近年来,随着人民生活水平的不断提高,对畜产品的需求量也与日俱增,奶牛产业发展迅速。在现代奶牛养殖中,随着良种化进程的推进,产奶量不断提高,而繁殖率却有所下降,尤其是高产奶牛的繁殖率下降更明显,繁殖障碍时有发生,因而制约奶业的发展。
奶牛妊娠后期(产前3周)至泌乳初期(产后3周)这段时间通常称为过渡期。该时期的奶牛由于经历生理、饲粮、环境和管理等变化,能量的维持需要与生产需要增加,干物质采食量急剧下降,易出现能量负平衡(NEB)。奶牛泌乳高峰期一般出现在产后30~45 d,而采食高峰期一般出现在产后70 d左右。因此,泌乳初期经常是产后奶牛NEB的高发期。一般来说,奶牛产后21~30 d易出现NEB。NEB不仅导致生产性能下降和营养代谢病发病率升高,而且可导致繁殖性能下降,制约奶业的发展。
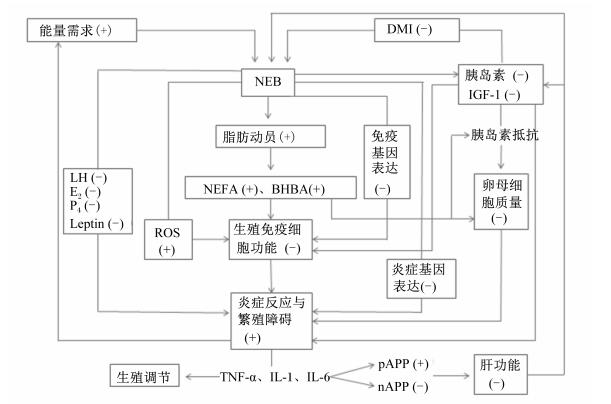
NEB对奶牛繁殖的影响早在20世纪80年代就有国外学者进行了研究[1],随后的研究中主要集中在饲粮能量和蛋白质水平对繁殖的影响[2-4]、NEB对繁殖性能的改变[5-7]以及NEB的改善研究[8-9],近年来的研究也涉及其深层次的机理[10-11],但目前国内在该方面的系统研究依然较少,故本文就NEB对奶牛繁殖的影响及作用机理进行综述(图 1),旨在揭示其影响模式,为改善奶牛尤其是过渡期奶牛的繁殖力研究提供借鉴与思路。
|
BHBA:β-羟丁酸β-hydroxybutyrate;DMI:干物质采食量dry matter intake;E2:雌二醇estradiol;IGF-1:胰岛素样生长因子-1 insulin-like growth factor-1;IL-1:白细胞介素-1 interleukin-1;IL-6:白细胞介素-6 interleukin-6;LH:促黄体生成素luetinizing hormone;Leptin:瘦素leptin;NEB:能量负平衡negative energy balalice;NEFA:非酯化脂肪酸non-esterified fatty acid;nAPP:负急性时相蛋白negative acute phase protein;P4:孕酮progesterone;pAPP:正急性时相蛋白positive acute phase protein;ROS:活性氧reactive oxygen;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α。“+”和“-”分别表示在分子水平(或相关功能)上的增加和降低。The symbols “+” and “-”indicate an increase and a decrease in biomolecule levels (or related functions), respectively. 图 1 NEB对奶牛繁殖影响的机理 Fig. 1 Mechanism of effects of NEB on reproduction of dairy cows |
由于产前胎儿对瘤胃的物理性压迫,同时受到营养代谢、激素等诸多方面的影响,奶牛干物质采食量下降,瘤胃发酵物的供给不足,造成丙酸缺乏,从而导致葡萄糖缺乏并上调脂肪分解信号,激活激素敏感脂肪酶(HSL)。磷酸化的HSL转移到脂滴中促进脂肪动员,并将脂肪所储存的甘油三酯(TG)水解成非脂化脂肪酸(NEFA)和甘油[12-13],一部分NEFA进入乳腺上皮细胞用于乳脂合成,另一部分NEFA作为能源物质进入肝脏细胞进行β-氧化供能以缓解NEB。若奶牛处于严重NEB状态,NEFA含量超出肝脏的氧化分解能力时则进行不完全β-氧化产生β-羟丁酸(BHBA)等酮体[14]。NEFA与BHBA是重要的能量代谢指标,反映机体能量代谢状态。当血浆NEFA的浓度高于0.70 mEq/L时,奶牛处于较严重的NEB状态[15]。
2 NEB诱发能量代谢疾病NEB引发动物机体能量代谢紊乱并由此进一步削弱动物繁殖性能。高含量的NEFA与BHBA可诱发奶牛一系列的能量代谢疾病。进入肝脏细胞氧化供能的NEFA,若超出肝脏氧化分解的能力,则会进行不完全β-氧化,产生酮体,进而诱发奶牛出现高酮血症或患酮症;另一部分NEFA进入肝脏后,被酯化合成TG。TG与载脂蛋白结合,形成极低密度脂蛋白(VLDL)后从肝脏输出进入血液。当生成的TG超出VLDL形式输出的量时,会沉积在肝脏,从而形成脂肪肝。至少50%的奶牛在产奶期的第1个月会经历亚临床酮症。酮症患牛的临床型子宫内膜炎和卵巢囊肿发生率显著增高,产后生殖器官恢复能力与受孕能力与酮症存在负相关[16]。酮症患牛血液中天冬氨酸转移酶(AST)活性升高,说明肝功能受到损伤,进而导致机体能量代谢障碍。脂肪肝患牛T细胞转化功能明显降低,免疫力下降,易感染其他疾病,并使产后第1次排卵延迟,孕酮(P4)分泌显著降低,进而导致奶牛繁殖力下降[17-18]。另有报道指出,脂肪肝患牛首次配种至产犊间隔天数、受胎至产犊间隔天数及配种次数显著增加,血液中胰岛素样生长因子(IGF)-1和促黄体生成素(LH)含量在首次配种后显著降低[19]。在产后早期的卵巢机能恢复过程中,胰岛素(INS)、IGF-1与LH的联合作用能促进优势卵泡的形成[20]。酮症脂肪肝综合征是奶牛过渡期易患的能量代谢病,能量代谢的紊乱将引起机体多方面的变化,并对繁殖机能产生显著影响。
3 NEB影响激素分泌激素代谢在奶牛繁殖过程中起重要的调节作用。与NEB相关的奶牛机体代谢变化导致血浆及卵泡液中激素代谢的变化,主要包括INS/IGF系统、促性腺激素(GnRH)、瘦素(Leptin)等的变化。
3.1 对INS/IGF系统的影响IGF家族主要有IGF-1、IGF-2。血浆中INS和IGF-1可以影响卵母细胞发育,促进卵巢颗粒细胞的增殖及类固醇的分泌;此外,IGF-1还可以调节P4及LH的分泌[21]。外周血中的IGF-1大部分在生长激素(GH)作用下从肝脏细胞释放出来,而在NEB条件下生长激素受体(GHR)表达下调,导致GH-IGF轴解偶联,使血液中IGF-1含量下降[22]。IGF-1对LH的分泌具有调节作用,是刺激P4分泌的强效因子[21, 23]。在重度NEB状态下,奶牛肝脏IGF-1、胰岛素样生长因子结合蛋白(IGFBP)-3、IGFBP-4、IGFBP-5、IGFBP-6和胰岛素样生长因子酸不稳定亚基(IGFALS)基因的表达显著降低,IGFBP-2基因的表达升高[24]。这些基因参与IGF-1的分泌和结合,并与半衰期有关。NEB状态下的奶牛血浆IGF-1的含量将持续走低,这将延缓卵泡的生长、减少雌二醇(E2)的合成及延迟排卵[6]。IGF-2能够促进卵泡的生长,也能促进类固醇激素的分泌,并对胚胎发育有显著影响,主要影响胚胎分裂、分化并可能对其代谢进行调节。NEB促进IGF-2分泌,干扰胚胎的发育,引起胚胎死亡。
INS能促进肝脏、肌肉和脂肪等组织摄取和利用葡萄糖,抑制肝糖原分解及糖异生作用,促进蛋白质和脂肪合成,抑制蛋白质、脂肪分解及酮体生成。INS同样与类固醇合成有关,能够调节颗粒细胞中与性腺激素合成和分泌相关的反应,在正常的生理浓度下能够促进颗粒细胞中E2的生成[25]。NEB奶牛血浆葡萄糖和INS含量均下降,而INS在体外和体内均有刺激奶牛卵泡细胞发育的作用[26]。此外,NEB还可能诱发奶牛机体INS抵抗。发生NEB时,奶牛血液中NEFA和BHBA含量升高,对INS的敏感性显著降低,即INS抵抗程度与血液中NEFA和BHBA含量存在显著的负相关[27]。在妊娠晚期,在奶牛皱胃中输入脂质可提高血浆NEFA含量,诱导奶牛全身性的INS抵抗[28]。INS抵抗的特征是靶组织或靶细胞对循环系统INS敏感性降低,损害细胞对葡萄糖的摄入,导致细胞凋亡。NEB状态下,血浆中高含量NEFA可以引起氧化应激,导致胰岛B细胞功能损害或凋亡[29-30]。高含量的NEFA进一步抑制胰岛B细胞分泌INS,抑制INS与肝脏中的特定受体结合,抑制细胞内葡萄糖转运蛋白的活性[31],诱发INS抵抗。Baruselli等[32]发现,对泌乳晚期奶牛进行人工授精时,受孕率较泌乳早期奶牛低,但将正常胚胎移植至泌乳晚期奶牛时,受孕率却正常,因此认为泌乳晚期的INS抵抗可能损伤卵母细胞,从而导致受孕率降低。
INS/IGF-1信号通路是重要的能源物质代谢信号。INS和IGF(IGF-1、IGF-2)具有相似的分子结构,二者通过与各自的酪氨酸激酶受体结合激发下游信号因子活化,胰岛素受体(IR)与胰岛素样生长因子1受体(IGF-1R)通过磷酸化胰岛素受体底物(IRSs)、Src同源性胶原蛋白等细胞因子完成信号转导作用,INS和IGF-1具有相同的信号转导通路,包括Ras-Raf-丝裂原活化蛋白激酶(MAPK)和磷脂酰肌醇3-激酶(PI3K)/蛋白激酶B(Akt)信号通路[33]。在能量缺乏的条件下,INS/IGF及其受体难以通过相关途径激活哺乳动物雷帕霉素靶蛋白(mTOR)信号通路,而mTOR信号通路与卵子和精子生成、胚胎发育过程及相关细胞中蛋白质代谢调节相关[33-34]。
3.2 对GnRH的影响NEB显著影响下丘脑-垂体性腺轴。LH脉冲分泌频率与能量平衡呈正相关,与血浆NEFA含量呈负相关[35]。在NEB条件下,反刍动物下丘脑GnRH受到抑制,从而抑制LH脉冲分泌频率,延长第1次发情时间,减缓生长卵泡的成熟,抑制排卵。NEB减少LH释放的机制可能涉及神经元氧化能源的供给和下丘脑-垂体的激素调制[36]。此外,血浆LH低频率脉冲促使E2负反馈增强,可能与NEB奶牛乏情有关[37]。外周血中P4受IGF-1的调节,NEB奶牛血液中IGF-1含量降低,导致P4分泌下降。P4诱导子宫组织营养素的分泌,对孕体的维持与养分供给至关重要。因此,P4含量下降对奶牛孕期胚胎发育产生负面影响[38]。
3.3 对Leptin的影响Leptin与繁殖有密切的关系。下丘脑、垂体、性腺、子宫均有Leptin受体。Leptin能刺激GnRH、LH、促卵泡生成素(FSH)、E2分泌。胎盘滋养层可以分泌瘦素,促进胚胎发育和附植,促进胎儿发育,对妊娠维持有重要作用[39]。Leptin通过影响下丘脑-垂体-性腺轴影响繁殖,是维持正常卵巢周期的必要因素。在分娩前35 d之前测量奶牛血浆中Leptin含量,发现血浆Leptin含量降低约50%,尽管在此后逐渐改善能量平衡,但在哺乳期间Leptin含量仍然保持较低水平;白色脂肪组织中Leptin mRNA的表达丰度也呈现相同趋势的变化[40]。Leptin影响采食量,同时促进围产期牛外周血中INS抵抗[41]。Leptin含量与机体能量水平呈正相关,在奶牛妊娠后期Leptin含量升高,分娩时降到最低,并在之后逐渐恢复,恢复的快慢主要取决于NEB的程度与持续时间。NEB状态下奶牛血浆Leptin含量降低,影响奶牛产后发情,增加Leptin可以缩短奶牛产后第1次发情时间间隔[37]。
4 NEB对生殖免疫的影响机体产生足够的免疫应答需要足够的能量支持。NEB奶牛繁殖机能的下降在一定程度上与内环境中高含量的NEFA与BHBA破坏机体免疫力和产后健康有关。在体外使用高产奶牛产后时期(NEB状态)相匹配的NEFA(0.12~1.00 mmol/L)处理免疫细胞后发现细胞功能及其生存能力降低[42]。增加在培养基中NEFA含量可减少外周血单核细胞γ-干扰素(IFN-γ)与免疫球蛋白M(IgM)的合成[43]。免疫球蛋白G(IgG)含量与NEFA含量呈现负相关[44]。不仅仅是NEFA,BHBA也与产后奶牛的免疫抑制有关。在高含量BHBA条件下,白细胞数量出现下降[45]。体外培养嗜中性粒细胞时,增加BHBA含量后细胞的吞噬能力、胞外陷阱形成能力与免疫杀伤能力均降低[46]。
许多奶牛在产后子宫遭受微生物侵染,经常发展为持续的子宫内膜炎,影响奶牛繁殖力。用微阵列技术分析严重NEB的产后奶牛子宫内膜细胞的差异表达基因,发现基质金属蛋白酶1(MMP1)、基质金属蛋白酶3(MMP3)、基质金属蛋白酶13(MMP13)、CXC趋化因子配体5(CXCL5)、人类白细胞抗原-DQB(HLA-DQB)、S100钙结合蛋白A8(S100A8)、S100钙结合蛋白A9(S100A9)、S100钙结合蛋白A12(S100A12)、胎球蛋白A(AHSG)、白细胞介素-1受体(IL-1R)、白细胞介素-8(IL-8)和白细胞介素8受体β(IL-8Rβ)等炎症反应相关基因的表达显著上调,这些基因涉及基质金属蛋白酶、趋化因子、细胞因子和钙粒蛋白等的编码;此外,ISG20、干扰素诱导与解旋酶C结构域1(IFIH1)、黏病毒耐药蛋白1(MX1)和黏病毒耐药蛋白2(MX2)等干扰素诱导相关基因的表达显著上调[10]。这是处于严重NEB的奶牛发生子宫炎症的原因。炎症因子诸如肿瘤坏死因子-α(TNF-α)、白细胞介素-1(IL-1)和白细胞介素-6(IL-6)等的释放,刺激肝脏正急性时相蛋白(pAPP)的合成,损害负急性时相蛋白(nAPP)的合成,从而干扰肝脏正常功能[47]。NEB可能通过改变子宫免疫反应环境而延长子宫恢复周期,降低奶牛繁殖力,并且通过改变子宫内环境代谢产物,或者间接上调AHSG基因的表达,损害INS受体信号诱发区域性INS抵抗,进而对子宫免疫系统造成不良影响[10]。此外,进行NEB代谢时,活性氧(ROS)水平增加,引发脂质过氧化,对其他组织细胞造成损伤。免疫细胞的膜具有高浓度的多不饱和脂肪酸,对过氧化作用非常敏感,受到刺激时能够产生大量的ROS[48]。NEB可能通过过氧化作用机制削弱机体免疫能力。
5 结语NEB通过多种途径影响奶牛繁殖机能,其通过影响脂肪动员,改变血液代谢指标,影响卵泡发育环境与子宫内环境,诱发动物机体能量代谢紊乱与能量代谢疾病,削弱奶牛的繁殖性能。NEB奶牛繁殖性能的下降归咎于机体多种神经激素、代谢调节激素的改变。NEB奶牛生殖免疫机能受到损害,不仅增加子宫疾病发病率,还将延缓产后子宫机能恢复。改善营养,适当提高奶牛过渡期饲粮非纤维碳水化合物(NFC)水平,可促进瘤胃乳头的发育以获得吸收足够挥发性脂肪酸(VFA)的能力,从而降低NEB对奶牛繁殖的影响[49]。饲粮添加剂的改良,如在奶牛饲粮中添加过瘤胃脂肪酸和甲基供体,能显著改善NEB,降低血液NEFA和BHBA含量,提高免疫机能[11, 50-51]。此外,改变瘤胃微生物发酵模式也能成为改善奶牛能量代谢,提升繁殖性能的新思路。
| [1] |
BUTLER W R, EVERETT R W, COPPOCK C E. The relationships between energy balance, milk production and ovulation in postpartum Holstein cows[J]. Journal of Animal Science, 1981, 53(3): 742-748. DOI:10.2527/jas1981.533742x |
| [2] |
LUCY M C, STAPLES C R, THATCHER W W, et al. Influence of diet composition, dry-matter intake, milk production and energy balance on time of post-partum ovulation and fertility in dairy cows[J]. Animal Science, 1992, 54(3): 323-331. |
| [3] |
TAMMINGA S. The effect of the supply of rumen degradable protein and metabolisable protein on negative energy balance and fertility in dairy cows[J]. Animal Reproduction Science, 2006, 96(3/4): 227-239. |
| [4] |
BURKE C R, KAY J K, PHYN C V C, et al. Short communication:effects of dietary nonstructural carbohydrates pre- and postpartum on reproduction of grazing dairy cows[J]. Journal of Dairy Science, 2010, 93(9): 4292-4296. DOI:10.3168/jds.2009-2869 |
| [5] |
BUTLER W R, SMITH R D. Interrelationships between energy balance and postpartum reproductive function in dairy cattle[J]. Journal of Dairy Science, 1989, 72(3): 767-783. DOI:10.3168/jds.S0022-0302(89)79169-4 |
| [6] |
WATHES D C, FENWICK M, CHENG Z, et al. Influence of negative energy balance on cyclicity and fertility in the high producing dairy cow[J]. Theriogenology, 2007, 68(Suppl.1): S232-S241. |
| [7] |
WATHES D C, CHENG Z R, FENWICK M A, et al. Influence of energy balance on the somatotrophic axis and matrix metalloproteinase expression in the endometrium of the postpartum dairy cow[J]. Reproduction, 2011, 141(2): 269-281. DOI:10.1530/REP-10-0177 |
| [8] |
GRUMMER R R, WILTBANK M C, FRICKE P M, et al. Management of dry and transition cows to improve energy balance and reproduction[J]. The Journal of Reproduction and Development, 2010, 56(Suppl.1): S22-S28. |
| [9] |
CARDOSO F C, LEBLANC S J, MURPHY M R, et al. Prepartum nutritional strategy affects reproductive performance in dairy cows[J]. Journal of Dairy Science, 2013, 96(9): 5859-5871. DOI:10.3168/jds.2013-6759 |
| [10] |
WATHES D C, CHENG Z R, CHOWDHURY W, et al. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows[J]. Physiological Genomics, 2010, 39(1): 1-13. |
| [11] |
ESPOSITO G, IRONS P C, WEBB E C, et al. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows[J]. Animal Reproduction Science, 2014, 144(3/4): 60-71. |
| [12] |
KOLTES D A, SPURLOCK D M. Coordination of lipid droplet-associated proteins during the transition period of Holstein dairy cows[J]. Journal of Dairy Science, 2011, 94(4): 1839-1848. DOI:10.3168/jds.2010-3769 |
| [13] |
LOCHER L F, MEYER N, WEBER E M, et al. Hormone-sensitive lipase protein expression and extent of phosphorylation in subcutaneous and retroperitoneal adipose tissues in the periparturient dairy cow[J]. Journal of Dairy Science, 2011, 94(9): 4514-4523. DOI:10.3168/jds.2011-4145 |
| [14] |
VAN DORLAND H A, SADRI H, MOREL I, et al. Coordinated gene expression in adipose tissue and liver differs between cows with high or low NEFA concentrations in early lactation[J]. Journal of Animal Physiology and Animal Nutrition, 2012, 96(1): 137-147. DOI:10.1111/jpn.2012.96.issue-1 |
| [15] |
RIBEIRO E S, LIMA F S, AYRES H, et al. Effect of postpartum diseases on reproduction of grazing dairy cows[J]. Journal of Dairy Science, 2011, 94(Suppl.1): 63. |
| [16] |
SHIN E K, JEONG J K, CHOI I S, et al. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows[J]. Theriogenology, 2015, 84(2): 252-260. DOI:10.1016/j.theriogenology.2015.03.014 |
| [17] |
周建平, 田文儒, 郑昌乐, 等. 产后脂肪肝奶牛外周血浆孕酮、前列腺素F2α变化规律的研究[J]. 黑龙江畜牧兽医, 1993(9): 1-3. |
| [18] |
周建平, 张俊育, 田文儒, 等. 脂肪肝影响围产期奶牛繁殖力的机理研究[J]. 畜牧兽医学报, 1997, 28(2): 115-119. DOI:10.3321/j.issn:0366-6964.1997.02.004 |
| [19] |
GOWRI B, PRATHABAN S, KATHIRESAN D, et al. Impact of the fatty infiltration of liver on fertility in cattle[J]. Indian Veterinary Journal, 2015, 92(9): 60-61. |
| [20] |
FENWICK M A, LLEWELLYN S, FITZPATRICK R, et al. Negative energy balance in dairy cows is associated with specific changes in IGF-binding protein expression in the oviduct[J]. Reproduction, 2008, 135(1): 63-75. DOI:10.1530/REP-07-0243 |
| [21] |
THATCHER W W, BILBY T R, BARTOLOME J A, et al. Strategies for improving fertility in the modern dairy cow[J]. Theriogenology, 2006, 65(1): 30-44. DOI:10.1016/j.theriogenology.2005.10.004 |
| [22] |
LUCY M C, JIANG H, KOBAYASHI Y. Changes in the somatotrophic axis associated with the initiation of lactation[J]. Journal of Dairy Science, 2001, 84(Suppl.1): E113-E119. |
| [23] |
BROWN K L, CASSELL B G, MCGILLIARD M L, et al. Hormones, metabolites, and reproduction in Holsteins, Jerseys, and their crosses[J]. Journal of Dairy Science, 2012, 95(2): 698-707. DOI:10.3168/jds.2011-4666 |
| [24] |
FENWICK M A, FITZPATRICK R, KENNY D A, et al. Interrelationships between negative energy balance (NEB) and IGF regulation in liver of lactating dairy cows[J]. Domestic Animal Endocrinology, 2008, 34(1): 31-44. DOI:10.1016/j.domaniend.2006.10.002 |
| [25] |
HEIN G J, PANZANI C G, RODRÍGUEZ F M, et al. Impaired insulin signaling pathway in ovarian follicles of cows with cystic ovarian disease[J]. Animal Reproduction Science, 2015, 156: 64-74. DOI:10.1016/j.anireprosci.2015.02.010 |
| [26] |
叶承荣, 张克春, 谭勋. 能量负平衡对高产奶牛繁殖性能影响的研究进展[J]. 上海交通大学学报(农业科学版), 2006, 24(4): 398-401. DOI:10.3969/j.issn.1671-9964.2006.04.018 |
| [27] |
OHTSUKA H, KOIWA M, HATSUGAYA A, et al. Relationship between serum TNF activity and insulin resistance in dairy cows affected with naturally occurring fatty liver[J]. Journal of Veterinary Medical Science, 2001, 63(9): 1021-1025. DOI:10.1292/jvms.63.1021 |
| [28] |
SALIN S, TAPONEN J, ELO K, et al. Effects of abomasal infusion of tallow or camelina oil on responses to glucose and insulin in dairy cows during late pregnancy[J]. Journal of Dairy Science, 2012, 95(7): 3812-3825. DOI:10.3168/jds.2011-5206 |
| [29] |
STEIN D T, STEVENSON B E, CHESTER M W, et al. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation[J]. Journal of Clinical Investigation, 1997, 100(2): 398-403. DOI:10.1172/JCI119546 |
| [30] |
RAVNSKJAER K, FRIGERIO F, BOERGESEN M, et al. PPARδ is a fatty acid sensor that enhances mitochondrial oxidation in insulin-secreting cells and protects against fatty acid-induced dysfunction[J]. Journal of Lipid Research, 2010, 51(6): 1370-1379. DOI:10.1194/jlr.M001123 |
| [31] |
KARLSSON H K R, CHIBALIN A V, KOISTINEN H A, et al. Kinetics of GLUT4 trafficking in rat and human skeletal muscle[J]. Diabetes, 2009, 58(4): 847-854. |
| [32] |
BARUSELLI P S, VIEIRA L M, SÁ FILHO M F, et al. Associations of insulin resistance later in lactation on fertility of dairy cows[J]. Theriogenology, 2016, 86(1): 263-269. DOI:10.1016/j.theriogenology.2016.04.039 |
| [33] |
DUPONT J, REVERCHON M, BERTOLDO M J, et al. Nutritional signals and reproduction[J]. Molecular and Cellular Endocrinology, 2014, 382(1): 527-537. DOI:10.1016/j.mce.2013.09.028 |
| [34] |
马勇, 罗海玲, 卢晓楠. 日粮能量和蛋白质对反刍动物繁殖机能影响的机理[J]. 中国草食动物科学, 2014(增刊): 14-16. |
| [35] |
KADOKAWA H, BLACHE D, MARTIN G B. Plasma leptin concentrations correlate with luteinizing hormone secretion in early postpartum Holstein cows[J]. Journal of Dairy Science, 2006, 89(8): 3020-3027. DOI:10.3168/jds.S0022-0302(06)72575-9 |
| [36] |
SCHNEIDER J E. Energy balance and reproduction[J]. Physiology & Behavior, 2004, 81(2): 289-317. |
| [37] |
王学君, 刘伟, 苗霆, 等. 能量对奶牛繁殖性能的影响与调控技术[J]. 中国奶牛, 2009(3): 31-33. DOI:10.3969/j.issn.1004-4264.2009.03.011 |
| [38] |
ROBINSON R S, FRAY M D, WATHES D C, et al. In vivo expression of interferon tau mRNA by the embryonic trophoblast and uterine concentrations of interferon tau protein during early pregnancy in the cow[J]. Molecular Reproduction and Development, 2006, 73(4): 470-474. DOI:10.1002/(ISSN)1098-2795 |
| [39] |
HOGGARD N, HUNTER L, TRAYHURN P, et al. Leptin and reproduction[J]. The Proceedings of the Nutrition Society, 1998, 57(3): 421-427. DOI:10.1079/PNS19980061 |
| [40] |
BLOCK S S, BUTLER W R, EHRHARDT R A, et al. Decreased concentration of plasma leptin in periparturient dairy cows is caused by negative energy balance[J]. Journal of Endocrinology, 2001, 171(2): 339-348. |
| [41] |
BLACHE D, CELI P, BLACKBERRY M A, et al. Decrease in voluntary feed intake and pulsatile luteinizing hormone secretion after intracerebroventricular infusion of recombinant bovine leptin in mature male sheep[J]. Reproduction, Fertility and Development, 2000, 12(7/8): 373-381. |
| [42] |
BISINOTTO R S, GRECO L F, RIBEIRO E S, et al. Influences of nutrition and metabolism on fertility of dairy cows[J]. Animal Reproduction, 2012, 9(3): 260-272. |
| [43] |
LACETERA N, SCALIA D, FRANCI O, et al. Short communication:effects of nonesterified fatty acids on lymphocyte function in dairy heifers[J]. Journal of Dairy Science, 2004, 87(4): 1012-1014. DOI:10.3168/jds.S0022-0302(04)73246-4 |
| [44] |
MÖSCH A.Parameters of energy metabolism and immunoglobulin G in the serum of dairy cows in the peripartal period[D]. Ph.D.Thesis.Berlin: Freie Universität Berlin, 2012.
|
| [45] |
SCALIA D, LACETERA N, BERNABUCCI U, et al. In vitro effects of nonesterified fatty acids on bovine neutrophils oxidative burst and viability[J]. Journal of Dairy Science, 2006, 89(1): 147-154. DOI:10.3168/jds.S0022-0302(06)72078-1 |
| [46] |
GRINBERG N, ELAZAR S, ROSENSHINE I, et al. β-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli[J]. Infection and Immunity, 2008, 76(6): 2802-2807. DOI:10.1128/IAI.00051-08 |
| [47] |
HUZZEY J M, DUFFIELD T F, LEBLANC S J, et al. Short communication:haptoglobin as an early indicator of metritis[J]. Journal of Dairy Science, 2009, 92(2): 621-625. DOI:10.3168/jds.2008-1526 |
| [48] |
SPEARS J W, WEISS W P. Role of antioxidants and trace elements in health and immunity of transition dairy cows[J]. The Veterinary Journal, 2008, 176(1): 70-76. DOI:10.1016/j.tvjl.2007.12.015 |
| [49] |
RABELO E, REZENDE R L, BERTICS S J, et al. Effects of transition diets varying in dietary energy density on lactation performance and ruminal parameters of dairy cows[J]. Journal of Dairy Science, 2003, 86(3): 916-925. DOI:10.3168/jds.S0022-0302(03)73674-1 |
| [50] |
CASTAÑEDA-GUTIÉRREZ E, BENEFIELD B C, DE VETH M J, et al. Evaluation of the mechanism of action of conjugated linoleic acid isomers on reproduction in dairy cows[J]. Journal of Dairy Science, 2007, 90(9): 4253-4264. DOI:10.3168/jds.2007-0117 |
| [51] |
FARRAN T B, REINHARDT C D, BLASI D A, et al. Source of dietary lipid may modify the immune response in stressed feeder cattle[J]. Journal of Animal Science, 2008, 86(6): 1382-1394. DOI:10.2527/jas.2007-0116 |




