2. 湖南农业大学动物科学技术学院, 长沙 410128
2. College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, China
炎症是机体应对感染、组织损伤等有害刺激的防御性反应。通常情况下,可控的炎症反应有利于机体清除入侵异物,保护自身免受侵害,但是严重的炎症反应会引起机体组织或器官变性、坏死、纤维化和功能障碍,转变为慢性炎症时还会影响机体的代谢,造成神经、免疫等系统功能紊乱。在畜禽生产中,炎症往往造成饲料转化率低、生长停滞、母猪繁殖性能下降等一系列问题。因此,如何合理控制炎症水平对促进畜禽生长、改善机体免疫力、提高养殖效益具有相当重要的意义。
炎症刺激可分为内源性和外源性2种,外源性刺激主要包括微生物病原体相关的分子模式(PAMPs)、病毒性致病因子、过敏原、异物和有毒成分等;内源性刺激主要来自受损的组织、内源性晶体、细胞外基质(ECM)分解产物和应激、紊乱、死亡状态下的细胞[1]。炎症过程中,诱导物通过刺激局部组织和器官中的感受器,如树突细胞(DCs)抗原呈递,进而诱导T淋巴细胞、巨噬细胞等免疫细胞产生并释放炎性介质[2]。这些炎性介质不仅激发局部炎症反应,而且可通过趋化因子的释放促使炎性细胞向炎症部位聚集,进而诱导系统性炎症[3]。炎性介质的产生受细胞信号通路的调控,而信号通路可通过受体被炎症诱导物或者不同炎性介质之间的交互作用激活[4]。因此,调节细胞信号通路可有效控制炎性介质的产生,进而调控机体炎症反应。
植物多酚是一类具有多种生物学活性的化合物,广泛存在于植物的根、茎、叶、花和果实中,在表皮含量尤为丰富。多酚的化学结构由数个酚环组成,在不同的植物中其结构也高度多样化,如黄酮类物质在其基本结构上因结合的基团及折叠方式不同便可形成多种不同的多酚(图 1)。这些酚环结构的数量和特性决定了多酚独特的物理、化学和生物特性[5-6]。一般而言,多酚不是机体必需营养素,虽然有研究指出多酚能替代部分维生素E等必需营养素[7]。然而,饮食或动物饲粮中的多酚对机体有多重有益作用,流行病学研究表明饮食中添加多酚可以预防心血管疾病、癌症、糖尿病、骨质疏松症和神经退行性疾病等慢性或代谢性疾病的发生[8-9],动物试验表明饲粮中添加多酚可有效缓解幼龄动物氧化应激并促进动物生长性能[10]。本研究团队近期的研究发现,植物多酚可通过与细胞炎症信号分子结合,并激活抗氧化信号通路,使机体维持稳态,缓解外界刺激造成的紊乱,从而达到减少炎症及氧化损伤的目的[11-12]。此外,多酚还可通过调节肠道菌群,减少脂多糖(LPS)的产生,从而降低炎症水平[13]。本文对多酚缓解炎症及氧化应激的调控机制做进一步阐述,为多酚的合理有效利用提供参考。
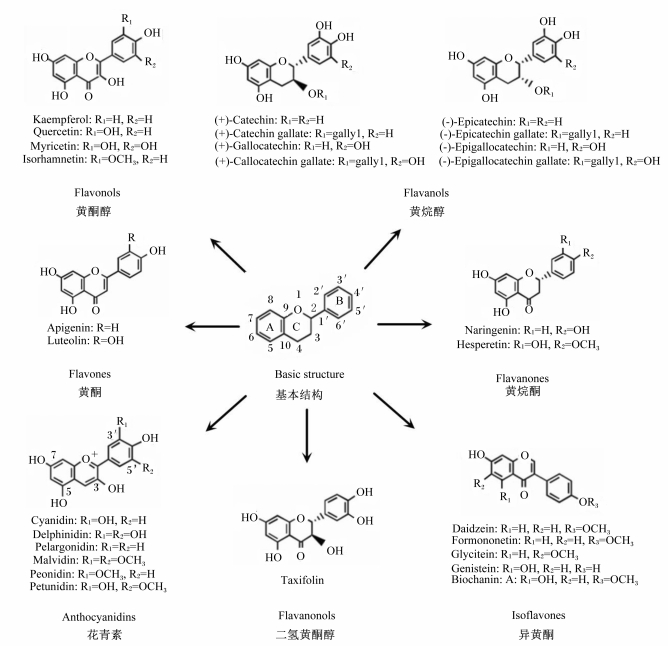
|
Kaempferol:山柰酚;Quercetin:槲皮素;Myricetin:杨梅酮;Isorhamnetin:异鼠李素;Catechin:儿茶素;Catechin gallate:儿茶素没食子酸酯;Gallocatechin:没食子儿茶素;Gallocatechin gallate:没食子儿茶素没食子酸酯;Epicatechin:表儿茶素;Epicatechin gallate:表儿茶素没食子酸酯;Epigallocatechin:表没食子儿茶素;Epigallocatechin gallate:表没食子儿茶素没食子酸酯;Apigenin:芹黄素;Luteolin:木犀草素;Naringenin:柚皮素;Hesperetin:橙皮素;Cyanidin:矢车菊素;Delphinidin:飞燕草色素;Pelargonidin:天竺葵色素;Malvidin:锦葵色素;Peonidin:芍药色素;Petunidin:牵牛花色素;Taxifolin:花旗松素;Daidzein:大豆黄酮;Formononetin:芒柄花黄素;Glycitein:黄豆黄素;Genistein:染料木素;Biochanin:鹰嘴豆素。 图 1 黄酮类物质的基本结构 Fig. 1 Basic structure of flavonoids |
血管活性胺、血管活性肽、补体成分片段、脂质介质、细胞因子和蛋白水解酶等炎性介质在调节炎症反应中发挥重要作用。血管活性胺和多肽对血管系统有复杂的影响,因为它们可根据具体情况调节血管通透性、血管舒张和收缩[1]。一氧化氮(NO)是一种强大的血管舒张剂和细胞毒性物质,在一氧化氮合酶(NOS)催化下由L-精氨酸形成[14]。一氧化氮合酶主要有3种亚型,包括神经元型一氧化氮合酶(nNOS)、内皮型一氧化氮合酶(eNOS)和诱导型一氧化氮合酶(iNOS)。nNOS主要在神经元组织中表达[15],eNOS主要存在于内皮细胞中,是脉管系统中主要的一氧化氮合酶[16-17]。nNOS和eNOS都在静息细胞中固有表达,由细胞内钙浓度控制以维持一氧化氮的稳态[18]。在大多数静息细胞中,iNOS不表达或者表达水平极低,当细胞受到细菌脂多糖等炎症诱导物刺激时,iNOS可以过表达,从而诱导大量一氧化氮产生并引起炎症[19]。
前列腺素是一类典型的炎性介质,它是在环氧合酶(COX-1和COX-2)催化下由花生四烯酸衍生而来[20]。COX-1是前列腺素合成过程中的关键酶,在大多数细胞中固有表达并作为稳态调节因子,而COX-2由炎症刺激、激素和生长因子诱导,在炎症和癌症等疾病中过度表达[21]。前列腺素E2(PGE2)是典型的COX-1和COX-2合成产物,被认为是促进炎症早期局部血管扩张和诱导中性粒细胞、巨噬细胞和肥大细胞聚集的介质[22]。
细胞因子是一类在炎症反应中发挥介导免疫细胞活化、增殖、渗透的重要信号蛋白,主要包括白细胞介素、干扰素、肿瘤坏死因子等。细胞因子可以作用于细胞本身(自分泌效应)、附近细胞(旁分泌效应)或远处细胞(内分泌效应)[23]。一般而言,白细胞介素-1(IL-1)、白细胞介素-6(IL-6)、肿瘤坏死因子-α(TNF-α)等大部分细胞因子在炎症中发挥促炎作用[24],少部分细胞因子,如白细胞介素-4(IL-4)、白细胞介素-10(IL-10)等被认为具有缓解炎症的作用[25]。但是,细胞因子在不同情况下可发挥不同功能,如在细胞因子信号转导抑制蛋白3(SOCS3)缺乏的情况下IL-6能抑制巨噬细胞炎症反应[26],而IL-4在敲除重组酶激活基因-2(RAG-2)的小鼠中能诱导结肠炎的发生[27]。此外,细胞因子在炎症循环中可彼此影响,如在关节炎等慢性炎症中TNF-α可诱导IL-1、IL-6、白细胞介素-17(IL-17)等细胞因子的产生[28],在自身免疫性脑脊髓炎中IL-1可诱导IL-17的产生并增加致病性辅助性T细胞数量[29]。因此,了解细胞因子之间的相互作用对于识别哪些介质驱动了特异性炎症发病机制非常重要。
1.2 主要炎症信号通路促炎介质的产生受炎症信号通路的调控,在炎症反应中,细菌脂多糖、紫外线、应激、酒精、食物中抗原物质、环境污染物、葡萄糖和脂类代谢异常等因素通过激活Toll样受体(TLRs)、肿瘤坏死因子受体(TNFRs)、胰岛素受体(IR)、表皮生长因子受体(EGFR)等细胞表面受体,进而活化炎症信号通路[30-31]。虽然炎症受多重信号通路的调控,丝裂原活化蛋白激酶(MAPK)和核因子-κB(NF-κB)通路被认为是主要的炎症信号通路[32-33]。哺乳动物MAPK家族由c-Jun氨基末端激酶(JNK)、细胞外信号调节激酶(ERK)和p38激酶组成[34]。JNK也被称为应激活化蛋白激酶(SAPK),主要对细胞应激(如毒性、缺氧或氧化应激)作出反应,并通过TLRs和TNFRs而被激活[35];ERK和p38激酶在JNK处于活化的状态下可同时被激活[36],但ERK在更多情况下是通过EGFR和IR被激活[37]。NF-κB在正常情况下在细胞质中与IκBs结合并保持静息状态,炎症刺激可通过激活TLRs、TNFRs、IR、EGFR等膜受体进而促进IκB蛋白的磷酸化和水解,从而使NF-κB进入核内参与转录[38]。而IκB蛋白的磷酸化受其激酶(IKK)和泛素介导的转化生长因子β活化激酶1(TAK1)的调控[39]。这些炎症信号通路的活化能促进iNOS、COX-2等多种促炎介质的产生,并促进炎性细胞因子的产生与释放,进而激发炎症[40-41]。图 2简单阐述了炎症发生过程中的主要促炎信号通路。
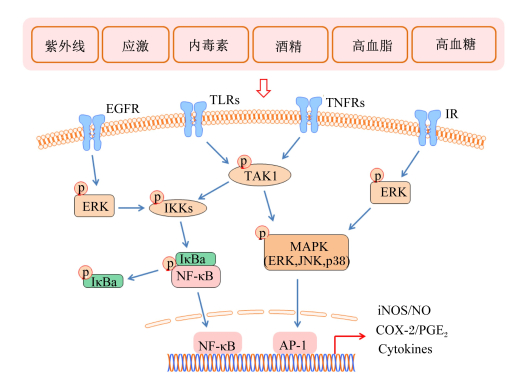
|
AP-1:激活蛋白-1 activator protein-1;COX-2:环氧合酶-2 cyclooxygenase-2;EGFR:表皮生长因子受体epidermal growth factor receptor;ERK:胞外信号调节激酶extracellular signal-regulated kinase;IKKs:IκB激酶IκB kinases; iNOS:诱导型一氧化氮合酶inducible nitric oxide synthase;IR:胰岛素受体insulin receptor; JNK:c-Jun氨基端激酶c-Jun N-terminal kinase; MAPK:丝裂原活化蛋白激酶mitogen-activated protein kinase; NF-κB:核因子-κB nuclear factor-κB; NO:一氧化氮nitric oxide; PGE2:前列腺素E2 prostaglandin E2;TAK1:转化生长因子β活化激酶1 transforming growth factor β activated kinase-1;TLRs:Toll样受体Toll-like receptors; TNFRs:肿瘤坏死因子受体tumor necrosis factor receptors;Cytokines:细胞因子;IκBa:核因子-κB抑制因子a nuclear factor B inhibitor。 图 2 炎症过程中的主要促炎信号通路 Fig. 2 Major proinflammatory pathways in inflammation |
活性氧(ROS)累积所引起的氧化应激在慢性炎症、衰老、癌症等多种病理过程中发挥着促进炎症作用[42],而还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶家族被认为是ROS的主要来源,另外线粒体电子传递酶、黄嘌呤氧化酶、环氧合酶、脂氧合酶、未偶联的一氧化氮合酶等其他因素也可以增加ROS的产生[43-44]。细菌脂多糖、酒精等炎症诱导物可以激活先天免疫系统的膜识别受体(如TLRs),进而通过髓样分化因子初次应答基因88(MYD88)激活NADPH氧化酶,产生超氧化物阴离子(O2-)[45-46]。超氧化物阴离子迅速被超氧化物歧化酶(SOD)转化为过氧化氢(H2O2)[43],而H2O2能激活红系衍生的核因子2相关因子2(Nrf2)等氧化应激反应通路[47],促进血红素氧合酶-1(HO-1)[48]、NADPH脱氢酶1(NQO1)[49]、谷胱甘肽过氧化物酶(GPx)[50]、锰型超氧化物歧化酶(MnSOD)[51]等抗氧化酶的表达,进而缓解氧化应激。H2O2可以激活NF-κB[52]、MAPK[53]、过氧化物氧化还原酶-2[54]等炎症信号通路,从而促进促炎细胞因子的释放并进一步激发炎症[55]。氧化应激与炎症信号通路在炎症发生过程中的交互作用如图 3所示。
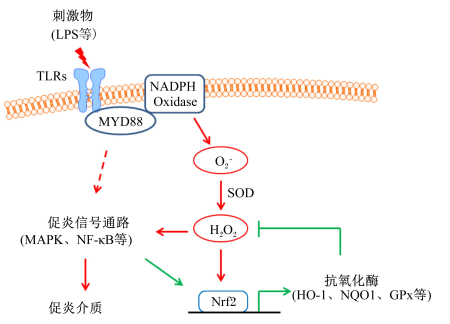
|
GPx:谷胱甘肽过氧化物酶glutathione peroxidase; HO-1:血红素氧合酶-1 heme oxygenase-1;LPS:脂多糖lipopolysaccharide; MAPK:丝裂原活化蛋白激酶mitogen-activated protein kinase;MYD88:髓样分化因子初次应答基因88 myeloid differentiation primary response gene 88;NF-κB:核因子-κB nuclear factor κB;NQO1:NADPH脱氢酶1 NADPH quinine oxidoreductase 1;NADPH Oxidase:还原型烟酰胺腺嘌呤二核苷酸磷酸氧化酶reduced nicotinamide adenine dinucleotide phosphate;Nrf2:红系衍生的核因子2相关因子2 nuclear factor (erythroid-derived 2)-like 2;SOD:超氧化物歧化酶superoxide dismutase;TLRs:Toll样受体Toll-like receptors。 图 3 氧化应激与炎症信号通路在炎症发生过程中的交互作用 Fig. 3 Crosstalk between oxidative stress and inflammatory pathways |
多酚因其强抗氧化和自由基清除能力而为人所熟知,有研究指出多酚的生理功能主要归功于其抗氧化活性[56]。所谓多酚的抗氧化能力,可以定义为清除等活性氧自由基簇以及低密度脂蛋白(LDL)等生物分子氧化生成的自由基的能力[57]。多酚抗氧化能力主要潜在机制被认为是基于活性氧的直接淬火和活性氧生成的抑制。首先,多酚的酚环结构能直接为脂质过氧化生成的自由基提供氢原子进行中和[58];其次,多酚能抑制黄嘌呤氧化酶和蛋白激酶C等氧化酶的作用,从而减少超氧阴离子的产生[59-60];第三,酚类物质可以螯合金属离子(如Fe2+/Fe3+和Cu2+),从而阻止O2-和H2O2变成具有更高侵袭性的羟自由基(·OH)[61];第四,多酚可上调转录因子Nrf2、线粒体MnSOD等介导的抗氧化反应通路,从而促进抗氧化酶的表达[62-63],例如,姜黄素可上调Nrf2介导的NQO1表达[64],白藜芦醇可通过活化腺苷酸活化蛋白激酶(AMPK)通路促进MnSOD的表达[62]。本研究团队前期研究结果表明,在高脂饲粮诱导的小鼠模型中,蓝靛果多酚可有效促进肝脏Nrf2、HO-1和MnSOD等抗氧化蛋白的表达,从而降低脂质过氧化水平[65]。多酚的这些抗氧化能力使其在预防或减少与氧化应激相关的疾病中被广泛应用[66]。
2.2 抗炎机制大量流行病学和试验数据表明,多酚类物质可有效缓解关节炎症[67]、自身免疫性脑脊髓炎[68]、炎症性肠病[69]、动脉粥样硬化[70]、皮肤炎症[71]、代谢综合征[72]等炎症相关的疾病。虽然多酚缓解炎症的能力得益于其抗氧化和自由基猝灭能力[73],但越来越多的证据表明多酚可通过直接参与调节细胞信号通路缓解炎症[74]。研究表明,多酚可下调MAPK[75]、NF-κB[76]、干扰素调节因子(IRF)[77]等多种炎症信号通路,进而抑制促炎介质的表达。近期研究表明,槲皮素、白藜芦醇和茶多酚等多酚类物质可以直接和ERK等蛋白激酶[78]以及miR-33a、miR-122等微小RNA(miRNAs)[79]发生特异性结合,从而抑制它们的活化与表达。本研究团队前期的研究结果也表明,表儿茶素和矢车菊素-3-葡萄糖苷可直接与巨噬细胞中TAK1蛋白激酶直接绑定,从而潜在地抑制MAPK和NF-κB炎症信号通路的活化并减少炎性细胞因子的生成,达到缓解炎症的目的[12]。此外,研究发现在体外模拟消化试验中,绿茶多酚能与醇溶蛋白结合从而降低其诱导的肠上皮细胞通透性,并减少IL-6和白细胞介素-8(IL-8)的释放,因此对乳糜泻等肠道疾病具有较好的防治作用[80]。这也从另一方面解释了多酚在较低浓度下仍可发挥生物学效应[81]。
多酚混合物的抗炎功能往往不能只归因于某一种作用机制,而是不同途径和机制之间的协调。代谢组学研究表明,在脂多糖诱导的小鼠胚胎成纤维细胞模型中,可可提取物的功效主要基于可可碱、黄烷-3-醇单体和原花青素的抗氧化特性,而柠檬过江籐(Lippia citriodora)提取物则主要基于毛蕊花苷能抑制NF-κB通路从而降低单核细胞趋化蛋白-1(MCP-1)的表达[82]。本研究团队前期研究也发现,脂多糖和多酚均能诱导巨噬细胞中抗氧化蛋白的表达,且多酚的诱导较脂多糖早,但脂多糖同时诱导了氧化应激和炎症相关蛋白的表达,而多酚则没有产生相应的诱导作用,可见多酚对氧化和炎症信号通路调节中存在某种平衡[12]。此外,研究指出从植物中提取的酚类物质混合物比单一成分具有更强的抗炎作用[83],这说明酚类物质彼此之间可能存在潜在的协同作用。
3 小结随着我国对饲用抗生素的进一步管控,寻找抗生素的有效替代途径是目前畜牧业所急需解决的问题之一。植物多酚具有抗氧化、抗炎、调节肠道菌群、促生长等潜在功效,且不会产生耐药性及药物残留,因此在新型饲料添加剂的开发中具有广阔的应用前景,但植物多酚在调控机制等方面的不明确使其难以得到合理有效利用。未来有关植物多酚在饲料中的应用及机制研究需重点关注以下几个方面:1)植物多酚在动物体内的代谢途径及其次级代谢产物的作用;2)植物多酚及其次级代谢产物在氧化应激及炎症信号通路中的作用靶点;3)不同植物多酚之间的协同作用;4)植物多酚与肠道菌群之间的相互作用。明确以上问题将使植物多酚在动物饲料中得到合理有效利用,从而减少乃至取代抗生素在饲料中的使用。
| [1] |
MEDZHITOV R. Origin and physiological roles of inflammation[J]. Nature, 2008, 454(7203): 428-435. DOI:10.1038/nature07201 |
| [2] |
ASAGIRI M, HIRAI T, KUNIGAMI T, et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis[J]. Science, 2008, 319(5863): 624-627. DOI:10.1126/science.1150110 |
| [3] |
NEURATH M F. Cytokines in inflammatory bowel disease[J]. Nature Reviews Immunology, 2014, 14(5): 329-342. DOI:10.1038/nri3661 |
| [4] |
HOTAMISLIGIL G S. Inflammation and metabolic disorders[J]. Nature, 2006, 444(7121): 860-867. DOI:10.1038/nature05485 |
| [5] |
DEL RIO D, RODRIGUEZ-MATEOS A, SPENCER J P E, et al. Dietary (poly)phenolics in human health:structures, bioavailability, and evidence of protective effects against chronic diseases[J]. Antioxidants & Redox Signalling, 2013, 18(14): 1818-1892. |
| [6] |
TSAO R. Chemistry and biochemistry of dietary polyphenols[J]. Nutrients, 2010, 2(12): 1231-1246. DOI:10.3390/nu2121231 |
| [7] |
IQBAL Z, KAMRAN Z, SULTAN J I, et al. Replacement effect of vitamin E with grape polyphenols on antioxidant status, immune, and organs histopathological responses in broilers from 1-to 35-d age[J]. The Journal of Applied Poultry Research, 2015, 24(2): 127-134. DOI:10.3382/japr/pfv009 |
| [8] |
ARTS I C, HOLLMAN P C. Polyphenols and disease risk in epidemiologic studies[J]. The American Journal of Clinical Nutrition, 2005, 81(1): 317S-325S. DOI:10.1093/ajcn/81.1.317S |
| [9] |
GRAF B A, MILBURY P E, BLUMBERG J B. Flavonols, flavones, flavanones, and human health:epidemiological evidence[J]. Journal of Medicinal Food, 2005, 8(3): 281-90. DOI:10.1089/jmf.2005.8.281 |
| [10] |
ZHAO J X, LI Q, ZHANG R X, et al. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs[J]. Animal Feed Science and Technology, 2018, 236: 76-85. DOI:10.1016/j.anifeedsci.2017.12.004 |
| [11] |
WU S, YANO S, HISANAGA A, et al. , HISANAGA A, et al.Polyphenols from Lonicera caerulea L. berry attenuate experimental nonalcoholic steatohepatitis by inhibiting proinflammatory cytokines productions and lipid peroxidation[J]. Molecular Nutrition and Food Research, 2017, 61(4). DOI:10.1002/mnfr.201600858 |
| [12] |
WU S S, YANO S, CHEN J H, et al. Polyphenols from Lonicera caerulea L.Berry inhibit LPS-induced inflammation through dual modulation of inflammatory and antioxidant mediators[J]. Journal of Agricultural and Food Chemistry, 2017, 65(25): 5133-5141. DOI:10.1021/acs.jafc.7b01599 |
| [13] |
WU S S, HU R Z, NAKANO H, et al. Modulation of gut microbiota by Lonicera caerulea L.Berry polyphenols in a mouse model of fatty liver induced by high fat diet[J]. Molecules, 2018, 23(12): 3213. DOI:10.3390/molecules23123213 |
| [14] |
KORHONEN R, LAHTI A, KANKAANRANTA H, et al. Nitric oxide production and signaling in inflammation[J]. Current Drug Targets:Inflammation and Allergy, 2005, 4(4): 471-479. DOI:10.2174/1568010054526359 |
| [15] |
ZHOU L, ZHU D Y. Neuronal nitric oxide synthase:structure, subcellular localization, regulation, and clinical implications[J]. Nitric Oxide, 2009, 20(4): 223-230. DOI:10.1016/j.niox.2009.03.001 |
| [16] |
SHAUL P W. Regulation of endothelial nitric oxide synthase:location, location, location[J]. Annual Review of Physiology, 2002, 64: 749-774. DOI:10.1146/annurev.physiol.64.081501.155952 |
| [17] |
FÖRSTERMANN U, MVNZEL T. Endothelial nitric oxide synthase in vascular disease:from marvel to menace[J]. Circulation, 2006, 113(3): 1708-1714. |
| [18] |
ALDERTON W K, COOPER C E, KNOWLES R G. Nitric oxide synthases:structure, function and inhibition[J]. Biochemical Journal, 2001, 357(3): 593-615. DOI:10.1042/bj3570593 |
| [19] |
AKTAN F. iNOS-mediated nitric oxide production and its regulation[J]. Life Sciences, 2004, 75(6): 639-653. DOI:10.1016/j.lfs.2003.10.042 |
| [20] |
MORITA I. Distinct functions of COX-1 and COX-2[J]. Prostaglandins & Other Lipid Mediators, 2002, 68/69: 165-175. |
| [21] |
RICCIOTTI E, FITZGERALD G A. Prostaglandins and inflammation[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2011, 31(5): 986-1000. DOI:10.1161/ATVBAHA.110.207449 |
| [22] |
KALINSKI P. Regulation of immune responses by prostaglandin E2[J]. Journal of Immunology, 2012, 188(1): 21-28. |
| [23] |
ZHANG J M, AN J X. Cytokines, inflammation, and pain[J]. International Anesthesiology Clinics, 2007, 45(2): 27-37. DOI:10.1097/AIA.0b013e318034194e |
| [24] |
MOSER B, WILLIMANN K. Chemokines:role in inflammation and immune surveillance[J]. Annals of the Rheumatic Diseases, 2004, 63(Suppl 2): ⅱ84-ⅱ89. |
| [25] |
WOODWARD E A, KOLESNIK T B, NICHOLSON S E, et al. The anti-inflammatory actions of IL-4 in human monocytes are not mediated by IL-10, RP105 or the kinase activity of RIPK2[J]. Cytokine, 2012, 58(3): 415-423. DOI:10.1016/j.cyto.2012.03.009 |
| [26] |
YASUKAWA H, OHISHI M, MORI H, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages[J]. Nature Immunology, 2003, 4(6): 551-556. DOI:10.1038/ni938 |
| [27] |
VAN KAMPEN C, GAULDIE J, COLLINS S M. Proinflammatory properties of IL-4 in the intestinal microenvironment[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2004, 288(1): G111-G117. |
| [28] |
BRENNAN F M, MCINNES I B. Evidence that cytokines play a role in rheumatoid arthritis[J]. Journal of Clinical Investigation, 2008, 118(11): 3537-3545. DOI:10.1172/JCI36389 |
| [29] |
SUTTON C, BRERETON C, KEOGH B, et al. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis[J]. Journal of Experimental Medicine, 2006, 203(7): 1685-1691. DOI:10.1084/jem.20060285 |
| [30] |
COGGINS M, ROSENZWEIG A. The fire within:cardiac inflammatory signaling in health and disease[J]. Circulation Research, 2012, 110(1): 116-125. DOI:10.1161/CIRCRESAHA.111.243196 |
| [31] |
NEWTON K, DIXIT V M. Signaling in innate immunity and inflammation[J]. Cold Spring Harbor Perspectives in Biology, 2012, 4(3): a006049. |
| [32] |
KAMINSKA B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits[J]. Biochimica et Biophysica Acta:Proteins and Proteomics, 2005, 1754(1/2): 253-262. |
| [33] |
LAWRENCE T. The nuclear factor NF-κB pathway in inflammation[J]. Cold Spring Harbor Perspectives in Biology, 2009, 1(6): a001651. |
| [34] |
KIM E K, CHOI E J. Pathological roles of MAPK signaling pathways in human diseases[J]. Biochimica et Biophysica Acta:Molecular Basis of Disease, 2010, 1802(4): 396-405. DOI:10.1016/j.bbadis.2009.12.009 |
| [35] |
WESTON C R, DAVIS R J. The JNK signal transduction pathway[J]. Current Opinion in Genetics & Development, 2002, 12(1): 14-21. |
| [36] |
SHAUL Y D, SEGER R. The MEK/ERK cascade:from signaling specificity to diverse functions[J]. Biochimica et Biophysica Acta:Molecular Cell Research, 2007, 1773(8): 1213-1226. DOI:10.1016/j.bbamcr.2006.10.005 |
| [37] |
WATANABE Y, NAGAI Y, TAKATSU K. Activation and regulation of the pattern recognition receptors in obesity-induced adipose tissue inflammation and insulin resistance[J]. Nutrients, 2013, 5(9): 3757-3778. DOI:10.3390/nu5093757 |
| [38] |
AGGARWAL B B, VIJAYALEKSHMI R V, SUNG B. Targeting inflammatory pathways for prevention and therapy of cancer:short-term friend, long-term foe[J]. Clinical Cancer Research, 2009, 15(2): 425-430. DOI:10.1158/1078-0432.CCR-08-0149 |
| [39] |
ADHIKARI A, XU M, CHEN Z J. Ubiquitin-mediated activation of TAK1 and IKK[J]. Oncogene, 2007, 26(22): 3214-3226. DOI:10.1038/sj.onc.1210413 |
| [40] |
KYRIAKIS J M, AVRUCH J. Mammalian MAPK signal transduction pathways activated by stress and inflammation:a 10-year update[J]. Physiological Reviews, 2012, 92(2): 689-737. DOI:10.1152/physrev.00028.2011 |
| [41] |
SHIH R H, WANG C Y, YANG C M. NF-kappaB signaling pathways in neurological inflammation:a mini review[J]. Frontiers in Molecular Neuroscience, 2015, 8: 77. |
| [42] |
REUTER S, GUPTA S C, CHATURVEDI M M, et al. Oxidative stress, inflammation, and cancer:how are they linked?[J]. Free Radical Biology and Medicine, 2010, 49(11): 1603-1616. DOI:10.1016/j.freeradbiomed.2010.09.006 |
| [43] |
PARAVICINI T M, TOUYZ R M. NADPH oxidases, reactive oxygen species, and hypertension:clinical implications and therapeutic possibilities[J]. Diabetes Care, 2008, 31(Suppl 2): S170-S180. |
| [44] |
BEDARD K, KRAUSE K H. The NOX family of ROS-generating NADPH oxidases:physiology and pathophysiology[J]. Physiological Reviews, 2007, 87(1): 245-313. DOI:10.1152/physrev.00044.2005 |
| [45] |
PANDAY A, SAHOO M K, OSORIO D, et al. NADPH oxidases:an overview from structure to innate immunity-associated pathologies[J]. Cellular and Molecular Immunology, 2014, 12(1): 5-23. |
| [46] |
CHO R L, YANG C C, LEE I T, et al. Lipopolysaccharide induces ICAM-1 expression via a c-Src/NADPH oxidase/ROS-dependent NF-κB pathway in human pulmonary alveolar epithelial cells[J]. American Journal of Physiology, 2016, 310(7): L639-L657. |
| [47] |
FOURQUET S, GUEROIS R, BIARD D, et al. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation[J]. Journal of Biological Chemistry, 2010, 285(11): 8463-8471. DOI:10.1074/jbc.M109.051714 |
| [48] |
RUSHWORTH S A, CHEN X L, MACKMAN N, et al. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C[J]. Journal of Immunology, 2005, 175(7): 4408-4415. DOI:10.4049/jimmunol.175.7.4408 |
| [49] |
TANIGAWA S, FUJII M, HOU D X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin[J]. Free Radical Biology and Medicine, 2007, 42(11): 1690-1703. DOI:10.1016/j.freeradbiomed.2007.02.017 |
| [50] |
SINGH A, RANGASAMY T, THIMMULAPPA R K, et al. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2[J]. American Journal of Respiratory Cell and Molecular Biology, 2006, 35(6): 639-650. DOI:10.1165/rcmb.2005-0325OC |
| [51] |
HUANG X S, CHEN H P, YU H H, et al. Nrf2-dependent upregulation of antioxidative enzymes:a novel pathway for hypoxic preconditioning-mediated delayed cardioprotection[J]. Molecular and Cellular Biochemistry, 2014, 385(1/2): 33-41. |
| [52] |
TAKADA Y, MUKHOPADHYAY A, KUNDU G C, et al. Hydrogen peroxide activates NF-κB through tyrosine phosphorylation of I κBα and serine phosphorylation of p65:evidence for the involvement of I κBα kinase and Syk protein-tyrosine kinase[J]. Journal of Biological Chemistry, 2003, 278(26): 24233-24241. DOI:10.1074/jbc.M212389200 |
| [53] |
CHEN K, KIRBER M T, XIAO H, et al. Regulation of ROS signal transduction by NADPH oxidase 4 localization[J]. Journal of Cell Biology, 2008, 181(7): 1129-1139. DOI:10.1083/jcb.200709049 |
| [54] |
SALZANO S, CHECCONI P, HANSCHMANN E M, et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(33): 12157-12162. DOI:10.1073/pnas.1401712111 |
| [55] |
BHATTACHARYYA A, CHATTOPADHYAY R, MITRA S, et al. Oxidative stress:an essential factor in the pathogenesis of gastrointestinal mucosal diseases[J]. Physiological Reviews, 2014, 94(2): 329-354. DOI:10.1152/physrev.00040.2012 |
| [56] |
HEIM K E, TAGLIAFERRO A R, BOBILYA D J. Flavonoid antioxidants:chemistry, metabolism and structure-activity relationships[J]. Journal of Nutritional Biochemistry, 2002, 13(10): 572-584. DOI:10.1016/S0955-2863(02)00208-5 |
| [57] |
NEUDÓRFFER A, DESVERGNE J P, BONNEFONT-ROUSSELOT D, et al. Protective effects of 4-hydroxycinnamic ethyl ester derivatives and related dehydrodimers against oxidation of LDL:radical scavengers or metal chelators?[J]. Journal of Agricultural and Food Chemistry, 2006, 54(5): 1898-1905. DOI:10.1021/jf052923p |
| [58] |
KUREK-GÓRECKA A, RZEPECKA-STOJKO A, GÓRECKI M, et al. Structure and antioxidant activity of polyphenols derived from propolis[J]. Molecules, 2013, 19(1): 78-101. DOI:10.3390/molecules19010078 |
| [59] |
WU N, ZU Y G, FU Y J, et al. Antioxidant activities and xanthine oxidase inhibitory effects of extracts and main polyphenolic compounds obtained from Geranium sibiricum L[J]. Journal of Agricultural and Food Chemistry, 2010, 58(8): 4737-4743. DOI:10.1021/jf904593n |
| [60] |
PIGNATELLI P, DI SANTO S, BUCHETTI B, et al. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation:effect on platelet recruitment[J]. The FASEB Journal, 2006, 20(8): 1082-1089. DOI:10.1096/fj.05-5269com |
| [61] |
ANDJELKOVIC M, VANCAMP J, DEMEULENAER B, et al. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups[J]. Food Chemistry, 2006, 98(1): 23-31. DOI:10.1016/j.foodchem.2005.05.044 |
| [62] |
GAN W T, DANG Y Q, HAN X, et al. ERK5/HDAC5-mediated, resveratrol-, and pterostilbene-induced expression of MnSOD in human endothelial cells[J]. Molecular Nutrition and Food Research, 2016, 60(2): 266-277. DOI:10.1002/mnfr.v60.2 |
| [63] |
LEON-GONZÁLEZ A J, AUGER C, SCHINI-KERTH V B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy[J]. Biochemical Pharmacology, 2015, 98(3): 371-80. DOI:10.1016/j.bcp.2015.07.017 |
| [64] |
ERLANK H, ELMANN A, KOHEN R, et al. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones[J]. Free Radical Biology and Medicine, 2011, 51(12): 2319-2327. DOI:10.1016/j.freeradbiomed.2011.09.033 |
| [65] |
LIU M, TAN J J, HE Z Y, et al. Inhibitory effect of blue honeysuckle extract on high-fat-diet-induced fatty liver in mice[J]. Animal Nutrition, 2018, 4(3): 288-293. DOI:10.1016/j.aninu.2018.06.001 |
| [66] |
QUIDEAU S, DEFFIEUX D, DOUAT-CASASSUS C, et al. Plant polyphenols:chemical properties, biological activities, and synthesis[J]. Angewandte Chemie International Edition, 2011, 50(3): 586-621. DOI:10.1002/anie.v50.3 |
| [67] |
JEAN-GILLES D, LI L Y, MA H, et al. Anti-inflammatory effects of polyphenolic-enriched red raspberry extract in an antigen-induced arthritis rat model[J]. Journal of Agricultural and Food Chemistry, 2012, 60(23): 5755-5762. DOI:10.1021/jf203456w |
| [68] |
GIACOPPO S, GALUPPO M, LOMBARDO G E, et al. Neuroprotective effects of a polyphenolic white grape juice extract in a mouse model of experimental autoimmune encephalomyelitis[J]. Fitoterapia, 2015, 103: 171-186. DOI:10.1016/j.fitote.2015.04.003 |
| [69] |
MARTIN D A, BOLLING B W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases[J]. Food & Function, 2015, 6(6): 1773-1786. |
| [70] |
LOKE W M, PROUDFOOT J M, HODGSON J M, et al. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2010, 30(4): 749-757. DOI:10.1161/ATVBAHA.109.199687 |
| [71] |
NAKAMURA Y, ISHII T, ABE N, et al. Thiol modification by bioactivated polyphenols and its potential role in skin inflammation[J]. Bioscience, Biotechnology, and Biochemistry, 2014, 78(6): 1067-1070. DOI:10.1080/09168451.2014.905190 |
| [72] |
AMIOT M J, RIVA C, VINET A. Effects of dietary polyphenols on metabolic syndrome features in humans:a systematic review[J]. Obesity Reviews, 2016, 17(7): 573-586. DOI:10.1111/obr.v17.7 |
| [73] |
CRASCÌ L, LAURO M R, PUGLISI G, et al. Natural antioxidant polyphenols on inflammation management:anti-glycation activity vs metalloproteinases inhibition[J]. Critical Reviews in Food Science and Nutrition, 2016, 58(6): 893-904. |
| [74] |
BYUN E H, FUJIMURA Y, YAMADA K, et al. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor[J]. The Journal of Immunology, 2010, 185(1): 33-45. |
| [75] |
QIAN Z J, WU Z Q, HUANG L, et al. Mulberry fruit prevents LPS-induced NF-κB/pERK/MAPK signals in macrophages and suppresses acute colitis and colorectal tumorigenesis in mice[J]. Scientific Reports, 2015, 5: 17348. DOI:10.1038/srep17348 |
| [76] |
ROMIER B, VAN DE WALLE J, DURING A, et al. Modulation of signalling nuclear factor-κB activation pathway by polyphenols in human intestinal Caco-2 cells[J]. British Journal of Nutrition, 2008, 100(3): 542-551. DOI:10.1017/S0007114508966666 |
| [77] |
KIM M H, YOO D S, LEE S Y, et al. The TRIF/TBK1/IRF-3 activation pathway is the primary inhibitory target of resveratrol, contributing to its broad-spectrum anti-inflammatory effects[J]. Pharmazie, 2011, 66(4): 293-300. |
| [78] |
HISANAGA A, MUKAI R, SAKAO K, et al. Anti-inflammatory effects and molecular mechanisms of 8-prenyl quercetin[J]. Molecular Nutrition and Food Research, 2016, 60(5): 1020-1032. DOI:10.1002/mnfr.201500871 |
| [79] |
BASELGA-ESCUDERO L, BLADE C, RIBAS-LATRE A, et al. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells[J]. Nucleic Acids Research, 2014, 42(2): 882-892. DOI:10.1093/nar/gkt1011 |
| [80] |
VAN BUITEN C B, LAMBERT J D, ELIAS R J. Green tea polyphenols mitigate gliadin-mediated inflammation and permeability in vitro[J]. Molecular Nutrition & Food Research, 2018, 62(12): 1700879. |
| [81] |
JOSEPH S V, EDIRISINGHE I, BURTON-FREEMAN B M. Fruit polyphenols:a review of anti-inflammatory effects in humans[J]. Critical Reviews in Food Science and Nutrition, 2016, 56(3): 419-444. DOI:10.1080/10408398.2013.767221 |
| [82] |
DE LA LUZ CÁDIZ-GURREA M, MICOL V, JOVEN J, et al. Different behavior of polyphenols in energy metabolism of lipopolysaccharide-stimulated cells[J]. Food Research International, 2018. DOI:10.1016/j.foodres.2018.02.027 |
| [83] |
JOSKOVA M, SADLONOVA V, NOSALOVA G, et al.Polyphenols and their components in experimental allergic asthma[M]//POKORSKI M.Respiratory regulation-the molecular approach.Dordrecht: Springer, 2013, 756: 91-98.
|




