由细胞间连接构成的相对封闭的单层肠道上皮细胞,除具有吸收电解质、水分以及各种小分子营养物质的功能外,还是阻挡肠腔内大量微生物及抗原物质进入体内的重要屏障。维持肠道上皮细胞的屏障功能对于动物的健康至关重要。乳酸杆菌属是人和动物肠道内的优势菌[1],在健康人体内常见的有50个以上的菌种[2],其中多个乳酸杆菌菌种被用做益生菌而得到广泛研究。2013版《饲料添加剂品种目录》中共有35个微生物菌种允许在饲料中使用,其中属于乳酸杆菌属的就占了10种。越来越多的研究表明,乳酸杆菌对预防和治疗肠道疾病具有重要价值。乳酸杆菌可以促进上皮细胞紧密连接蛋白的表达和合理分布,维持上皮细胞的屏障功能;同时通过竞争性抑制病原菌对上皮细胞的黏附,缓解病原菌诱导的炎性细胞因子对紧密连接的损伤,改善肠上皮屏障功能,维持黏膜的完整性。本文就肠道上皮细胞紧密连接的生理性和病理性调控以及乳酸杆菌的影响进行了总结,旨在增进我们对于乳酸杆菌益生机制更全面、更清晰的认识。
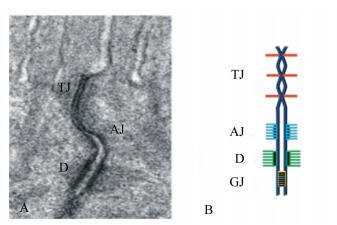
1 肠道上皮细胞间连接的基本结构肠道单层上皮细胞紧密排列,通过细胞间连接,构成了一道相对封闭的屏障。一方面阻挡肠腔内复杂的微生物、大分子代谢物进入体内,另一方面选择性地允许小分子营养物质的吸收以及体液的分泌[3]。上皮细胞间连接主要包括紧密连接、黏着连接、桥粒和间隙连接[4](图 1)。桥粒和间隙连接位于上皮细胞连接的下端。桥粒类似钮扣状结构,将相邻细胞紧密地连接在一起。间隙连接由间隙连接蛋白(Cx)家族构成,主要介导细胞内交流的通道。而紧密连接和黏着连接共同构成上皮细胞顶端连接复合体,负责胞间运输的调控[5]。黏着连接由钙黏蛋白(cadherin)和连环蛋白(catenin)构成,与胞内的细胞骨架-连接相关肌动球蛋白环(perijunctional actomyosin ring)相连,构成顶端连接复合体的基础。紧密连接位于黏着连接的上方,是调控胞间运输的关键[6-7]。紧密连接由至少50个不同的膜相关蛋白组成[8],主要包括跨膜蛋白和支架蛋白,跨膜蛋白如闭合蛋白(claudin)和闭锁蛋白(occludin),通过胞外环的相互作用连接2个相邻细胞[9],支架蛋白如闭合小环蛋白(ZO)-1、2、3,将跨膜连接蛋白和细胞骨架-连接相关肌动球蛋白环以及其他胞质调节蛋白连接在一起,同时还与连环蛋白互作,将紧密连接、黏着连接沟通在一起[9]。这些蛋白共同构成了复杂的紧密连接复合体。
|
透射电镜(A)和示意图(B)显示:紧密连接(TJ)和黏着连接(AJ)位于上皮细胞连接复合体的顶端,而桥粒(D)和间隙连接(GJ)位于上皮连接的下端。 Transmission electron microscope (A) and diagrammatic sketch (B) showed: tight junction (TJ) and adhering junction (AJ) located at the top of the epithelial cell junction complex, and desmosome (D) and gap junction (GJ) located at the lower end of epithelial junction. 图 1 细胞连接 Fig. 1 Cell junction |
紧密连接并不是一道静止的屏障,在各种生理性和病理性因子的作用下,通过改变各种连接蛋白的表达、分布以及细胞骨架-连接相关肌动球蛋白环的收缩,调控紧密连接的结构,影响上皮细胞屏障的渗透性。在上皮细胞紧密连接的调控中肌球蛋白轻链激酶(myosin light chain kinase,MLCK)发挥重要的作用。
2.1 生理性调控在研究胞间运输时发现,钠-葡萄糖共运输体系可以诱导紧密连接渗透性增加,主要是增加小分子物质的渗透性,而大分子物质的渗透性不增加[10]。进一步研究发现,钠-葡萄糖共运输体系调控紧密连接与连接相关肌动球蛋白的收缩有关[11-13],推测连接相关肌动球蛋白的收缩可能调控了紧密连接的屏障功能。随后发现,Ⅱ型肌球蛋白轻链(myosin light chain,MLC)磷酸化增加,这是肌动球蛋白收缩的生化标志物[10]。而抑制MLCK活性可以阻止钠-葡萄糖共运输对紧密连接的调控[10]。这些研究表明,MLCK诱导的MLC磷酸化是紧密连接生理性调控的必要途径。生理性因素还通过影响紧密连接蛋白的分布,调控紧密连接。研究发现,钠-葡萄糖共运输体系可影响胞内游离ZO-1和紧密连接复合体中ZO-1的交换,而抑制MLCK活性可以阻止这种交换[14]。
2.2 病理性调控 2.2.1 病原菌可直接影响上皮细胞之间的紧密连接病原菌与上皮细胞上的模式识别受体结合,直接影响上皮细胞间紧密连接蛋白的表达和分布。给小鼠灌服肠出血性大肠杆菌(EHEC)O157 : H7显著降低了隐窝底部紧密连接蛋白occludin和ZO-1的表达,增加肠上皮的通透性[15]。体外将肠毒性大肠杆菌(ETEC)K88与猪上皮细胞系IPEC-J2细胞共培养后,同样降低了紧密连接蛋白occludin、claudin-1和ZO-1的表达,增加单层上皮细胞的通透性[16]。研究人员还发现,鼠伤寒沙门氏菌[17]、内毒素(LPS)[18]和致病性大肠杆菌(EPEC)[19]均可影响紧密连接蛋白的表达,导致上皮细胞层通透性增加。病原菌还通过调控紧密连接蛋白的分布影响上皮细胞间的通透性。Roselli等[20]报道,ETEC诱导猪上皮细胞系IPEC-1细胞损伤与ZO-1蛋白重排有关。Yu等[21]也研究发现,ETEC K88导致Caco2细胞系上紧密连接蛋白claudin-1及E-cadherin的重排及缺失。病原菌对上皮细胞通透性的影响可能通过调控MLCK的表达与活性有关。Long等[22]研究发现,小鼠感染旋毛虫后,结肠黏膜中MLCK表达显著升高,MLC磷酸化水平升高。服用MLCK抑制剂均能降低小鼠肠道通透性的增加。Gu等[23]也报道,小鼠受到LPS攻击后,MLCK表达及活性增加,紧密连接屏障功能受损。而抑制MLCK后,可以缓解LPS对上皮屏障的有害影响[24]。
2.2.2 病原菌诱导炎性细胞因子分泌,间接调控上皮细胞紧密连接病原菌与上皮细胞上的模式识别受体结合,诱导上皮细胞分泌白细胞介素(IL)-6、IL-8等细胞因子以及趋化因子等[25],这些细胞因子和趋化因子可以诱导体内粒细胞、单核细胞、树突细胞募集到炎症部位[26],进一步产生更多的炎性细胞因子,如肿瘤坏死因子-α(TNF-α)、γ-干扰素(IFN-γ)、IL-1β等。这些细胞因子与上皮细胞上的相关受体结合,直接影响上皮细胞间的紧密连接[27],增加上皮细胞屏障的渗透性。其中研究较多的是TNF-α和IFN-γ。
TNF-α可以增加人肠道上皮细胞Caco2、T84和HT29的通透性[28-30]。这种影响主要通过MLCK的调控[31]。使用MLCK抑制剂可以消除TNF-α诱导的单层上皮细胞渗透性的升高[32]。进一步研究发现,TNF-α通过增加MLCK转录,增加MLCK的活性,破坏紧密连接[33]。TNF-α可能通过诱导occludin的内吞,调控紧密连接[34],使用MLCK抑制剂可以阻止TNF-α诱导的occludin的内吞[34-35]。这些研究表明,occludin的内吞可能是病理性紧密连接调控中的重要过程。
IFN-γ影响紧密连接蛋白的表达[36-38]。IFN-γ还可以引起紧密连接蛋白occludin和claudin-1的内吞。通过共聚焦显微镜可以发现,IFN-γ通过巨胞饮(macropinocytosis)诱导T84上皮细胞紧密连接复合体中的occludin进入胞内[39-40]。另外,IFN-γ诱导紧密连接蛋白内吞以及紧密连接渗透性增加,需要Rho激酶诱导的MLC磷酸化[41]。
3 乳酸杆菌对肠道上皮细胞紧密连接的调控 3.1 乳酸杆菌对肠道上皮细胞紧密连接的直接调控乳酸杆菌可以直接提高紧密连接蛋白的表达,增强上皮紧密连接的完整性。有研究表明,给健康仔猪口服罗伊氏乳杆菌I5007后,空肠和回肠上皮occludin和ZO-1的蛋白水平显著增加[18]。饲喂鼠李糖乳杆菌[15]和植物乳杆菌[42]也有类似的报道。给断奶仔猪饲喂弗氏乳杆菌,显著增加了十二指肠、空肠和回肠紧密连接蛋白ZO-1、occludin和claudin-1 mRNA的表达水平,但没有上调蛋白的表达[43]。体外试验发现,植物乳杆菌通过增加Caco2细胞系上跨膜电阻(TER)值来提高上皮屏障的完整性[44]。Anderson等[45]报道,植物乳杆菌MB452对Caco2细胞系TER值的影响呈现剂量依赖性,随植物乳杆菌添加剂量增大,TER值升高。另外,嗜酸乳杆菌通过激活HT29细胞和Caco2细胞系上p38丝裂原活化蛋白激酶(p38 mitogen-activated protein kinases,p38MAPK)、细胞外信号调节激酶(extracellular signal-regulated kinases,ERK)和c-jun氨基末端激酶(c-jun N-terminal kinases,JNK)信号分子通路,提高ZO-1和occludin蛋白表达[46]。乳酸杆菌还通过影响上皮紧密连接蛋白的分布,调控紧密连接的完整性。有研究表明,无论对健康者[47]还是在Caco2细胞系上[45],植物乳杆菌均减少了occludin的内吞,降低了ZO-1蛋白从紧密连接复合体中解离,增加了occludin和ZO-1蛋白在紧密连接中的分布,从而提高了紧密连接屏障功能。
3.2 乳酸杆菌对病理条件下肠道上皮细胞紧密连接的调控当致病菌和其他毒素致使肠道损伤时,乳酸杆菌通过调节紧密连接蛋白的表达与分布,提高上皮屏障功能,缓解黏膜损伤。活体试验发现,罗伊氏乳杆菌R2LC显著增加结肠炎小鼠隐窝底部紧密连接蛋白occludin和ZO-1的表达,改善肠道上皮的完整性[42]。体外试验也报道,罗伊氏乳杆菌I5007[18]和植物乳杆菌[48]都阻止了毒素诱导下紧密连接蛋白occludin和ZO-1表达的下降。Qin等[49]研究发现,侵袭性大肠杆菌(EIEC)可以诱导Caco2细胞上紧密连接蛋白claudin-1、occludin和ZO-1和细胞骨架纤维状肌动蛋白(F-actin)的重排。植物乳杆菌加入后,可以缓解上皮细胞的紧密连接损伤。Yu等[21]也报道,嗜淀粉乳杆菌D14能够抑制ETEC K88诱导的Caco2细胞上claudin-1及E-cadherin的重排及缺失。鼠李糖乳杆菌[15]和干酪乳杆菌[50]同样也可以阻止上皮细胞感染病原菌后ZO-1的重分布。乳酸杆菌可以改变MLC的磷酸化,调节肌动球蛋白环收缩和紧密蛋白的紊乱。罗伊氏乳杆菌LR1在IPEC-1细胞感染ETEC K88前加入,能够增加ZO-1和occludin蛋白水平,降低上皮细胞通透性。使用MLCK抑制剂ML-7细胞后,罗伊氏乳杆菌LR1对紧密连接屏障的促进作用被阻断,这表明罗伊氏乳杆菌可能通过MLCK信号通路来发挥作用[51]。除此之外,罗伊氏乳杆菌ZJ617通过抑制LPS诱导下p38MAPK和ERK1/2的磷酸化,减少MLC磷酸化,从而恢复紧密连接蛋白的表达,维持紧密连接的结构[52]。鼠李糖乳杆菌在结肠炎小鼠和LPS诱导的Caco2细胞上也有类似的报道[53]。
乳酸杆菌还可以调节炎性和抗炎性细胞因子间的平衡,提高炎症时紧密连接的完整性。Wu等[54]研究发现,植物乳杆菌通过降低ETEC K88诱导下IPEC-J2细胞炎性细胞因子IL-1β、IL-8及TNF-α的表达,提高抗炎性细胞因子转化生长因子-β(TGF-β)的表达,恢复claudin、occludin和ZO-1的表达水平。Karimi等[55]也报道,IPEC-J2细胞感染ETEC后,炎性细胞因子IL-6和TNF-α的表达水平显著增加,而罗伊氏乳杆菌预处理后,可以恢复炎性细胞因子的表达水平。鼠李糖乳杆菌上也有相似的发现[15, 56-57]。还有研究发现,罗伊氏乳杆菌I5007对LPS诱导下IPEC-J2细胞炎性细胞因子的影响与作用时间有关[18]。罗伊氏乳杆菌添加1~4 h对TNF-α和IL-6的表达无显著影响,添加4或8 h后,罗伊氏乳杆I5007显著抑制了LPS诱导下TNF-α和IL-6表达的增加。
4 小结维持肠道上皮细胞的屏障功能对于动物的健康至关重要。紧密连接是构成肠道上皮屏障的重要组成部分。病原菌及其诱导的炎性细胞因子可以调控紧密连接蛋白的表达、分布以及细胞骨架-连接相关肌动球蛋白环的收缩,影响紧密连接的结构,其中MLCK是关键的调控环节。多种乳酸杆菌作为益生素,可以促进上皮细胞紧密连接蛋白的表达和合理分布,维持上皮细胞的屏障功能;同时可以缓解病原菌及其诱导的炎性细胞因子对紧密连接的损伤,改善肠上皮的屏障功能。有关乳酸杆菌对上皮细胞MLCK的调控以及相关信号途径报道较少。
| [1] |
YAN W, SUN C J, YUAN J W, et al. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency[J]. Scientific Reports, 2017, 7: 45308. DOI:10.1038/srep45308 |
| [2] |
DE VRIES M C, VAUGHAN E E, KLEEREBEZEM M, et al. Lactobacillus plantarum-survival, functional and potential probiotic properties in the human intestinal tract[J]. International Dairy Journal, 2006, 16(9): 1018-1028. DOI:10.1016/j.idairyj.2005.09.003 |
| [3] |
CUNNINGHAM K E, TURNER J R. Myosin light chain kinase:pulling the strings of epithelial tight junction function[J]. Annals of the New York Academy of Sciences, 2012, 1258(1): 34-42. DOI:10.1111/j.1749-6632.2012.06526.x |
| [4] |
SRINIVASARAO M, GALLIFORD C V, LOW P S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents[J]. Nature reviews Drug Discovery, 2015, 14(3): 203-219. DOI:10.1038/nrd4519 |
| [5] |
LUISSINT A C, PARKOS C A, NUSRAT A. Inflammation and the intestinal barrier:leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair[J]. Gastroenterology, 2016, 151(4): 616-632. DOI:10.1053/j.gastro.2016.07.008 |
| [6] |
MA T Y, NIGHOT P, AL-SADI R.Tight junctions and the intestinal barrier[M]//SAID H M.Physiology of the Gastrointestinal Tract.6th ed.Amsterdam: Elsevier, 2018: 587-639.
|
| [7] |
FRIEDL P, MAYOR R. Tuning collective cell migration by cell-cell junction regulation[J]. Cold Spring Harbor Perspectives in Biology, 2017, 39(4): a029199. |
| [8] |
CEREIJIDO M, CONTRERAS R G, FLORES-BENÍTEZ D, et al. New diseases derived or associated with the tight junction[J]. Archives of Medical Research, 2007, 38(5): 465-478. DOI:10.1016/j.arcmed.2007.02.003 |
| [9] |
HARTSOCK A, NELSON W J. Adherens and tight junctions:structure, function and connections to the actin cytoskeleton[J]. Biochimica et Biophysica Acta (BBA):Biomembranes, 2008, 1778(3): 660-669. DOI:10.1016/j.bbamem.2007.07.012 |
| [10] |
TURNER J R, BUSCHMANN M M, ROMERO-CALVO I, et al. The role of molecular remodeling in differential regulation of tight junction permeability[J]. Seminars in Cell & Developmental Biology, 2014, 36: 204-212. |
| [11] |
SHEN L. Tight junctions on the move:molecular mechanisms for epithelial barrier regulation[J]. Annals of the New York Academy of Sciences, 2012, 1258(1): 9-18. DOI:10.1111/j.1749-6632.2012.06613.x |
| [12] |
CHOI W, YERUVA S, TURNER J R. Contributions of intestinal epithelial barriers to health and disease[J]. Experimental Cell Research, 2017, 358(1): 71-77. DOI:10.1016/j.yexcr.2017.03.036 |
| [13] |
DU L J, KIM J J, SHEN J H, et al. Crosstalk between inflammation and ROCK/MLCK signaling pathways in gastrointestinal disorders with intestinal hyperpermeability[J]. Gastroenterology Research and Practice, 2016, 2016: 7374197. |
| [14] |
YU D, MARCHIANDO A M, WEBER C R, et al. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(18): 8237-8241. DOI:10.1073/pnas.0908869107 |
| [15] |
JOHNSON-HENRY K C, DONATO K A, SHEN-TU G, et al. Lactobacillus rhamnosus strain GG prevents Enterohemorrhagic Escherichia coli O157 : H7-induced changes in epithelial barrier function[J]. Infection and Immunity, 2008, 76(4): 1340-1348. DOI:10.1128/IAI.00778-07 |
| [16] |
YU H T, DING X L, SHANG L J, et al. Protective ability of biogenic antimicrobial peptide microcin J25 against Enterotoxigenic Escherichia coli-induced intestinal epithelial dysfunction and inflammatory responses IPEC-J2 cells[J]. Frontiers in Cellular and Infection Microbiology, 2018, 8: 242. DOI:10.3389/fcimb.2018.00242 |
| [17] |
KESSLER S P, OBERY D R, NICKERSON K P, et al. Multifunctional role of 35 kilodalton hyaluronan in promoting defense of the intestinal epithelium[J]. Journal of Histochemistry & Cytochemistry, 2018, 66(4): 273-287. |
| [18] |
YANG F J, WANG A N, ZENG X F, et al. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions[J]. BMC Microbiology, 2015, 15: 32. DOI:10.1186/s12866-015-0372-1 |
| [19] |
JARIWALA R, MANDAL H, BAGCHI T. Indigenous lactobacilli strains of food and human sources reverse enteropathogenic E. coli O26 : H11-induced damage in intestinal epithelial cell lines:effect on redistribution of tight junction proteins[J]. Microbiology, 2017, 163(9): 1263-1272. DOI:10.1099/mic.0.000507 |
| [20] |
ROSELLI M, FINAMORE A, BRITTI M S, et al. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage[J]. The Journal of Nutrition, 2007, 137(12): 2709-2716. DOI:10.1093/jn/137.12.2709 |
| [21] |
YU Q, WANG Z, YANG Q. Lactobacillus amylophilus D14 protects tight junction from enteropathogenic bacteria damage in Caco-2 cells[J]. Journal of Dairy Science, 2012, 95(10): 5580-5587. DOI:10.3168/jds.2012-5540 |
| [22] |
LONG Y, DU L, KIM J J, et al. MLCK-mediated intestinal permeability promotes immune activation and visceral hypersensitivity in PI-IBS mice[J]. Neurogastroenterology & Motility, 2018, 30(9): e13348. |
| [23] |
GU L L, LI N, GONG J F, et al. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia[J]. The Journal of Infectious Diseases, 2011, 203(11): 1602-1612. DOI:10.1093/infdis/jir147 |
| [24] |
NIGHOT M, AL-SADI R, GUO S H, et al. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression[J]. The American Journal of Pathology, 2017, 187(12): 2698-2710. DOI:10.1016/j.ajpath.2017.08.005 |
| [25] |
KEARNEY C J, CULLEN S P, TYNAN G A, et al. Necroptosis suppresses inflammation via termination of TNF-or LPS-induced cytokine and chemokine production[J]. Cell Death and Differentiation, 2015, 22(8): 1313-1327. DOI:10.1038/cdd.2014.222 |
| [26] |
PLATZER B, BAKER K, VERA M P, et al. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites[J]. Mucosal Immunology, 2015, 8(3): 516-532. DOI:10.1038/mi.2014.85 |
| [27] |
LEE S H. Intestinal permeability regulation by tight junction:implication on inflammatory bowel diseases[J]. Intestinal Research, 2015, 13(1): 11-18. DOI:10.5217/ir.2015.13.1.11 |
| [28] |
AL-SADI R, GUO S H, YE D M, et al. TNF-α modulation of intestinal tight junction permeability is mediated by NIK/IKK-α axis activation of the canonical NF-κB pathway[J]. The American Journal of Pathology, 2016, 186(5): 1151-1165. DOI:10.1016/j.ajpath.2015.12.016 |
| [29] |
JENNIS M, CAVANAUGH C R, LEO G C, et al. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo[J]. Neurogastroenterology & motility, 2018, 30(2): e13178. |
| [30] |
HERING N A, LUETTIG J, KRUG S M, et al. Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunction in vitro[J]. Annals of the New York Academy of Sciences, 2017, 1405(1): 177-188. DOI:10.1111/nyas.13405 |
| [31] |
LANDY J, RONDE E, ENGLISH N, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer[J]. World Journal of Gastroenterology, 2016, 22(11): 3117-3126. DOI:10.3748/wjg.v22.i11.3117 |
| [32] |
ZOLOTAREVSKY Y, HECHT G, KOUTSOURIS A, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease[J]. Gastroenterology, 2002, 123(1): 163-172. DOI:10.1053/gast.2002.34235 |
| [33] |
WANG F J, GRAHAM W V, WANG Y M, et al. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression[J]. The American Journal of Pathology, 2005, 166(2): 409-419. DOI:10.1016/S0002-9440(10)62264-X |
| [34] |
CLAYBURGH D R, BARRETT T A, TANG Y M, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo[J]. The Journal of Clinical Investigation, 2005, 115(10): 2702-2715. DOI:10.1172/JCI24970 |
| [35] |
MARCHIANDO A M, SHEN L, GRAHAM W V, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo[J]. Journal of Cell Biology, 2010, 189(1): 111-126. |
| [36] |
PRASAD S, MINGRINO R, KAUKINEN K, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells[J]. Laboratory Investigation, 2005, 85(9): 1139-1162. DOI:10.1038/labinvest.3700316 |
| [37] |
WISNER D M, HARRIS Ⅲ L R, GREEN C L, et al. Opposing regulation of the tight junction protein claudin-2 by interferon-γ and interleukin-4[J]. Journal of Surgical Research, 2008, 144(1): 1-7. DOI:10.1016/j.jss.2007.03.059 |
| [38] |
WILLEMSEN L E M, HOETJES J P, VAN DEVENTER S J H, et al. Abrogation of IFN-γ mediated epithelial barrier disruption by serine protease inhibition[J]. Clinical & Experimental Immunology, 2005, 142(2): 275-284. |
| [39] |
BRUEWER M, UTECH M, IVANOV A I, et al. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process[J]. The FASEB Journal, 2005, 19(8): 923-933. DOI:10.1096/fj.04-3260com |
| [40] |
IVANOV A I, NUSRAT A, PARKOS C A. Endocytosis of the apical junctional complex:mechanisms and possible roles in regulation of epithelial barriers[J]. Bioessays, 2005, 27(4): 356-365. DOI:10.1002/(ISSN)1521-1878 |
| [41] |
UTECH M, IVANOV A I, SAMARIN S N, et al. Mechanism of IFN-γ-induced endocytosis of tight junction proteins:myosin Ⅱ-dependent vacuolarization of the apical plasma membrane[J]. Molecular Biology of the Cell, 2005, 16(10): 5040-5052. DOI:10.1091/mbc.e05-03-0193 |
| [42] |
LIU H Y, ROOS S, JONSSON H, et al. Effects of Lactobacillus johnsonii and Lactobacillus reuteri on gut barrier function and heat shock proteins in intestinal porcine epithelial cells[J]. Physiological Reports, 2015, 3(4): e12355. DOI:10.14814/phy2.12355 |
| [43] |
HU J, CHEN L L, ZHENG W Y, et al. Lactobacillus frumenti facilitates intestinal epithelial barrier function maintenance in early-weaned piglets[J]. Frontiers in Microbiology, 2018, 9: 897. DOI:10.3389/fmicb.2018.00897 |
| [44] |
ANDERSON R C, COOKSON A L, MCNABB W C, et al. Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function[J]. FEMS Microbiology Letters, 2010, 309(2): 184-192. |
| [45] |
ANDERSON R C, COOKSON A L, MCNABB W C, et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation[J]. BMC Microbiology, 2010, 10: 316. DOI:10.1186/1471-2180-10-316 |
| [46] |
RESTA-LENERT S, BARRETT K E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC)[J]. Gut, 2003, 52(7): 988-997. DOI:10.1136/gut.52.7.988 |
| [47] |
KARCZEWSKI J, TROOST F J, KONINGS I, et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2010, 298(6): G851-G859. DOI:10.1152/ajpgi.00327.2009 |
| [48] |
吴云鹏.植物乳杆菌调节猪肠上皮细胞屏障功能和转运载体的研究[D].硕士学位论文.广州: 华南农业大学, 2016.
|
| [49] |
QIN H L, ZHANG Z W, HANG X M, et al. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells[J]. BMC Microbiology, 2009, 9: 63. DOI:10.1186/1471-2180-9-63 |
| [50] |
PARASSOL N, FREITAS M, THOREUX K, et al. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells[J]. Research in Microbiology, 2005, 156(2): 256-262. DOI:10.1016/j.resmic.2004.09.013 |
| [51] |
YI H B, WANG L, XIONG Y X, et al. Lactobacillus reuteri LR1 improved expression of genes of tight junction proteins via the MLCK pathway in IPEC-1 cells during infection with enterotoxigenic Escherichia coli K88[J]. Mediators of Inflammation, 2018, 2018: 6434910. |
| [52] |
CUI Y J, LIU L, DOU X X, et al. Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide[J]. Oncotarget, 2017, 8(44): 77489-77499. |
| [53] |
MIYAUCHI E, MORITA H, TANABE S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo[J]. Journal of Dairy Science, 2009, 92(6): 2400-2408. DOI:10.3168/jds.2008-1698 |
| [54] |
WU Y P, ZHU C, CHEN Z, et al. Protective effects of Lactobacillus plantarum on epithelial barrier disruption caused by enterotoxigenic Escherichia coli in intestinal porcine epithelial cells[J]. Veterinary Immunology and Immunopathology, 2016, 172: 55-63. DOI:10.1016/j.vetimm.2016.03.005 |
| [55] |
KARIMI S, JONSSON H, LUNDH T, et al. Lactobacillus reuteri strains protect epithelial barrier integrity of IPEC-J2 monolayers from the detrimental effect of enterotoxigenic Escherichia coli[J]. Physiological Reports, 2018, 6(2): e13514. DOI:10.14814/phy2.13514 |
| [56] |
MAO X B, GU C S, HU H Y, et al. Dietary Lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus[J]. PLoS One, 2016, 11(1): e0146312. DOI:10.1371/journal.pone.0146312 |
| [57] |
KANDASAMY S, CHATTHA K S, VLASOVA A N, et al. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model[J]. Gut Microbes, 2014, 5(5): 639-651. DOI:10.4161/19490976.2014.969972 |




