在现代集约化养殖生产中,畜禽易遭受各种环境或生理应激(高温、寒冷、饥饿、食物中毒、分娩等),通常引起动物采食量下降、饲粮消化率降低,造成动物生产性能降低、抗病力下降,甚至导致动物死亡[1-2]。腺苷酸激活蛋白激酶(AMPK)是一种丝氨酸/苏氨酸蛋白激酶,被称为“能量感受器”,在机体能量代谢平衡方面发挥着关键作用[3-4]。AMPK激活是对应激因素的反应,研究显示,当动物在营养缺乏、高温、缺氧等应激状态时,AMPK通路信号会被加强,促进葡萄糖转运、脂肪酸氧化及糖酵解等代谢,抑制蛋白质、脂肪和糖原合成代谢,改善机体能量物质代谢,降低应激所带来的危害[5-7]。本文就AMPK通路激活机制及其在应激环境中对动物能量代谢的调控进行阐述,为缓解或治疗动物应激综合征和代谢疾病提供新的思路。
1 AMPK的活性调节AMPK是由1个催化亚基(α亚基)、2个调节亚基(β亚基和γ亚基)组成的异源三聚体蛋白,每个亚基都存在由2~3种基因所编码的异构体(α1、α2、β1、β2、γ1、γ2、γ3)。α亚基靠近N末端含有1个高度保守的丝氨酸/苏氨酸激酶结构域,该结构域内172位点上苏氨酸残基的磷酸化对酶的活性起关键作用,C末端主要负责与β和γ亚基结合。β亚基为AMPK的结构核心,含有糖原结合域(CBM),该区域可以结合糖原,当糖原大量储备时,AMPKβ1上的CBM与糖原结合,抑制AMPK活性[8]。γ亚基含有一磷酸腺苷(AMP)或者三磷酸腺苷(ATP)的结合位点,AMP或ATP通过与AMPK的γ亚基Bateman域结合磷酸化或去磷酸化α亚基上的苏氨酸172(Thr 172)残基,提高或降低AMPK活性[9]。不同物种间AMPK的基因序列具有高度保守性[10]。AMPK活性主要受细胞内AMP/ATP水平、上游激酶活性以及激素和药物激活剂的调节。
1.1 AMP/ATP在大多数真核细胞内,AMPK的活性主要受AMP/ATP水平的调节。在正常生理条件下,AMP/ATP约为1:100,且变化范围很小[9]。当ATP消耗增加或ATP合成受到抑制时,AMP浓度显著增加,进一步造成AMPK Thr 172的磷酸化或者变构激活AMPK(图 1)。最新研究发现,醛缩酶不仅是一种重要的糖酵解酶,同时也是葡萄糖的感受器,一旦醛缩酶“吃不到”由葡萄糖衍生的果糖1, 6-二磷酸,可直接激活AMPK,该过程完全不涉及AMP/ATP水平[11]。
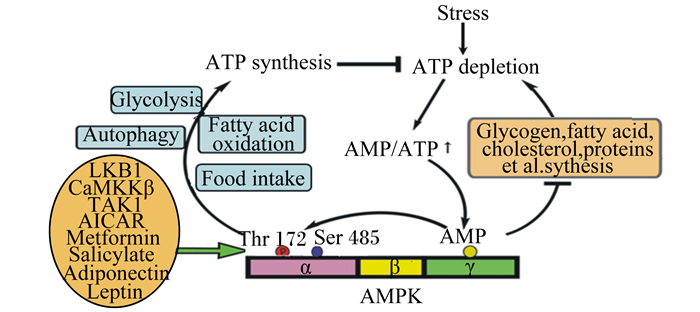
|
Stress:应激;ATP synthesis:ATP合成;ATP depletion:ATP消耗;Glycolysis:糖酵解;Autophagy:自噬;Fatty acid oxidation:脂肪酸氧化;Food intake:采食量;AMP;一磷酸腺苷adenosine monophosphate;ATP:三磷酸腺苷adenosine triphosphate;Glycogen:糖原;fatty acid:脂肪酸;cholesterol:胆固醇;proteins:蛋白质;LKB1:肝激酶B1 liver kinase B1;CaMKK-β:Ca2+/钙调蛋白依赖蛋白激酶-β calmodulin-dependent kinase kinase β;TAK1:转化生长因子-β激活激酶1 transforming growth factor β-activated kinase 1;AICAR:5-氨基咪唑-4-甲酰胺核糖核苷酸5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside;Metformin:二甲双胍;Salicylate:水杨酸盐;Adiponectin:脂联素;Leptin:瘦素;Thr 172:苏氨酸172位点;Ser 485:丝氨酸485位点;AMPK:腺苷酸激活蛋白激酶AMP-activated protein kinase。 图 1 AMPK激活机制 Fig. 1 Mechanisms of AMPK activation |
已有的研究表明,至少有3种AMPK的上游激酶,分别为肝激酶B1(LKB1)、Ca2+/钙调蛋白依赖蛋白激酶-β(CaMKK-β)和转化生长因子-β激活激酶1(TAK1)。LKB1是一个肿瘤抑制蛋白,主要分布于肝脏和肌肉等外周组织,可通过磷酸化AMPKα亚基上Thr 172位点激活AMPK(图 1)[12]。有研究报道,LKB1对AMPK的激活过程发生于细胞溶酶体表面,说明溶酶体对机体代谢也发挥了重要调节作用[13]。CaMKK-β主要分布于中枢神经系统,通过改变细胞内Ca2+浓度实现AMPKα亚基Thr 172的磷酸化,该过程与AMP浓度无关[14]。TAK1的活性受多种细胞因子影响,如白细胞介素-1(IL-1)、转化生长因子-β(TGF-β)和CD40等。过去研究表明TAK1以及TAK1相关蛋白(TAB1)可通过磷酸化AMPKα亚基Thr 172直接激活AMPK[15],然而近几年研究报道TAK1可能是通过激活LKB1间接激活AMPK[16]。
1.3 激素和药物激活剂在机体处于静息状态下,瘦素、脂联素、5-氨基咪唑-4-甲酰胺核糖核苷酸(AICAR)、白藜芦醇、二甲双胍、水杨酸盐、罗格列酮等都可直接或间接激活AMPK,但相关机制不详(图 1)[17]。在骨骼肌中,瘦素选择性激活AMPKα2亚基,抑制乙酰CoA羧化酶(ACC)活性,加速脂肪酸氧化,减少甘油三酯沉积[18]。脂联素是一种由脂肪组织产生的细胞因子,与脂联素受体1结合后激活AMPK,AMPK激活后进一步引起下游靶点丝裂原活化蛋白激酶(MAPK)活性的增加,增强过氧化物酶增殖体激活受体(PPARα)及靶基因的表达,促进肌肉脂肪酸的β氧化,降低脂质在骨骼肌的沉积[19]。AICAR是嘌呤从头合成的中间体,在腺苷激酶的作用下形成一磷酸衍生物(ZMP),ZMP与AMP类似,可模拟AMP与AMPK的γ亚基结合,变构激活AMPK[17]。白藜芦醇是一种广泛存在于植物中的天然多酚复合物,研究表明,白藜芦醇通过激活AMPK抑制哺乳动物雷帕霉素靶蛋白(mTOR)信号通路,引起mTOR下游的真核生物翻译起始因子4E结合蛋白1(4E-BP1)和核糖体蛋白S6激酶分子(S6K)磷酸化失活,最终造成肿瘤细胞蛋白表达量的降低[16]。
1.4 应激近年来研究发现,几乎所有的应激,如饥饿、缺氧[6]、缺血[20]、电刺激[21]、热应激[7]、冷应激[22-23]、氧化应激[24-25]以及一氧化氮(NO)[26]等均会导致机体AMP/ATP水平或AMPK的上游激酶急剧升高,从而增强AMPK活性(图 1)。在低温环境下,机体通过增强LKB1-AMPK和PPARα-AMPKα通路促进糖脂代谢,增加机体产热[22-23]。热应激显著增加奶牛血浆AMPK活性[27]。体外试验也发现,热应激通过降低大鼠骨骼肌ATP浓度快速增加AMPK活性,促进骨骼肌葡萄糖转运和代谢[28]。NO则可通过提高内皮细胞Ca2+/CaMKK-β水平激活AMPK[29]。此外,应激产生的活性氧(ROS)会消耗大量ATP而间接增强AMPK活性。AMPK可通过抑制脂肪酸合成来维持机体原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)和谷胱甘肽(GSH)浓度在一个高水平范围内,减少ROS积累,最终保护细胞免受氧化应激的破坏[30-31]。Guo等[32]进一步研究发现,AMPK通过增加细胞NADPH的合成及限制NADPH的分解来抑制地塞米松产生ROS,减缓氧化应激。
2 AMPK对应激环境中机体物质代谢的调控 2.1 食欲和采食量应激可引起畜禽采食量急剧下降[5, 7]。AMPK在哺乳动物下丘脑弓状核、外侧区、腹内侧核和视上核等摄食调节中心区域均有表达,并参与机体摄食和能量调节[33-34]。提高下丘脑AMPK活性会促进摄食,而抑制AMPK活性则引起厌食反应,并且这些作用主要是通过激素实现的,如脂联素、瘦素和胃饥饿素等激素都影响AMPK活性[35]。在下丘脑弓状核中,脂联素可增强AMPK活性,AMPK激活后进一步增加刺鼠相关蛋白(AgRP)和神经肽Y(NPY)的表达,促进食欲,增加采食量[19]。在外侧区下丘脑中,瘦素通过抑制AMPK活性增加抑制食欲的神经肽前阿黑皮素原(POMC)的释放,降低食欲[18]。当机体处于饥饿状态时,胃饥饿素可通过Ca2+/CaMKKβ途径激活下丘脑AMPK,进一步增加AgRP/NPY释放,促进食欲[36]。而在低温环境下,三碘甲状腺氨酸(T3)和胰高血糖素样肽-1(GLP-1)的浓度会急剧升高,激活下丘脑腹内侧核神经细胞AMPK的活性,促进交感神经释放肾上腺素,引起脂肪酸氧化,使机体产热的快速增加[35, 37-38]。
2.2 糖代谢研究报道,应激会通过增强AMPK活性,加快糖代谢来维持机体能量平衡[27, 39]。AMPK对糖代谢的影响主要表现在以下几个方面。
2.2.1 促进葡萄糖的吸收当机体处于应激状态下(缺氧、热休克),葡萄糖的吸收会成倍增加,说明增强AMPK的活性能够促进葡萄糖的转运[23, 28]。葡萄糖需要借助葡萄糖转运蛋白(GLUT)才能进入细胞内,介导葡萄糖转运的主要是GLUT1和GLUT4。硫氧还蛋白互作蛋白与GLUT1结合会降低GLUT1活性,直接抑制葡萄糖的摄取,AMPK则通过磷酸化抑制硫氧还蛋白互作蛋白的表达,以提高GLUT1活性[40]。TBC1域家族蛋白成员4(TBC1D4)处于非磷酸化状态可阻止GLUT4的移位,AMPK通过磷酸化TBC1D4促进GLUT4从胞质囊泡转移至细胞膜表面[4],进而促进葡萄糖转运。另外,组蛋白去乙酰化酶5(HDAC5)可抑制GLUT4基因表达,AMPKα2/AICAR则通过磷酸化抑制HDAC5,进而降低HDAC5与核细胞增强因子2A(MEF2A)的结合,引起GLUT4基因表达的增加[3]。
2.2.2 抑制糖异生激活的AMPK不仅可通过磷酸化激活2,6-二磷酸果糖激酶(PFKFB3)、L型丙酮酸激酶(L-PK)等促进葡萄糖酵解,还能通过抑制葡萄糖-6-磷酸酶(G6Pase)和磷酸烯醇式丙酮酸羧激酶(PEPCK)活性抑制糖异生[3],上述作用主要涉及到以下过程实现:AMPK通过磷酸化抑制cAMP应答元件结合蛋白(CREB)转录共激活因子2(CRTC2)和组蛋白去乙酰酶Ⅱ家族(HDACS)活性,降低叉头转录因子家族(FOXO)转录因子活性以及糖异生酶的表达。此外,AMPK还可通过抑制糖原合成酶(GS)磷酸化来降低糖原合成水平[41]。
2.3 脂质代谢研究报道,冷应激、热应激、氧化应激或AICAR处理均会激活AMPK,AMPK激活后会降低脂肪酸合成,同时增加脂肪酸氧化分解,引起能量供给的快速增加,应激反应因此得到缓解[22, 31, 42]。AMPK对脂质代谢的调控主要在以下几个方面。
2.3.1 磷酸化β-羟基-β-甲基戊二酸单酰辅酶A还原酶(HMGR)和固醇调节元件结合蛋白(SREBP-1)HMGR是胆固醇合成的关键酶,AMPK通过磷酸化HMGR肽链丝氨酸(Ser)871位点使HMGR失活,从而抑制胆固醇合成[34]。碳水化合物反应元件结合蛋白(ChREBP)是肝脏特异性转录因子,可促进糖酵解以及脂肪酸合成基因ACC和脂肪酸合酶(FAS)的表达,AMPK通过磷酸化ChREBP的Ser 568位点阻止其与DNA结合并且降低其转录活性,进一步降低脂肪酸合成[31]。SREBP-1是脂肪合成基因的重要转录调节因子,AMPK通过下调SREBP-1c的表达,抑制其靶基因ACC和FAS的表达,进而降低脂质合成[43]。
2.3.2 磷酸化ACCACC是催化脂肪酸合成的限速酶,有ACC-1和ACC-2 2种同工型存在,其中ACC-1催化生成丙二酰辅酶A(MCA),主要参与脂肪酸的从头合成,可反馈抑制肉碱脂酰转移酶1(CPT-1)的活性;ACC-2催化生成的丙二酰辅酶A脱羧酶(MCD),主要抑制CPT-1的活性,调节脂肪酸的β-氧化,促进脂肪酸合成。AMPK通过磷酸化ACC1/2的3个位点Ser 79、Ser 1 200和Ser 1 215来抑制ACC活性,引起MCA表达水平的下降;MCA表达水平下降则会降低对CPT-1的抑制,提高脂肪酸的氧化并减少脂肪酸合成[4, 43](图 2)。
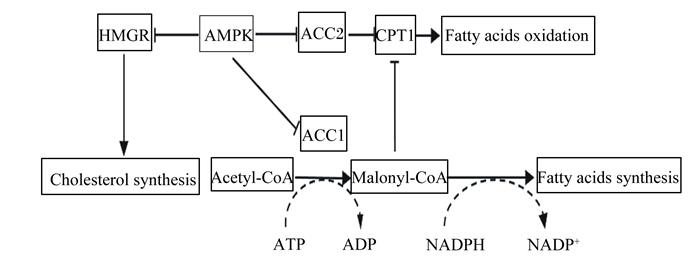
|
HMGR:β-羟基-β-甲基戊二酸单酰辅酶A还原酶β-hydroxy-β-methyl-glutaryl-coA reductase;AMPK:腺苷酸激活蛋白激酶AMP-activated protein kinase;ACC:乙酰CoA羧化酶acetyl-CoA carboxylase;CPT1:肉碱脂酰转移酶1 carnitine palmitoyltransferase 1;Fatty acids oxidation:脂肪酸氧化;Cholesterol synthesis:胆固醇合成;Acetyl-CoA:乙酰辅酶A;ATP:三磷酸腺苷adenosine triphosphate;ADP:磷酸腺苷adenosine diphosphate;Malonyl-CoA:丙二酰辅酶A;NADPH:还原型烟酰胺腺嘌呤二核苷酸磷酸reduced nicotinamide adenine dinucleotide phosphate;NADP+:烟酰胺腺嘌呤二核苷酸磷酸nicotinamide adenine dinucleotide phosphate;Fatty acids synthesis:脂肪酸合成。 图 2 AMPK调控脂质代谢 Fig. 2 Regulation of lipid metabolism by AMPK |
应激能够增强AMPK活性,AMPK激活后会抑制mTOR信号通路,引起蛋白质合成降低[44-46]。AMPK抑制哺乳动物类雷帕霉素靶蛋白C1(mTORC1)机制总结如下(图 3):1)AMPK被激活后直接磷酸化mTORC1相关受体(raptor)而抑制其活性;2)结节性硬化症复合物2(TSC2)是三磷酸鸟苷(GTP)结合蛋白(Rheb)的激活蛋白,AMPK通过磷酸化TSC2激活结节性硬化症复合物(TSC1-TSC2)催化Rheb与二磷酸鸟苷(GDP)结合而间接抑制mTOR的活性;3)通过p38蛋白激酶β-p38调节活化蛋白激酶(p38β-PRAK)通路下调Rheb活性来抑制mTORC1。mTORC1活性下降导致其下游分子S6K、4EBP1及半胱氨酸天冬氨酸蛋白酶3(caspase3)磷酸化过程受阻,进而抑制蛋白质合成,使细胞生长、增殖停滞[47]。磷酸化的AMPK也能通过激活真核延伸因子2激酶(eEF2K)使真核细胞延伸因子2(eEF-2)磷酸化失活,进而抑制肽链的延伸,降低真核生物蛋白质的合成[48]。转录起始因子1A(TIF-1A)是RNA聚合酶1的转录因子,研究表明,AMPK可通过磷酸化TIF-1A来抑制核糖体RNA合成,减少蛋白质合成[48]。综上所述,AMPK能够通过多条途径来抑制mTOR通路,降低蛋白质合成,从而减少不必要的能量消耗,减缓应激反应。
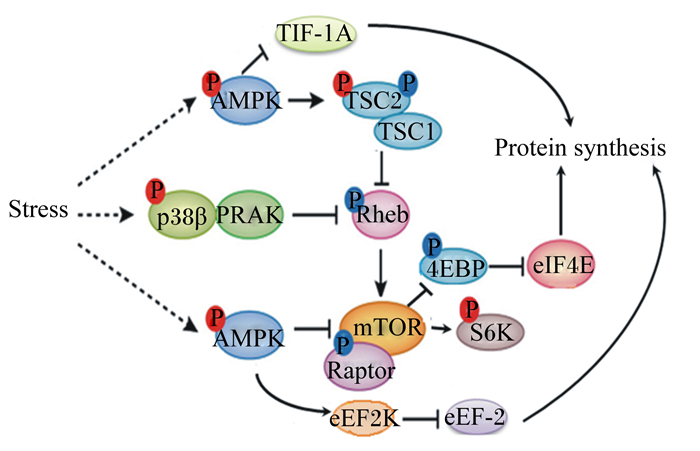
|
TIF-1A:转录起始因子transcription initiation factor 1A;AMPK:腺苷酸激活蛋白激酶AMP-activated protein kinase;TSC:结节性硬化症复合物tuberous sclerosis complex;Stress:应激;p38β:p38蛋白激酶β;PRAK:p38调节活化蛋白激酶p38 mitogen-activated protein kinases;Rheb:GTP结合蛋白GTP binding protein;4EBP:真核生物翻译起始因子4E结合蛋白eukaryotic translation initiation factor 4E-binding protein;eIF4E:真核翻译起始因子4E eukaryotic translation initiation factor 4e;mTOR:哺乳动物类雷帕霉素靶蛋白mammalian rapamycin target protein;raptor:哺乳动物类雷帕霉素靶蛋白C1相关受体;S6K:核糖体S6激酶ribosomal s6 kinase;eEF2K:真核延伸因子2激酶eukaryotic elongation factor 2 kinase;Protein synthesis:蛋白质合成。 图 3 AMPK对mTOR信号通路的调控 Fig. 3 Regulation of mTOR signaling by AMPK |
自噬不仅能够消灭细胞内有害物质,还为合成新的细胞提供所需的原料,因此,自噬作为一种细胞的自我保护性机制,有利用缓解应激反应。许多研究表明,当细胞在缺乏营养或发生应激反应时,可增强AMPK活性促进细胞自噬,减缓线粒体的损伤[6, 24, 30]。AMPK可通过Unc-51样蛋白激酶1(ULK1)路径调控细胞自噬,具体机制如下(图 4):通过抑制mTORC1活性降低mTORC1对ULK1的磷酸化抑制作用,间接激活ULK1[49]。也有研究报道,AMPK通过磷酸化液泡蛋白分选蛋白34(VPS34)复合体和bcl-2同源结构域蛋白(beclin-1)增强beclin-1-VPS34-Atg14L复合体活性,促进细胞自噬[50]。FOXO能够上调自噬受体的表达,在热应激状态下,AMPK通过磷酸化激活FOXO3A,促进细胞自噬[51]。AMPK还可通过磷酸化细胞周期调控因子p53和p27来抑制细胞G1/S转化,促进细胞自噬[52]。当细胞营养缺乏时,通过线粒体生物合成产生能量对维持细胞正常生理功能极为重要。过氧化物酶体增生激活受体γ协同刺激因子-1α(PGC-1α)是细胞核编码线粒体生物合成基因主要的辅助因子,AMPK可通过磷酸化PGC-1α并促进去乙酰化酶活性来调节细胞线粒体生物合成[53]。也有研究报道,热应激通过AMPK-SIRT1-PGC-1α信号通路诱导C2C12骨骼肌细胞线粒体生物合成[54]。
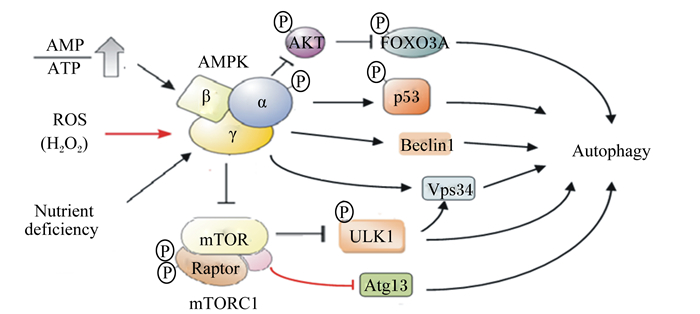
|
AKT:蛋白激酶B protein kinase B;FOXO3A:叉头转录因子3A forkhead box O 3a;AMP:一磷腺苷酸adenosine monophosphate;ATP:三磷酸腺苷adenosine triphosphate;AMPK:腺苷酸激活蛋白激酶AMP-activated protein kinase;p53:细胞周期调控因子p53;ROS:活性氧reactive oxygen species;H2O2:过氧化氢;Beclin-1:bcl-2同源结构域蛋白;Autophagy:自噬;Nutrient deficiency:营养缺乏;mTOR:哺乳动物雷帕霉素靶蛋白mammalian rapamycin target protein;ULK1:Unc-51样蛋白激酶1 Unc-51-like autophagy-activating kinase 1;Raptor:哺乳动物类雷帕霉素靶蛋白C1相关受体;mTORC1:哺乳动物类雷帕霉素靶蛋白C1 mammalian target protein of rapamycin C1;Atg13:自噬相关基因13 autophagy associated gene 13;Vps34:液泡蛋白分选蛋白34 vacuolar protein sorting 34。 图 4 AMPK对细胞自噬的调控 Fig. 4 Regulation of cell autophagy by AMPK |
综上所述,热应激、冷应激、氧化应激等均会增强体内AMPK信号,AMPK激活后可抑制糖原、蛋白质、甘油三酯和胆固醇的合成,提高脂肪、葡萄糖等氧化供能,促进NADPH的合成,并引起细胞自噬,最终缓解应激反应。因此,研究应激状态下AMPK对畜禽物质代谢的调节作用及机制对缓解或治疗应激综合征具有十分重要的意义。
| [1] |
LU Z, HE X F, MA B B, et al. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism[J]. Journal of Agricultural and Food Chemistry, 2017, 65(51): 11251-11258. DOI:10.1021/acs.jafc.7b04428 |
| [2] |
GANESAN S, SUMMERS C M, PEARCE S C, et al. Short-term heat stress causes altered intracellular signaling in oxidative skeletal muscle[J]. Journal of Animal Science, 2017, 95(6): 2438-2451. |
| [3] |
HARDIE D G. AMP-activated protein kinase:a key regulator of energy balance with many roles in human disease[J]. Journal of Internal Medicine, 2014, 276(6): 543-559. DOI:10.1111/joim.2014.276.issue-6 |
| [4] |
KE R, XU Q C, LI C, et al. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism[J]. Cell Biology International, 2018, 42(4): 384-392. DOI:10.1002/cbin.v42.4 |
| [5] |
TSAI Y, LAM K, PENG Y, et al. Heat shock protein 70 and AMP-activated protein kinase contribute to 17-DMAG-dependent protection against heat stroke[J]. Journal of Cellular & Molecular Medicine, 2016, 20(10): 1889-1897. |
| [6] |
JIANG L B, CAO L, YIN X F, et al. Activation of autophagy via Ca2+-dependent AMPK/mTOR pathway in rat notochordal cells is a cellular adaptation under hyperosmotic stress[J]. Cell Cycle, 2015, 14(6): 867-879. DOI:10.1080/15384101.2015.1004946 |
| [7] |
RAJAEI-SHARIFABADI H, ELLESTAD L, PORTER T, et al. Noni (Morinda citrifolia) modulates the hypothalamic expression of stress-and metabolic-related genes in broilers exposed to acute heat stress[J]. Frontiers in Genetics, 2017, 5: 192. |
| [8] |
XIAO B, SANDERS M J, UNDERWOOD E, et al. Structure of mammalian AMPK and its regulation by ADP[J]. Nature, 2011, 472(7342): 230-233. DOI:10.1038/nature09932 |
| [9] |
RAJAMOHAN F, REYES A R, FRISBIE R K, et al. Probing the enzyme kinetics, allosteric modulation and activation of α1-and α2-subunit-containing AMP-activated protein kinase (AMPK) heterotrimeric complexes by pharmacological and physiological activators[J]. Biochemical Journal, 2016, 473(5): 581-592. DOI:10.1042/BJ20151051 |
| [10] |
BENKEL B, KOLLERS S, FRIES R, et al. Characterization of the bovine ampkγ1 gene[J]. Mammalian Genome, 2005, 16(3): 194-200. DOI:10.1007/s00335-004-2426-9 |
| [11] |
ZHANG C S, HAWLEY S A, ZONG Y, et al. Fructose-1, 6-bisphosphate and aldolase mediate glucose sensing by AMPK[J]. Nature, 2017, 548(7665): 112-116. DOI:10.1038/nature23275 |
| [12] |
HAWLEY S A, BOUDEAU J, REID J L, et al. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade[J]. The Journal of Biology, 2003, 2: 28. DOI:10.1186/1475-4924-2-28 |
| [13] |
ZHANG C S, JIANG B, LI M P, et al. The lysosomal v-ATPase-ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism[J]. Cell Metabolism, 2014, 20(3): 526-540. DOI:10.1016/j.cmet.2014.06.014 |
| [14] |
HAWLEY S A, PAN D A, MUSTARD K J, et al. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase[J]. Cell Metabolism, 2005, 2(1): 9-19. DOI:10.1016/j.cmet.2005.05.009 |
| [15] |
CHEN Z Y, SHEN X L, SHEN F Y, et al. TAK1 activates AMPK-dependent cell death pathway in hydrogen peroxide-treated cardiomyocytes, inhibited by heat shock protein-70[J]. Molecular and Cellular Biochemistry, 2013, 377(1/2): 35-44. |
| [16] |
HERRERO-MARTÍN G, HØYER-HANSEN M, GARCÍA-GARCÍA C, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells[J]. Embo Journal, 2009, 28(6): 677-685. DOI:10.1038/emboj.2009.8 |
| [17] |
DAY E A, FORD R J, STEINBERG G R. AMPK as a therapeutic target for treating metabolic diseases[J]. Trends in Endocrinology & Metabolism, 2017, 28(8): 545-560. |
| [18] |
MINOKOSHI Y, ALQUIER T, FURUKAWA N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus[J]. Nature, 2004, 428(6982): 569-574. DOI:10.1038/nature02440 |
| [19] |
CHEN H, ZHANG L, LI X W, et al. Adiponectin activates the AMPK signaling pathway to regulate lipid metabolism in bovine hepatocytes[J]. The Journal of Steroid Biochemistry and Molecular Biology, 2013, 138: 445-454. DOI:10.1016/j.jsbmb.2013.08.013 |
| [20] |
LI J, MCCULLOUGH L D. Effects of AMP-activated protein kinase in cerebral ischemia[J]. Journal of Cerebral Blood Flow & Metabolism, 2010, 30(3): 480-492. |
| [21] |
MATSUYAMA S, MORIUCHI M, SUICO M A, et al. Mild electrical stimulation increases stress resistance and suppresses fat accumulation via activation of LKB1-AMPK signaling pathway in C. elegans[J]. PLoS One, 2014, 9(12): e114690. DOI:10.1371/journal.pone.0114690 |
| [22] |
ZHANG Z W, BI M Y, YAO H D, et al. Effect of cold stress on expression of AMPKα-PPARα pathway and inflammation genes[J]. Avian Diseases, 2014, 58(3): 415-426. DOI:10.1637/10763-010814-Reg.1 |
| [23] |
LU Y L, NIE M M, WANG L, et al. Energy response and modulation of AMPK pathway of the olive flounder Paralichthys olivaceus in low-temperature challenged[J]. Aquaculture, 2018, 484: 205-213. DOI:10.1016/j.aquaculture.2017.11.031 |
| [24] |
ESSICK E E, WILSON R M, PIMENTEL D R, et al. Adiponectin modulates oxidative stress-induced autophagy in cardiomyocytes[J]. PLoS One, 2013, 8(7): e68697. DOI:10.1371/journal.pone.0068697 |
| [25] |
MIN H, BO L. Resveratrol via activation of LKB1-AMPK signaling suppresses oxidative stress to prevent endothelial dysfunction in diabetic mice[J]. Clinical and Experimental Hypertension, 2016, 38(4): 381-387. |
| [26] |
CARDACI S, FILOMENI G, CIRIOLO M R. Redox implications of AMPK-mediated signal transduction beyond energetic clues[J]. Journal of Cell Science, 2012, 125(9): 2115-2125. DOI:10.1242/jcs.095216 |
| [27] |
MIN L, CHENG J B, SHI B L, et al. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows[J]. Journal of Zhejiang University-Science B, 2015, 16(6): 541-548. DOI:10.1631/jzus.B1400341 |
| [28] |
GOTO A, EGAWA T, SAKON I, et al. Heat stress acutely activates insulin-independent glucose transport and 5'-AMP-activated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle[J]. Physiological Reports, 2015, 3(11): e12601. DOI:10.14814/phy2.12601 |
| [29] |
ZHANG J H, XIE Z L, DONG Y Z, et al. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells[J]. Journal of Biological Chemistry, 2008, 283(41): 27452-27461. DOI:10.1074/jbc.M802578200 |
| [30] |
SHE C, ZHU L Q, ZHEN Y F, et al. Activation of AMPK protects against hydrogen peroxide-induced osteoblast apoptosis through autophagy induction and NADPH maintenance:new implications for osteonecrosis treatment[J]. Cellular Signalling, 2014, 26(1): 1-8. |
| [31] |
JEON S M, CHANDEL N S, HAY N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress[J]. Nature, 2012, 485(7400): 661-665. DOI:10.1038/nature11066 |
| [32] |
GUO S G, MAO L, JI F, et al. Activating AMP-activated protein kinase by an α1 selective activator compound 13 attenuates dexamethasone-induced osteoblast cell death[J]. Biochemical and Biophysical Research Communications, 2016, 471(4): 545-552. DOI:10.1016/j.bbrc.2016.02.036 |
| [33] |
MINOKOSHI Y, SHIUCHI T, LEE S, et al. Role of hypothalamic AMP-kinase in food intake regulation[J]. Nutrition, 2008, 24(9): 786-790. DOI:10.1016/j.nut.2008.06.002 |
| [34] |
HARDIE D G. AMPK:positive and negative regulation, and its role in whole-body energy homeostasis[J]. Current Opinion in Cell Biology, 2015, 33: 1-7. |
| [35] |
LÓPEZ M, VARELA L, VÁZQUEZ M J, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance[J]. Nature Medicine, 2010, 16(9): 1001-1008. DOI:10.1038/nm.2207 |
| [36] |
LÓPEZ M, LAGE R, SAHA A K, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin[J]. Cell Metabolism, 2008, 7(5): 389-399. DOI:10.1016/j.cmet.2008.03.006 |
| [37] |
QIN B, SUN W Y, XIA H Z, et al. Effects of cold stress on mRNA level of uncoupling protein 2 in liver of chicks[J]. Pakistan Veterinary Journal, 2014, 34(3): 309-313. |
| [38] |
BEIROA D, IMBERNON M, GALLEGO R, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK[J]. Diabetes, 2014, 63(10): 3346-3358. DOI:10.2337/db14-0302 |
| [39] |
QIU S L, XIAO Z C, PIAO C M, et al. AMP-activated protein kinase α2 protects against liver injury from metastasized tumors via reduced glucose deprivation-induced oxidative stress[J]. Journal of Biological Chemistry, 2014, 289(13): 9449-9459. DOI:10.1074/jbc.M113.543447 |
| [40] |
WU N, ZHENG B, SHAYWITZ A, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1[J]. Molecular Cell, 2013, 49(6): 1167-1175. DOI:10.1016/j.molcel.2013.01.035 |
| [41] |
HA J, GUAN K L, KIM J. AMPK and autophagy in glucose/glycogen metabolism[J]. Molecular Aspects of Medicine, 2015, 46: 46-62. DOI:10.1016/j.mam.2015.08.002 |
| [42] |
YANG M, HUANG Y, CHEN J, et al. Activation of AMPK participates hydrogen sulfide-induced cyto-protective effect against dexamethasone in osteoblastic MC3T3-E1 cells[J]. Biochemical and Biophysical Research Communications, 2014, 454(1): 42-47. DOI:10.1016/j.bbrc.2014.10.033 |
| [43] |
LI Y, XU S Q, MIHAYLOVA M M, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice[J]. Cell Metabolism, 2011, 13(4): 376-388. DOI:10.1016/j.cmet.2011.03.009 |
| [44] |
INOKI K, KIM J, GUAN K L. AMPK and mTOR in cellular energy homeostasis and drug targets[J]. Annual Review of Pharmacology and Toxicology, 2012, 52: 381-400. DOI:10.1146/annurev-pharmtox-010611-134537 |
| [45] |
GROGAN P T, SLEDER K D, SAMADI A K, et al. Cytotoxicity of Withaferin A in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways[J]. Investigational New Drugs, 2013, 31(3): 545-557. DOI:10.1007/s10637-012-9888-5 |
| [46] |
BURGOS S A, KIM J J M, DAI M, et al. Energy depletion of bovine mammary epithelial cells activates AMPK and suppresses protein synthesis through inhibition of mTORC1 signaling[J]. Hormone and Metabolic Research, 2013, 45(3): 183-189. |
| [47] |
LEPRIVIER G, REMKE M, ROTBLAT B, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation[J]. Cell, 2013, 153(5): 1064-1079. DOI:10.1016/j.cell.2013.04.055 |
| [48] |
HOPPE S, BIERHOFF H, CADO I, et al. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(42): 17781-17786. DOI:10.1073/pnas.0909873106 |
| [49] |
KIM J, KUNDU M, VIOLLET B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1[J]. Nature Cell Biology, 2011, 13(2): 132-141. |
| [50] |
KIM J, KIM Y C, FANG C, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy[J]. Cell, 2013, 152(1/2): 290-303. |
| [51] |
YOSHIHARA T, KOBAYASHI H, KAKIGI R, et al. Heat stress-induced phosphorylation of FOXO3a signalling in rat skeletal muscle[J]. Acta Physiologica, 2016, 218(3): 178-187. DOI:10.1111/apha.2016.218.issue-3 |
| [52] |
GENG Y A, ZHANG C, LEI J L, et al. Walsuronoid B induces mitochondrial and lysosomal dysfunction leading to apoptotic rather than autophagic cell death via ROS/p53 signaling pathways in liver cancer[J]. Biochemical Pharmacology, 2017, 142: 71-86. DOI:10.1016/j.bcp.2017.06.134 |
| [53] |
O'NEILL H M, MAARBJERG S J, CRANE J D, et al. AMP-activated protein kinase (AMPK) β1β2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(38): 16092-16097. DOI:10.1073/pnas.1105062108 |
| [54] |
LIU C T, BROOKS G A. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes[J]. Journal of Applied Physiology, 2012, 112(3): 354-361. DOI:10.1152/japplphysiol.00989.2011 |




