胆汁酸(bile acid, BA)是由高度不溶于水的胆固醇在肝脏通过分解代谢产生的可溶于水的化合物。BA分子通常由4个固醇环和1条由5个碳原子构成的酸性支链组成,其中固醇环形成的晶格结构包含1个凸起的疏水面和1个凹陷的亲水面,酸性支链则通常与甘氨酸或牛磺酸发生结合反应,形成不同种类的BA(图 1)。BA的两性分子结构使其可以通过胶束的形成促进肠道对食物中脂类和脂溶性维生素的消化吸收。此外,BA分子中羟基的数量和位置决定其具有不同的疏水性,强的疏水性通常伴随着强的细胞毒性。近年来,BA受体法尼醇X受体(farnesoid X receptor,FXR)、G蛋白偶联BA受体1(G protein-coupled bile acid receptor 1,GPBAR1,又称TGR5)和维生素D受体(vitamin D receptor,VDR)的相继发现以及这些受体对葡萄糖、脂类和BA代谢的调节作用[1-3]赋予了BA更多的生理调控功能。本文主要围绕BA的分类、BA的营养生理功能、BA稳态及营养对BA稳态的调节进行简要综述,以期为动物营养研究及动物生产调控提供新的视觉和参考。
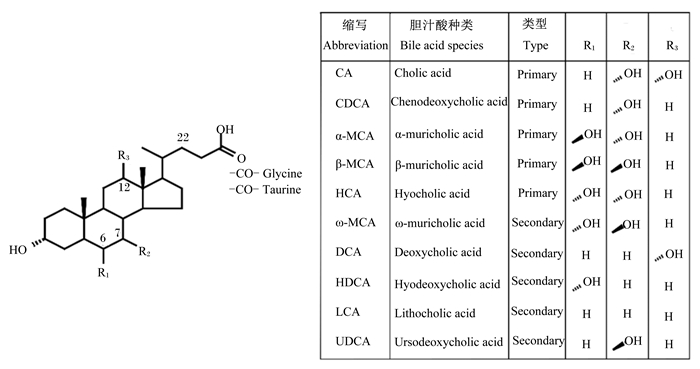
|
CA:胆酸;CDCA:鹅脱氧胆酸;α-MCA:α-鼠胆酸;β-MCA:β-鼠胆酸;HCA:猪胆汁酸;ω-MCA:ω-鼠胆酸;DCA:脱氧胆酸;HDCA:猪脱氧胆酸;LCA:石胆酸;UDCA:熊脱氧胆酸;Glycine:甘氨酸;Taurine:牛磺酸;Primary:初级;Secondary:次级。 图 1 胆汁酸结构与组成 Fig. 1 Structure and composition of bile acids[14] |
根据合成部位的不同,BA通常分为初级BA和次级BA。肝脏合成的BA被称为初级BA,这个过程分别由胆固醇7α羟化酶(cholesterol 7α-hydroxylase, CYP7A1)及下游酶介导的经典途径和固醇27α羟化酶(cholesterol 27-xylase, CY27A1)及下游酶介导的替代途经组成,这2条通路分别合成了大约75%和25%的BA[3]。初级BA在不同物种间存在较大差异,人上主要由胆酸(cholic acid, CA)和鹅脱氧胆酸(chenodeoxycholic acid, CDCA)组成,啮齿动物上主要由CA、α-鼠胆酸(α-muricholic acid, α-MCA)和β-鼠胆酸(β-muricholic acid, β-MCA)组成[4],猪上主要由猪胆酸(hyocholic acid,HCA)、CDCA和CA组成[5](图 2)。由于BA具有两性性质,因此,在其向胆管腔分泌之前,初级BA通常与牛磺酸或甘氨酸结合形成甘氨结合型胆汁酸(glycine conjugated bile acid, G-BA)或牛磺结合型胆汁酸(taurine conjugated bile acid, T-BA),从而阻止非结合型BA以被动扩散方式重新返回肝脏。不同物种上结合型BA组成也不尽相同,例如人血液中BA均以G-BA为主,而啮齿动物上血液中BA以T-BA为主[6]。进入肠道后,结合型BA经由肠道微生物加工修饰后生成的BA我们称之为次级BA。由于次级BA均由初级BA代谢产生,故其组成也存在物种间差异。例如, 人上主要包括石胆酸(lithocholic acid, LCA)和脱氧胆酸(deoxycholic acid, DCA),啮齿动物上主要包括ω-鼠胆酸(ω-muricholic acid, ω-MCA)、熊脱氧胆酸(ursodeoxycholic acid, UDCA)、DCA和LCA[7],猪上则主要包括猪脱氧胆酸(hyodeoxycholic acid,HDCA)、DCA、LCA和UDCA[8]。重吸收返回肝脏的次级BA通常以T-BA或G-BA形式存在。
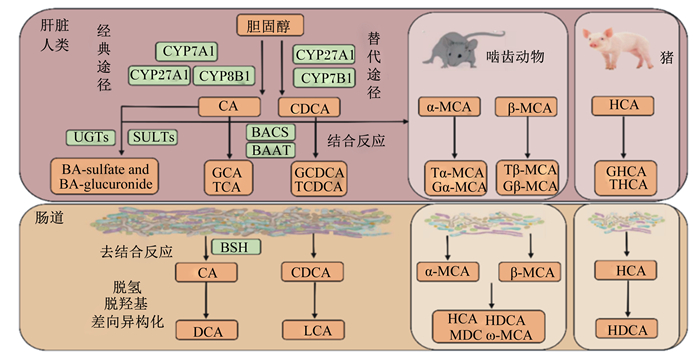
|
CYP7A1:胆固醇7α羟化酶;CYP27A1:固醇27α羟化酶;CYP8B1:固醇12α羟化酶;CYP7B1:氧固醇7α羟化酶;BACS:胆汁酸辅酶A合成酶;BAAT:胆汁酸辅酶A氨基酸N-乙酰转移酶;UGTs:尿苷二磷酸葡萄糖醛酸基转移酶;SULTs:磺基转移酶;BSH:胆盐水解酶;CA:胆酸;CDCA:鹅脱氧胆酸;GCA:甘氨结合型胆酸;TCA:牛磺结合型胆酸;GCDCA:甘氨结合型鹅脱氧胆酸;TCDCA:牛磺结合型鹅脱氧胆酸;BA-sulfate:硫酸化胆汁酸;BA-glucuronide:葡萄糖醛酸化胆汁酸;DCA:脱氧胆酸;LCA:石胆酸;α-MCA:α-鼠胆酸;β-MCA:β-鼠胆酸;Gα-MCA:甘氨结合型α-鼠胆酸;Tα-MCA:牛磺结合型α-鼠胆酸;Gβ-MCA:甘氨结合型β-鼠胆酸;Tβ-MCA:牛磺结合型β-鼠胆酸;HCA:猪胆汁酸;HDCA:猪脱氧胆酸;ω-MCA:ω-鼠胆酸;MDCA:鼠脱氧胆酸。 图 2 胆汁酸合成与代谢 Fig. 2 Bile acids synthesis and metabolism[22-24] |
脂类是动物主要的能量储存、信号传导以及质膜骨架成分,对动物健康具有至关重要的作用。BA通过形成混合胶束促进脂类和脂溶性维生素的溶解、消化和吸收[7]。小肠中,胶束的形成使脂肪酸和甘油一酯的含量提升大约1 000倍,进而使脂类物质的扩散速度加快大约100倍。但是,只有当BA浓度超过其临界胶束浓度,混合胶束才可以有效发挥其功能。因此,小肠中BA浓度对于食物中脂类和脂溶性维生素溶解、消化和吸收具有至关重要的作用。
此外,BA对脂类和脂溶性维生素消化吸收的作用存在剂量和组成差异。在不影响肉鸡平均日采食量的前提下,饲粮添加猪胆汁酸可以显著提高脂蛋白连接酶、脂肪酶活性和第1~42天平均日增重,显著降低第1~42天料重比[9]。人食物中额外添加CA也可显著提高胆固醇吸收[10]。然而,较低剂量(40 mg/kg)猪胆汁酸并不会提高饲粮脂肪和脂溶性维生素消化吸收,而只有较高剂量(60和80 mg/kg)猪胆汁酸可以显著改善肉鸡生长性状。此外,与CA相比,人食物中添加CDCA并不能显著提高胆固醇吸收,添加DCA甚至产生相反效果[11],这与家禽和猪上研究结果[12-13]一致。这些BA效果的不同可能与机体对不同BA的应答有关。对上述结果分析发现,CA在显著提高肠腔内CA以及总胆汁酸(total bile acid, TBA)浓度的同时,对其他种类BA影响很小[10],相反,CDCA并不能提高肠腔内CDCA浓度以及餐后TBA浓度峰值,DCA虽然提高了肠腔中DCA浓度,但是其肠腔内TBA浓度峰值并未改变[11]。
2.2 BA对肝脏代谢稳态及健康的作用肝脏位于消化道和全身循环的中央位置,在机体葡萄糖代谢和脂代谢上发挥着关键作用。肝脏一方面可以通过肝糖原和糖异生作用维持葡萄糖稳态,另一方面通过吸收食物中甘油三酯和胆固醇合成新的胆固醇,新合成的胆固醇在肝脏作用下合成BA,进一步作用于肠道,发挥其促进脂类代谢作用。肝脏代谢异常会导致包括糖尿病和动脉粥样硬化在内的多种疾病的发生,因此,维持肝脏代谢稳态对动物和人类健康具有十分重要作用。
2.2.1 BA与葡萄糖代谢作为FXR的内源配体,BA通过激活FXR调节葡萄糖代谢[15](图 3),该调节存在时间和组织效应。FXR激动剂CA和人工合成GW4064短期(5~16 d)处理小鼠均可通过抑制糖异生途径进而显著降低葡萄糖含量[15-16]。意料之外的是,GW4064长期处理小鼠却会产生相反效果[16]。GW4064长期(3个月)处理高脂饲粮饲喂小鼠可显著提高血液葡萄糖含量和葡萄糖不耐受性,这种相反的结果与小鼠BA合成降低和BA池减少有关,使用CA替代GW4064可以逆转上述代谢异常。以上结果提示,长期使用合成的FXR激动剂并不利于葡萄糖代谢的长期调节,天然BA在葡萄糖代谢长期调节中效果更趋于稳定。肝脏和肠道是FXR基因表达水平最高的部位[3],然而肠道与肝脏FXR的激活会产生截然不同的功效。小鼠上研究发现,BA可通过激活肝脏FXR降低禁食葡萄糖水平,主要表现为促进肝糖原合成和降低糖异生[16-17](图 3)。相反,阻止肠道FXR基因表达反而有助于机体葡萄糖稳态的维持[18-20]。
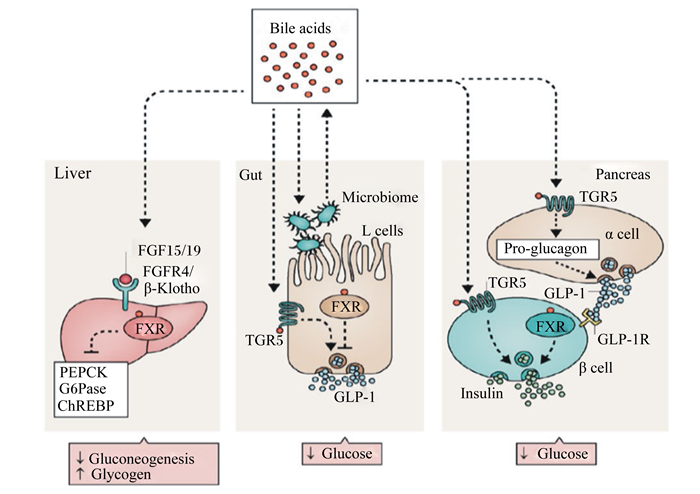
|
Bile acids:胆汁酸;Liver:肝脏;Gut:肠道;Pancreas:胰腺;Microbiome:微生物;L cells:肠道内分泌L细胞;FGF15/FGF19:成纤维细胞生长因子15/19;FGFR4/β-Klotho:成纤维生长因子受体4/成纤维细胞生长因子21(FGF21)的辅助因子;PEPCK:磷酸烯醇式丙酮酸羧基酶;G6Pase:葡萄糖-6-磷酸酶;ChREBP:碳水化合物应答元件-结合蛋白;Gluconeogenesis:糖异生作用;Glycogen:糖原;FXR:法尼醇X受体;TGR5:G蛋白偶联胆汁酸受体1;GLP-1:胰高血糖素样肽-1;Glucose:葡萄糖;α cell:胰腺α细胞;Pro-glucagon:胰高血糖素原;GLP-1R:胰高血糖素样肽-1受体;β cell:胰腺β细胞;Insulin:胰岛素。 图 3 胆汁酸信号调控机体血糖响应 Fig. 3 Bile acids signaling control systemic glycemic response[37] |
BA还可通过激活TGR5调节葡萄糖代谢[21]。TGR5是一种G蛋白偶联受体,在肠道、胆囊、棕色和白色脂肪组织、骨骼肌、大脑和胰腺中广泛表达。BA激活肠道FXR会抑制胰高血糖素样肽-1(glucagon-like peptide 1,GLP-1)的分泌,相反,BA激活TGR5可促进肠道L细胞分泌GLP-1,后者可作用于胰腺β细胞,促进胰岛素分泌,调节葡萄糖代谢[21]。肠道FXR与TGR5对GLP-1的相反调控意味着肠道L细胞TGR5的激活可能是采食后的一种快速响应,而FXR的激活可能是一种延迟的响应。
2.2.2 BA与脂类代谢除了参与食物中脂类和脂溶性维生素溶解、消化和吸收之外,BA还以其他途径参与调节脂类代谢。与健康人相比,胆结石病人有更高的胆固醇合成、更低的BA合成和更少的BA池,进而导致更多的胆固醇分泌进入胆汁[25]。早期研究发现,采用CA、CDCA和UDCA可以溶解胆结石[26]。一方面,BA(例如CDCA)可以在胆囊中溶解胆固醇进而破坏其结晶和胆结石的形成[27-28];另一方面,BA还可通过激活FXR调节脂类代谢[29-33]。与葡萄糖代谢相似的是,BA或GW4064短期处理均可通过激活FXR改变参与脂肪酸、甘油三酯和脂蛋白代谢的基因表达,显著降低血浆甘油三酯和胆固醇含量,维持正常脂质代谢[1, 30, 32]。GW4064长期处理反而会显著提高高脂饲粮诱导的血液中甘油三酯累积,使用CA替代GW4064可以完全阻止这种现象发生[34]。这意味着长期使用合成的FXR激动剂也不利于脂类代谢的长期调节,天然BA在脂类代谢长期调节中效果更趋稳定。此外,BA还可通过激活FXR调节自身代谢[35-36],使BA维持在相对恒定水平,该部分将在3.1详细描述。综合前述,BA在调节机体脂类代谢中具有极其重要的作用。
2.3 BA对肠道发育及健康的作用不同BA对于肠道细胞的增殖可能具有不同的调节作用。与野生型小鼠相比,FXR敲除小鼠结肠细胞增殖速度更快。与此一致的是,CA、CDCA、DCA和LCA均可抑制大鼠小肠细胞增殖[38]。相反的是,TCA处理可以促进大鼠小肠细胞增殖[38],而CDCA处理可显著提高全静脉营养仔猪小肠重量及回肠绒隐比,同时改善肠外营养引起的FXR下游标志因子成纤维生长因子(fibroblast growth factor, FGF)19和促肠道生长营养因子胰高血糖素样肽-2(glucagon-like peptide-2, GLP-2)分泌的下降[39]。这些不一致的结果提示,除了FXR之外,还有其他途径参与调节肠道细胞的增殖。BA对肠道细胞增殖的调控可能是通过影响酪氨酸激酶、表皮生长因子受体、细胞外信号调节激酶、TGR5以及FXR信号通路共同起作用。
除了调节肠细胞的增殖外,维持FXR的活性还有助于缓解炎症并维持肠道上皮屏障的完整性,抑制微生物进入肠道,进而调控肠道微生物生长。研究显示,FXR激动剂可抑制肠道巨噬细胞肿瘤坏死因子α、白细胞介素-1β、白细胞介素-6、环氧合酶-1和环氧合酶-2等基因表达。同时,野生型小鼠灌喂FXR激动剂INT747可以显著缓解葡聚糖硫酸钠诱导的肠道炎症反应增强、形态学评分下降和杯状细胞损失等结肠炎症状,而对于FXR敲除小鼠则无效[40]。此外,INT747还显著降低了肠道的通透性并抑制了肠道炎症因子的表达。以上结果表明激活FXR有助于缓解肠道炎症反应。
2.4 BA对胎儿存活的影响及机制综合上文,BA对脂类吸收、糖脂代谢、肠道发育和健康均有正向调控作用。但不容忽视的是,BA的疏水性特征使其表现出细胞毒性,BA的大量累积会损害机体健康。大量研究显示,BA代谢与胎儿存活之间存在密切关联[41-42]。阐明BA与胎儿存活之间关系的典型临床案例是妊娠期肝内胆汁淤积(intrahepatic cholestasis of pregnancy, ICP)。研究发现,母体血清TBA含量≥40 μmol/L的孕妇比TBA含量 < 40 μmol/L的孕妇有更高的胎儿综合征发生率[43]。此外,怀双胞胎孕妇的ICP发生率(20%~22%)远高于怀单胎孕妇(0.5%~1.5%)[41],提示孕育的胎儿数可能会影响孕妇胆汁淤积发生。本实验室前期在妊娠母猪上的研究也证实母体BA代谢紊乱严重威胁胎猪存活[44]。
对BA与胎儿存活之间关系研究发现,妊娠早期胎儿肝脏即可利用胆固醇合成BA[45],然而妊娠期胎儿和新生儿的BA肠肝循环尚未完全成熟,因此,虽然母体、胎儿之间BA转运是双向的,但是传统观点认为,胎儿很大程度上依赖胎盘将BA转运进入母体进行代谢。ICP患者升高的母体BA浓度损害胎盘BA转运能力,导致胎儿BA浓度升高[46-47]。升高的胎儿BA浓度可能通过以下途径导致胎儿死亡:途径1,BA可显著提高分泌型磷脂酶A2活性,导致肺泡表面活性剂失活[48];途径2,BA(尤其是TCA)可以通过引起心肌细胞收缩异常,导致胎儿宫内猝死[49]。
3 BA代谢稳态及调控如前文所述,BA营养生理作用具有两面性:一方面,适宜浓度的BA有助于肠道对脂类的消化吸收,维持肝脏葡萄糖代谢和脂类代谢平衡;另一方面,当BA代谢尤其是BA分泌和脱毒(硫酸化、羟基化和葡萄糖醛酸化)机能受损时,肝脏合成的BA无法有效转运出肝脏或通过肾脏排泄,导致大量BA或胆盐在肝脏细胞和血液中累积,此时,BA会表现出有害的一面,机体过高浓度的BA会威胁母体健康和胎儿存活。因此,维持BA稳态对机体健康具有重要意义。
3.1 BA稳态及其调控的分子机制机体主要通过FXR信号通路维持BA稳态[35-36]。FXR调节BA代谢主要通过以下2条途径:途径1,BA激活肝脏FXR,提高小异源二聚体分子伴侣(small heterodimer partner,SHP)基因表达,进而抑制CYP7A1和固醇12α羟化酶(sterol 12α-hydroxylase,CYP8B1)基因表达,需要指出的是,肝脏FXR对CYP7A1调节作用似乎要弱于CYP8B1;途径2,BA激活肠道FXR,提高FGF15(鼠)或FGF19(人和猪)表达和分泌,进而抑制肝脏CYP7A1和CYP8B1表达。此外,近期研究发现,BA还可以通过激活肠道VDR进而抑制肝脏CYP7A1表达[50],该部分将在3.4.3详细描述。因此,BA也可能通过VDR调节BA合成。
3.2 繁殖激素对BA稳态的调控临床研究发现,繁殖激素中雌激素与胆汁淤积疾病密切相关。大鼠上一系列研究发现,雌激素代谢产物雌二醇17-β葡萄糖醛酸、雌三醇-17β(β-D-葡萄糖醛酸)和雌三醇-16α(β-D-葡萄糖醛酸)均可通过抑制胆盐输出泵(bile salt export pump,BSEP)基因表达阻止BA分泌进入胆囊,进而诱导胆汁淤积发生[51-53],而硫酸化雌激素D-环葡萄糖醛酸可以消除其致胆汁淤积作用[54]。
虽然雌激素D-环葡萄糖醛酸在啮齿动物BA代谢上具有重要作用,然而孕酮似乎对BA稳态具有更强的调控作用。早期研究揭示,胆汁淤积孕妇有更高的血清孕酮含量[54]和更高的硫酸化孕酮代谢产物含量[55-57]。最新研究进一步揭示了硫酸化孕酮代谢产物与BA代谢之间的关系。作为FXR的部分激动剂,硫酸化孕酮代谢产物可以竞争性抑制BA介导的FXR激活,影响肝脏BA吸收[58]和转运[52],导致胆汁淤积发生。
3.3 肠道微生物对BA稳态的调控肠道微生物在次级BA合成中发挥至关重要作用。在肠道微生物作用下,初级结合型BA先后经过去结合反应,C3、C7和C12位羟基氧化和7α、7β脱羟基以及差向异构化反应,合成次级BA[3]。具有去结合作用的肠道菌属主要有拟杆菌、梭菌、乳杆菌、双歧杆菌和李氏杆菌,具有7α脱羟基作用的肠道菌属主要为梭菌属和真杆菌属。此外,拟杆菌属、真杆菌属、梭菌属、肠杆菌属、消化链球菌属等还具有催化C3、C7和C12位羟基氧化和异构化的功能[59]。此外,经由肠道微生物代谢产生的次级BA还可进一步通过FXR调节BA代谢。近期研究揭示了小鼠肠道微生物可以通过降低牛磺β-鼠胆酸(taurine conjugated beta-muricholic,TβMCA)含量,缓解其对FXR的抑制作用,进而降低机体TBA浓度[4]。
作为介导微生物-宿主之间相互作用的媒介,BA可以影响微生物菌群结构和宿主代谢通路。一方面,BA通过促进BA代谢菌生长,从而抑制其他BA敏感菌生长;另一方面,胆汁流动受阻会导致肠道微生物过度增殖和肠黏膜损伤,进而导致细菌易位通过黏膜屏障,引起系统性感染[60-61]。额外摄入BA可以抑制胆管堵塞导致的细菌过度增殖和易位[62],BA这种功能发挥与其发挥洗涤剂特性摧毁细菌质膜和通过FXR诱导免疫系统产生抗菌剂(例如白细胞介素-8)有关[63]。
3.4 营养对BA稳态的调控 3.4.1 葡萄糖BA可以调节葡萄糖代谢,这个过程反之亦然。采食后血液TBA和肝脏CYP7A1表达水平增加的同时,BA-FXR-FGF15介导的BA合成负反馈调节却并未发挥作用,该发现表明食物中营养参与BA合成调节[64]。采用葡萄糖处理禁食小鼠后发现,其肝脏CYP7A1表达可以达到采食后水平,这意味着采食后BA合成能力的增加可能是由升高的血液葡萄糖含量引起的,而甘油三酯处理却并未取得同样效果。这种采食后葡萄糖和BA代谢之间的相互调节对于采食后葡萄糖稳态可能具有重要作用,机体对禁食和餐后BA合成的响应异常可能会导致糖尿病和肥胖的发生。
3.4.2 脂类在胆固醇分解代谢中,大约50%的胆固醇在肝脏转化为BA,因此,作为BA合成前体,胆固醇也可通过调节BA代谢维持自身平衡。此外,剩下大约40%胆固醇进入胆汁最终以粪便形式排出。早期研究发现,高胆固醇或高脂摄入可以显著提高粪便中BA的排泄[65-66],然而其是否可以提高肝脏BA合成能力,目前尚无确切结论。但是,高脂食物可以改变粪便中BA组成,降低UDCA/DCA值[63],因而对BA代谢有潜在的调节作用。
3.4.3 维生素A和维生素DBA在促进肠道对食物中脂溶性维生素消化吸收的同时,脂溶性维生素A和维生素D也可反过来调节BA代谢。维生素A和维生素D调节BA代谢主要通过影响BA合成和BA脱毒2方面。近期研究发现,维生素A和维生素D均可通过降低CYP7A1表达抑制BA合成[50]。然而,两者的具体机制又不尽相同。维生素D活性形式(1α,25-二羟基维生素D3)可通过VDR提高FGF15表达进而抑制CYP7A1表达。维生素A可通过同时提高肠道FGF15和肝脏SHP表达进而抑制CYP7A1表达。维生素A双重调节机制为病理性肠道负反馈受损条件下维持BA稳态提供了新的可能。此外,1α,25-二羟基维生素D3可通过激活VDR,诱导BA脱毒基因细胞色素P4503A4基因表达,降低肝脏和肠道中LCA毒性[2],缓解LCA引发的结肠癌[67]。因此,维生素A和维生素D可通过BA合成和脱毒2方面维持BA代谢稳态,保护机体健康。
3.4.4 果胶果胶是在植物细胞壁的初生壁和细胞中间片层中广泛存的一类杂多糖,根据其分子主链和支链结构的不同,果胶主要分为同型半乳糖醛酸聚糖、鼠李半乳糖醛酸聚糖、鼠李半乳糖醛酸聚糖Ⅱ和木糖半乳糖醛酸聚糖。作为一种可溶性膳食纤维,人和啮齿动物上研究均表明,果胶可以调节BA代谢[68-70]。其途径主要包括:1)提高肝脏BA合成限速酶CYP7A1活性,增加BA合成;2)降低BA重吸收,增加粪便中BA排出。
4 小结与展望BA作为FXR、TGR5和VDR等关键信号分子的天然配体,使BA在营养、生理及免疫方面的调控作用越来越受到人们的关注,但是目前的研究主要集中在人和模式动物(主要是大鼠和小鼠)上,关于BA代谢与动物生产的关系及其调控在畜禽上还缺乏系统深入的研究。值得重视的是,对于新生期、快速生长期或特殊营养干预(如肠外营养)动物,适宜的BA浓度有助于肠道对脂类和脂溶性维生素的消化吸收,促进肠道生长发育,并有调节肠道微生物和免疫力的作用;对于成年非妊娠动物,BA不仅促进食物中脂类的消化和吸收,还可通过调节糖脂代谢维持机体健康;然而,对于孕妇和妊娠动物,机体BA淤积会损害母体和胎儿健康,提高胎儿宫内发育迟缓、早产甚至死亡等不良结局的风险。因此,客观全面地认识BA的营养生理作用、科学合理地调控BA代谢,对维护人和动物健康、提高动物生产均具有重要意义。
| [1] |
SINAL C J, TOHKIN M, MIYATA M, et al. Targeted Disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis[J]. Cell, 2000, 102(6): 731-744. DOI:10.1016/S0092-8674(00)00062-3 |
| [2] |
MAKISHIMA M, LU T T, XIE W, et al. Vitamin D receptor as an intestinal bile acid sensor[J]. Science, 2002, 296(5571): 1313-1316. |
| [3] |
LEFEBVRE P, CARIOU B, LIEN F, et al. Role of bile acids and bile acid receptors in metabolic regulation[J]. Physiological Reviews, 2009, 89(1): 147-191. DOI:10.1152/physrev.00010.2008 |
| [4] |
SAYIN S I, WAHLSTRÖM A, FELIN J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist[J]. Cell Metabolism, 2013, 17(2): 225-235. |
| [5] |
PEREIRA-FANTINI P M, LAPTHORNE S, JOYCE S A, et al. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease[J]. Journal of Hepatology, 2014, 61(5): 1115-1125. |
| [6] |
GARCÍA-CAÑAVERAS J C, DONATO M T, CASTELL J V, et al. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method[J]. The Journal of Lipid Research, 2012, 53(10): 2231-2241. DOI:10.1194/jlr.D028803 |
| [7] |
DE AGUIAR VALLIM T Q, TARLING E J, EDWARDS P A. Pleiotropic roles of bile acids in metabolism[J]. Cell Metabolism, 2013, 17(5): 657-669. |
| [8] |
宋雨默.万古霉素对妊娠母猪肠道菌群及胆汁酸稳态的影响研究[D].硕士学位论文.成都: 四川农业大学, 2017: 26-32.
|
| [9] |
LAI W Q, HUANG W G, DONG B, et al. Effects of dietary supplemental bile acids on performance, carcass characteristics, serum lipid metabolites and intestinal enzyme activities of broiler chickens[J]. Poultry Science, 2018, 97(1): 196-202. DOI:10.3382/ps/pex288 |
| [10] |
WOOLLETT L A, BUCKLEY D D, YAO L H, et al. Cholic acid supplementation enhances cholesterol absorption in humans[J]. Gastroenterology, 2004, 126(3): 724-731. |
| [11] |
WANG Y W, JONES P J H, WOOLLETT L A, et al. Effects of chenodeoxycholic acid and deoxycholic acid on cholesterol absorption and metabolism in humans[J]. Translational Research, 2006, 148(1): 37-45. |
| [12] |
PIEKARSKI A, DECUYPERE E, BUYSE J, et al. Chenodeoxycholic acid reduces feed intake and modulates the expression of hypothalamic neuropeptides and hepatic lipogenic genes in broiler chickens[J]. General and Comparative Endocrinology, 2016, 229: 74-83. |
| [13] |
DE DIEGO-CABERO N, MEREU A, MENOYO D, et al. Bile acid mediated effects on gut integrity and performance of early-weaned piglets[J]. BMC Veterinary Research, 2015, 11: 111. |
| [14] |
SWANN J R, WANT E J, GEIER F M, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(Suppl.1): 4523-4530. |
| [15] |
MA K, SAHA P K, CHAN L, et al. Farnesoid X receptor is essential for normal glucose homeostasis[J]. Journal of Clinical Investigation, 2006, 116(4): 1102-1109. DOI:10.1172/JCI25604 |
| [16] |
ZHANG Y Q, LEE F Y, BARRERA G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(4): 1006-1011. DOI:10.1073/pnas.0506982103 |
| [17] |
POTTHOFF M J, BONEY-MONTOYA J, CHOI M, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway[J]. Cell Metabolism, 2011, 13(6): 729-738. |
| [18] |
LI F, JIANG C T, KRAUSZ K W, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity[J]. Nature Communications, 2013, 4: 2384. DOI:10.1038/ncomms3384 |
| [19] |
JIANG C T, XIE C, LI F, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease[J]. Journal of Clinical Investigation, 2015, 125(1): 386-402. |
| [20] |
JIANG C T, XIE C, LV Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction[J]. Nature Communications, 2015, 6: 10166. |
| [21] |
THOMAS C, GIOIELLO A, NORIEGA L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis[J]. Cell Metabolism, 2009, 10(3): 167-177. DOI:10.1016/j.cmet.2009.08.001 |
| [22] |
WAHLSTRÖM A, SAYIN S I, MARSCHALL H U, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism[J]. Cell Metabolism, 2016, 24(1): 41-50. DOI:10.1016/j.cmet.2016.05.005 |
| [23] |
EYSSEN H J, DE PAUW G, VAN ELDERE J. Formation of hyodeoxycholic acid from muricholic acid and hyocholic acid by an unidentified gram-positive rod termed HDCA-1 isolated from rat intestinal microflora[J]. Applied and Environmental Microbiology, 1999, 65(7): 3158-3163. |
| [24] |
BERGSTRÖM S, DANIELSSON H, GÖRANSSON Å. On the bile acid metabolism in the pig.Bile acids and steroids.81[J]. Acta Chemica Scandinavica, 1959, 13: 776-783. DOI:10.3891/acta.chem.scand.13-0776 |
| [25] |
KERN F, Jr. Effects of dietary cholesterol on cholesterol and bile acid homeostasis in patients with cholesterol gallstones[J]. The Journal of Clinical Investigation, 1994, 93(3): 1186-1194. DOI:10.1172/JCI117072 |
| [26] |
HOFMANN A F, HAGEY L R. Key discoveries in bile acid chemistry and biology and their clinical applications:history of the last eight decades[J]. The Journal of Lipid Research, 2014, 55(8): 1553-1595. |
| [27] |
ISER J H, SALI A. Chenodeoxycholic acid:a review of its pharmacological properties and therapeutic use[J]. Drugs, 1981, 21(2): 90-119. |
| [28] |
BELL G D, WHITNEY B, DOWLING R H. Gallstone dissolution in man using chenodeoxycholic acid[J]. The Lancet, 1972, 300(7789): 1213-1216. DOI:10.1016/S0140-6736(72)92266-0 |
| [29] |
KAST H R, NGUYEN C M, SINAL C J, et al. Farnesoid X-activated receptor induces apolipoprotein C-Ⅱ transcription:a molecular mechanism linking plasma triglyceride levels to bile acids[J]. Molecular Endocrinology, 2001, 15(10): 1720-1728. DOI:10.1210/mend.15.10.0712 |
| [30] |
LAMBERT G, AMAR M J A, GUO G, et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis[J]. The Journal of Biological Chemistry, 2003, 278(4): 2563-2570. |
| [31] |
HANNIMAN E A, LAMBERT G, MCCARTHY T C, et al. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice[J]. The Journal of Lipid Research, 2005, 46(12): 2595-2604. |
| [32] |
WATANABE M, HOUTEN S M, WANG L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c[J]. Journal of Clinical Investigation, 2004, 113(10): 1408-1418. DOI:10.1172/JCI21025 |
| [33] |
ZHANG Y Q, WANG X P, VALES C, et al. FXR deficiency causes reduced atherosclerosis in Ldlr-/- mice[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2006, 26(10): 2316-2321. DOI:10.1161/01.ATV.0000235697.35431.05 |
| [34] |
WATANABE M, HORAI Y, HOUTEN S M, et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure[J]. The Journal of Biological Chemistry, 2011, 286(30): 26913-26920. DOI:10.1074/jbc.M111.248203 |
| [35] |
KONG B, WANG L, CHIANG J Y L, et al. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice[J]. Hepatology, 2012, 56(3): 1034-1043. DOI:10.1002/hep.25740 |
| [36] |
INAGAKI T, CHOI M, MOSCHETTA A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis[J]. Cell Metabolism, 2005, 2(4): 217-225. |
| [37] |
SHAPIRO H, KOLODZIEJCZYK A A, HALSTUCH D, et al. Bile acids in glucose metabolism in health and disease[J]. The Journal of Experimental Medicine, 2018, 215(2): 383-396. |
| [38] |
DOSSA A Y, ESCOBAR O, GOLDEN J, et al. Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2016, 310(2): G81-G92. DOI:10.1152/ajpgi.00065.2015 |
| [39] |
JAIN A K, STOLL B, BURRIN D G, et al. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2012, 302(2): G218-G224. |
| [40] |
GADALETA R M, VAN ERPECUM K J, OLDENBURG B, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease[J]. Gut, 2011, 60(4): 463-472. DOI:10.1136/gut.2010.212159 |
| [41] |
GEENES V, WILLIAMSON C. Intrahepatic cholestasis of pregnancy[J]. World Journal of Gastroenterology, 2009, 15(17): 2049-2066. DOI:10.3748/wjg.15.2049 |
| [42] |
ZECCA E, DE LUCA D, MARRAS M, et al. Intrahepatic cholestasis of pregnancy and neonatal respiratory distress syndrome[J]. Pediatrics, 2006, 117(5): 1669-1672. DOI:10.1542/peds.2005-1801 |
| [43] |
GLANTZ A, MARSCHALL H U, MATTSSON L Å. Intrahepatic cholestasis of pregnancy:relationships between bile acid levels and fetal complication rates[J]. Hepatology, 2004, 40(2): 467-474. |
| [44] |
王朋.母猪妊娠中后期胆汁酸代谢及其对繁殖性能的影响[D].硕士学位论文.成都: 四川农业大学, 2016: 1-10.
|
| [45] |
SMALLWOOD R A, JABLONSKI P, WATTS J M. Bile acid synthesis in the developing sheep liver[J]. Clinical Science, 1973, 45(3): 403-406. DOI:10.1042/cs0450403 |
| [46] |
GEENES V, LÖVGREN-SANDBLOM A, BENTHIN L, et al. The reversed feto-maternal bile acid gradient in intrahepatic cholestasis of pregnancy is corrected by ursodeoxycholic acid[J]. PLoS One, 2014, 9(1): e83828. DOI:10.1371/journal.pone.0083828 |
| [47] |
SERRANO M A, BRITES D, LARENA M G, et al. Beneficial effect of ursodeoxycholic acid on alterations induced by cholestasis of pregnancy in bile acid transport across the human placenta[J]. Journal of Hepatology, 1998, 28(5): 829-839. |
| [48] |
DE LUCA D, MINUCCI A, ZECCA E, et al. Bile acids cause secretory phospholipase A2 activity enhancement, revertible by exogenous surfactant administration[J]. Intensive Care Medicine, 2009, 35(2): 321-326. |
| [49] |
WILLIAMSON C, MIRAGOLI M, KADIR S S A, et al. Bile acid signaling in fetal tissues:implications for intrahepatic cholestasis of pregnancy[J]. Digestive Diseases, 2011, 29(1): 58-61. DOI:10.1159/000324130 |
| [50] |
SCHMIDT D R, HOLMSTROM S R, FON TACER K, et al. Regulation of bile acid synthesis by fat-soluble vitamins A and D[J]. The Journal of Biological Chemistry, 2010, 285(19): 14486-14494. DOI:10.1074/jbc.M110.116004 |
| [51] |
STIEGER B, FATTINGER K, MADON J, et al. Drug-and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver[J]. Gastroenterology, 2000, 118(2): 422-430. DOI:10.1016/S0016-5085(00)70224-1 |
| [52] |
VALLEJO M, BRIZ O, SERRANO M A, et al. Potential role of trans-inhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy[J]. Journal of Hepatology, 2006, 44(6): 1150-1157. |
| [53] |
MEYERS M, SLIKKER W, VORE M. Steroid D-ring glucuronides:characterization of a new class of cholestatic agents in the rat[J]. Journal of Pharmacology and Experimental Therapeutics, 1981, 218(1): 63-73. |
| [54] |
ESTIÚMÁ C, MONTE M J, RIVAS L, et al. Effect of ursodeoxycholic acid treatment on the altered progesterone and bile acid homeostasis in the mother-placenta-foetus trio during cholestasis of pregnancy[J]. British Journal of Clinical Pharmacology, 2015, 79(2): 316-329. |
| [55] |
MENG L J, REYES H, PALMA J, et al. Profiles of bile acids and progesterone metabolites in the urine and serum of women with intrahepatic cholestasis of pregnancy[J]. Journal of Hepatology, 1997, 27(2): 346-357. DOI:10.1016/S0168-8278(97)80181-X |
| [56] |
ABU-HAYYEH S, PAPACLEOVOULOU G, LÖVGREN-SANDBLOM A, et al. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype[J]. Hepatology, 2013, 57(2): 716-726. |
| [57] |
REYES H, SJÖVALL J. Bile acids and progesterone metabolites intrahepatic cholestasis of pregnancy[J]. Annals of Medicine, 2000, 32(2): 94-106. |
| [58] |
ABU-HAYYEH S, MARTINEZ-BECERRA P, KADIR S H S A, et al. Inhibition of Na+-taurocholate co-transporting polypeptide-mediated bile acid transport by cholestatic sulfated progesterone metabolites[J]. The Journal of Biological Chemistry, 2010, 285(22): 16504-16512. DOI:10.1074/jbc.M109.072140 |
| [59] |
JIA W, XIE G X, JIA W P. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis[J]. Nature Reviews Gastroenterology & Hepatology, 2018, 15(2): 111-128. |
| [60] |
BERG R D. Bacterial translocation from the gastrointestinal tract[J]. Trends in Microbiology, 1995, 3(4): 149-154. DOI:10.1016/S0966-842X(00)88906-4 |
| [61] |
CLEMENTS W D B, PARKS R, ERWIN P, et al. Role of the gut in the pathophysiology of extrahepatic biliary obstruction[J]. Gut, 1996, 39(4): 587-593. DOI:10.1136/gut.39.4.587 |
| [62] |
LORENZO-ZÚÑIGAÁ V, BARTOLÍ R, PLANAS R, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats[J]. Hepatology, 2003, 37(3): 551-557. DOI:10.1053/jhep.2003.50116 |
| [63] |
INAGAKI T, MOSCHETTA A, LEE Y K, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(10): 3920-3925. |
| [64] |
LI T G, FRANCL J M, BOEHME S, et al. Glucose and insulin induction of bile acid synthesis:mechanisms and implication in diabetes and obesity[J]. The Journal of Biological Chemistry, 2012, 287(3): 1861-1873. DOI:10.1074/jbc.M111.305789 |
| [65] |
LIN D S, CONNOR W E. The long term effects of dietary cholesterol upon the plasma lipids, lipoproteins, cholesterol absorption, and the sterol balance in man:the demonstration of feedback inhibition of cholesterol biosynthesis and increased bile acid excretion[J]. Journal of Lipid Research, 1980, 21(8): 1042-1052. |
| [66] |
STENMAN L K, HOLMA R, KORPELA R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids[J]. World Journal of Gastroenterology, 2012, 18(9): 923-929. DOI:10.3748/wjg.v18.i9.923 |
| [67] |
WELSH J, WIETZKE J A, ZINSER G M, et al. Vitamin D-3 receptor as a target for breast cancer prevention[J]. The Journal of Nutrition, 2003, 133(7 Suppl): 2425S-2433S. |
| [68] |
BOSAEUS I, CARLSSON N G, SANDBERG A S, et al. Effect of wheat bran and pectin on bile acid and cholesterol excretion in ileostomy patients[J]. Human Nutrition Clinical Nutrition, 1986, 40(6): 429-440. |
| [69] |
GARCIA-DIEZ F, GARCIA-MEDIAVILLA V, BAYON J E, et al. Pectin feeding influences fecal bile acid excretion, hepatic bile acid and cholesterol synthesis and serum cholesterol in rats[J]. The Journal of Nutrition, 1996, 126(7): 1766-1771. |
| [70] |
LEVEILLE G A, SAUBERLICH H E. Mechanism of the cholesterol-depressing effect of pectin in the cholesterol-fed rat[J]. The Journal of Nutrition, 1966, 88(2): 209-214. DOI:10.1093/jn/88.2.209 |




