [36] |
MARTELLI A M, FAENZA I, BILLI A M, et al. Nuclear protein kinase C isoforms:key players in multiple cell functions?[J]. Histology & Histopathology, 2003, 18(4): 1301-1312. |
| [37] |
ROTMANN A, STRAND D, MARTINÉ U, et al. Protein kinase C activation promotes the internalization of the human cationic amino acid transporter hCAT-1.A new regulatory mechanism for hCAT-1 activity[J]. Journal of Biological Chemistry, 2004, 279(52): 54185-54192. DOI:10.1074/jbc.M409556200 |
| [38] |
ROTMANN A, VÉKONY N, GASSNER D, et al. Activation of classical protein kinase C reduces the expression of human cationic amino acid transporter 3 (hCAT-3) in the plasma membrane[J]. Biochemical Journal, 2006, 395(1): 117-123. DOI:10.1042/BJ20051558 |
| [39] |
KROTOVA K Y, ZHARIKOV S I, BLOCK E R. Classical isoforms of PKC as regulators of CAT-1 transporter activity in pulmonary artery endothelial cells[J]. American Journal of Physiology:Lung Cellular and Molecular Physiology, 2003, 284(6): L1037-L1044. DOI:10.1152/ajplung.00308.2002 |
| [40] |
YOSHIMOTO T, YOSHIMOTO E, MERUELO D. Enhanced gene expression of the murine ecotropic retroviral receptor and its human homolog in proliferating cells[J]. Journal of Virology, 1992, 66(7): 4377-4381. |
| [41] |
GRÄF P, FÖRSTERMANN U, CLOSS E I. The transport activity of the human cationic amino acid transporter hCAT-1 is downregulated by activation of protein kinase C[J]. British Journal of Pharmacology, 2001, 132(6): 1193-1200. DOI:10.1038/sj.bjp.0703921 |
| [42] |
PERKINS C P, MAR V, SHUTTER J R, et al. Anemia and perinatal death result from loss of the murine ecotropic retrovirus receptor mCAT-1[J]. Genes & Development, 1997, 11(7): 914-925. |
| [43] |
PAN M, MENG Q H, WOLFGANG C L, et al. Activation of intestinal arginine transport by protein kinase C is mediated by mitogen-activated protein kinases[J]. Journal of Gastrointestinal Surgery, 2002, 6(6): 876-882. DOI:10.1016/S1091-255X(02)00052-5 |
| [44] |
PAN M, STEVENS B R. Protein kinase C-dependent regulation of L-arginine transport activity in Caco-2 intestinal cells[J]. Biochimica Biophysica Acta:Biomembranes, 1995, 1239(1): 27-32. DOI:10.1016/0005-2736(95)00136-Q |
| [45] |
FERNANDEZ J, YAMAN I, MISHRA R, et al. Internal ribosome entry site-mediated translation of a mammalian mRNA is regulated by amino acid availability[J]. Journal of Biological Chemistry, 2001, 276(15): 12285-12291. DOI:10.1074/jbc.M009714200 |
| [46] |
HATZOGLOU M, FERNANDEZ J, YAMAN I, et al. Regulation of cationic amino acid transport:the story of the CAT-1 transporter[J]. Annual Review of Nutrition, 2004, 24(1): 377-399. DOI:10.1146/annurev.nutr.23.011702.073120 |
| [47] |
FERNANDEZ J, LOPEZ A B, WANG C P, et al. Transcriptional control of the arginine/lysine transporter, CAT-1, by physiological stress[J]. Journal of Biological Chemistry, 2003, 278(50): 50000-50009. DOI:10.1074/jbc.M305903200 |
| [48] |
BRUHAT A, AVEROUS J, CARRARO V, et al. Differences in the molecular mechanisms involved in the transcriptional activation of the CHOP and asparagine synthetase genes in response to amino acid deprivation or activation of the unfolded protein response[J]. Journal of Biological Chemistry, 2002, 277(50): 48107-48114. DOI:10.1074/jbc.M206149200 |
| [49] |
SIU F, BAIN P J, LEBLANC-CHAFFIN R, et al. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene[J]. Journal of Biological Chemistry, 2002, 277(27): 24120-24127. DOI:10.1074/jbc.M201959200 |
| [50] |
SIU F, CHEN C, ZHONG C, et al. CCAAT/enhancer-binding protein-β is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene[J]. Journal of Biological Chemistry, 2001, 276(51): 48100-48107. DOI:10.1074/jbc.M109533200 |
| [51] |
FERNANDEZ J, YAMAN I, SARNOW P, et al. Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2α[J]. Journal of biological chemistry, 2002, 277(21): 19198-19205. DOI:10.1074/jbc.M201052200 |
| [52] |
SOOD R, PORTER A C, OLSEN D A, et al. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2α[J]. Genetics, 2000, 154(2): 787-801. |
| [53] |
AULAK K S, MISHRA R, ZHOU L Y, et al. Post-transcriptional regulation of the arginine transporter CAT-1 by amino acid availability[J]. Journal of Biological Chemistry, 1999, 274(43): 30424-30432. DOI:10.1074/jbc.274.43.30424 |
| [54] |
KIM J W, CLOSS E I, ALBRITTON L M, et al. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor[J]. Nature, 1991, 352(6337): 725-728. DOI:10.1038/352725a0 |
| [55] |
O'DELL B L, SAVAGE J E. Arginine-lysine antagonism in the chick and its relationship to dietary cations[J]. The Journal of Nutrition, 1966, 90(4): 364-370. DOI:10.1093/jn/90.4.364 |
| [56] |
TEWS J K, BRADFORD A M, HARPER A E. Induction of lysine imbalance in rats:relationships between tissue amino acids and diet[J]. The Journal of Nutrition, 1981, 111(6): 968-978. DOI:10.1093/jn/111.6.968 |
| [57] |
ANTHONY J C, YOSHIZAWA F, ANTHONY T G, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway[J]. The Journal of Nutrition, 2000, 130(10): 2413-2419. DOI:10.1093/jn/130.10.2413 |
| [58] |
GARCÍA H, MORALES A, ARAIZA A, et al. Gene expression, serum amino acid levels, and growth performance of pigs fed dietary leucine and lysine at different ratios[J]. Genetics and Molecular Research, 2015, 14(1): 1589-1601. DOI:10.4238/2015.March.6.6 |




