2. 湖南农业大学动物医学院, 长沙 410128
2. College of Veterinary Medicine, Hunan Agricultural University, Changsha 410128, China
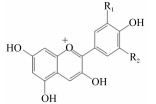
花青素是自然界中最常见的水溶性色素之一,其主要结构是2-苯基苯并吡喃阳离子,属于类黄酮化合物。花青素在自然状态下不稳定,通常以花色苷的形式存在,自然界中已发现的花色苷有600余种。花青素的基本结构如表 1所示,6种主要的花青素为天竺葵素、矢车菊素、芍药素、飞燕草素、牵牛素和锦葵素[1]。花青素具有抗炎、抗氧化等生理功能,应用于畜禽生产中可促进畜禽生长、提高免疫力,且具有来源广泛、低毒、无残留、无污染等特点,在新型无抗饲料添加剂中具有很好的应用前景[2]。
|
|
表 1 常见花青素的化学结构 Table 1 Chemical structure of common anthocyanidins |
矢车菊素-3-葡萄糖苷(cyanidin-3-glucoside, C3G)是自然界分布最广的花色苷,大量存在于黑米、黑豆、紫甘蓝、紫薯、黑接骨木、黑莓、黑葡萄、黑树莓、血橙、桑葚等有色谷物、水果和蔬菜中[1]。大量研究表明,C3G具有抗炎[3]、抗氧化[4]、抗癌[5]、预防心血管疾病[6]、调节脂肪代谢的作用[7],但C3G进入体内后极容易降解,真正进入血液循环的大部分为其代谢产物[8]。因此,了解C3G在体内的吸收代谢以及代谢产物的生理功能对其有重要意义。
1 C3G的吸收代谢 1.1 吸收率C3G在消化道内极少以母体结构被机体吸收,Miyazawa等[9]给大鼠饲喂320 mg/kg的C3G后,通过紫外-高效液相色谱(UV-HPLC)法检测血浆中C3G含量约为1%。Ichiyanagi等[10]使用越橘提取物(含有15种花色苷)饲喂大鼠,通过高效液相色谱(HPLC)法测定得出C3G的吸收率为0.92%。Marczylo等[11]使用500 mg/kg的C3G饲喂小鼠, 得出C3G的吸收率为1.7%。不同研究结果存在差异,这主要基于以下几方面的原因:第一,C3G在体内会产生广泛的代谢,如甲基化、葡萄糖醛酸化等过程并降解成一系列酚类代谢产物,因此进入血液循环的C3G母体水平较低[12-13],而其代谢产物在体内的含量远高于C3G母体,如甲基化和葡萄糖醛酸化产物在血浆中的含量能达到C3G含量的2倍[14]。第二,在进入血液后C3G迅速被机体再次代谢,静脉注射C3G 15 s后检测到甲基化产物,1 min后,C3G及其甲基化产物几乎都从血浆消失,因此通过测定血浆中C3G含量不能准确反映出C3G的利用率[15]。Czank等[16]最新研究通过同位素标记C3G,检测其在呼吸、尿液中的排泄量,得到C3G的相对利用率为12.38%。这种方法能避免体内代谢产物动态变化的干扰,但总回收率(粪便、呼吸、尿液)只有43.9%,远低于摄入量,因此C3G的总利用率应大于12.38%。
1.2 C3G在体内的分布C3G在进入体内后能被快速吸收,摄入C3G 15 min后即在血浆和肾脏中达到峰值[17]。在C3G进入体内后主要分布于胃壁和十二指肠壁中,肝脏、肾脏、胆囊、脂肪组织、膀胱、睾丸、肺脏、前列腺中也能检测到C3G分布[11, 18]。其中胃壁、前列腺、肺脏、肾脏的C3G含量均能达到相应药理浓度[11]。口服摄入的C3G能穿过血脑屏障到达大脑[11, 19]。C3G也能通过日常饮食在组织器官中积累[20-21]。
1.3 C3G在胃内的吸收C3G在胃液酸性环境中相对稳定[22-23]。对小鼠进行胃灌注C3G,仅6 min后就在血浆中检测到C3G,表明花青素可以从胃部被吸收[17, 24]。通过鼻管分别将C3G引入大肠癌转移患者胃和小肠分析其尿液中C3G含量发现,直接导入胃中患者尿液中C3G含量是导入小肠患者的5倍[25]。大鼠的原位胃灌注模型也证实经原位灌注后门静脉和体循环中均出现高含量的C3G[26],使用该模型得出C3G在胃的吸收率为23%[22]。C3G进入胃后能被完整地吸收进入胃壁内[27],但大部分存在于胃黏膜中[11],而在血浆中的含量较低。考虑到C3G在血浆中较低的含量和原位灌注模型中较高的吸收率,有学者提出C3G存在首过代谢[28]。C3G在胃内的主要转运方式是主动转运,载体为bilitranslocase[26]。
1.4 C3G在小肠中的吸收C3G进入小肠后能被完整吸收进入肠壁内[27]。小鼠肠段尤斯灌流(Ussing chamber)表明,花青素在空肠段的吸收率最高[(55.3±7.6)%],其次为十二指肠段[(10.4±7.6)%][25],但极少在回肠或结肠段吸收[23, 29-30]。大鼠空肠回肠原位灌注后C3G吸收率为22.4%。且在小肠肠道内C3G存在相对稳定,45 min仅降解2.3%[31]。同样结果也在Felgines等[32]的试验中得到验证。C3G在小肠的吸收可能主要由钠依赖性葡萄糖转运蛋白(SGLT1)或葡萄糖转运蛋白2(GLUT2)进行转运[33]。根皮苷(SGLT1抑制剂)或根皮素(GLUT2抑制剂)能显著抑制C3G的吸收。通过外翻囊模型同样发现,C3G可通过钠依赖性葡萄糖转运蛋白穿过肠上皮细胞[17]。
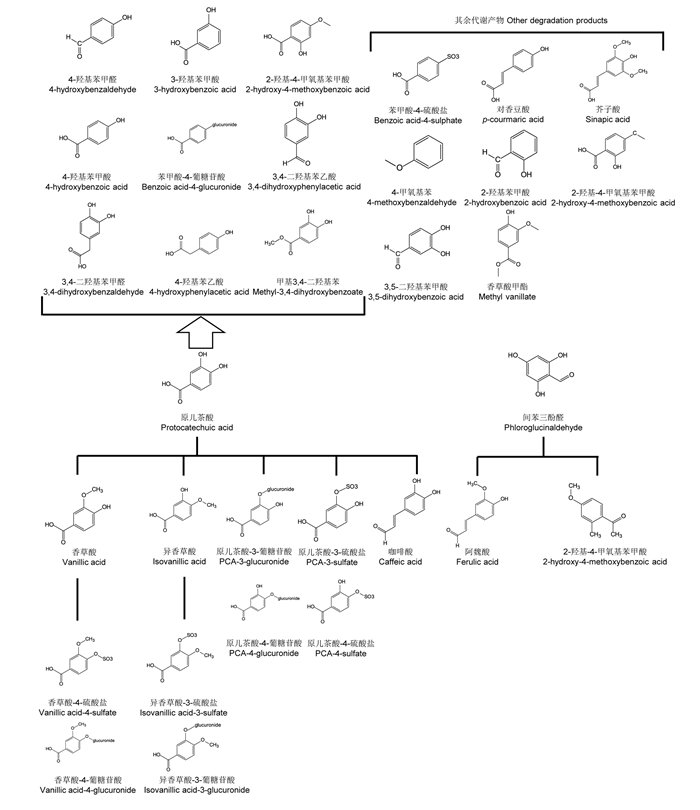
2 C3G的降解代谢及生理功能 2.1 C3G的降解代谢C3G的代谢产物种类繁多,目前已鉴定出41种代谢产物,主要分为C3G的甲基化、葡萄糖醛酸化、硫酸盐化和去糖基化产物,以及C3G降解生成的酚酸原儿茶酸、间苯三酚醛(表 2)和原儿茶酸、间苯三酚醛的衍生物和其余代谢产物(图 1)。如图 1所示,原儿茶酸在体内会发生甲基化生成香草酸、异香草酸;葡萄糖醛酸化生成原儿茶酸-3-葡萄苷、原儿茶酸-4-葡萄苷;此外,生成的香草酸还会被硫酸盐化生成香草酸-3-硫酸盐、异香草酸-4-硫酸盐;原儿茶酸还会直接衍生生成咖啡酸。间苯三酚醛会代谢生成阿魏酸、2-羟基-4甲氧基苯甲酸[13]。De Ferrars等[15]使用同位素标记C3G发现,原儿茶酸在体内还会代谢生成4-甲基苯甲醛、4甲基苯甲酸、4-羟基苯乙酸、3-羟基苯甲酸、2-羟基-4-甲氧基苯甲酸、苯甲-4-葡糖苷酸、3, 4-二羟基苯乙酸、3, 4-二羟基苯甲醛、甲基-3, 4, -二羟基苯共9种物质,但尚不清楚其在体内代谢过程。此外,还检测到苯甲酸-4-硫酸盐、对香豆酸、4-甲氧基苯、2-羟基苯甲酸、2-羟基-4甲氧基苯甲酸、3, 5-二羟基苯甲酸、香豆酸甲脂、芥子酸共8种C3G在体内的代谢产物[13, 16]。
|
|
表 2 矢车菊素-3-葡萄糖苷的亲本代谢产物及降解产物 Table 2 Parental metabolites and degradation products of C3G |
|
图 1 原儿茶酸和间苯酚醛衍生物及矢车菊素-3-葡萄糖苷其余代谢产物 Fig. 1 Derivatives of protocatechuic acid, phloroglucinaldehyde and other degradation products of C3G[13, 15] |
葡萄糖醛酸化是目前研究报道的花青素主要代谢途径,其次是花青素的甲基化[11, 14]。C3G在体内的葡萄糖醛酸化主要有2种途径:第一,C3G在到达肠道后在β-葡糖苷酶作用下形成矢车菊素后在尿苷二磷酸(UDP)葡萄糖醛酸转移酶作用下发生葡萄糖醛酸化[31, 35]。第二,在肝脏中发生葡萄糖醛酸化。通过体外小鼠和人的肝微粒体温育试验可发现矢车菊素、C3G、原儿茶酸的葡萄糖醛酸化产物[23, 38]。Ichiyanagi等[36]通过对比静脉注射C3G和口服C3G后尿液与血浆中C3G的葡糖糖醛酸化产物,进一步验证了C3G、甲基化C3G的葡萄糖醛酸化发生部位在肝脏。
C3G的甲基化主要发生在肝脏[15, 39],在儿茶酚O-甲基转移酶作用下发生甲基化[37, 40]。大量研究表明不仅C3G会在肝脏发生甲基化,而且在β-葡糖苷酶作用下生成的矢车菊素、葡萄糖醛酸化代谢物、降解产物均会在肝脏儿茶酚-O-甲基转移酶作用下发生甲基化[18, 41]。
此外,微生物代谢也是C3G降解的主要途径之一。在模拟肠液孵育中C3G可降解成原儿茶酸、间苯三酚醛[12],肠道中的乳杆菌属和双歧杆菌属具有β-葡萄糖苷酶活性,从而可以将C3G的糖苷部分切割形成苷元[38, 42]。肠道微生物还表现出儿茶酚-O-甲基转移酶活性,从而将C3G的甲基化代谢产物去甲基化[12, 30]。体外试验表明,C3G在微生物作用下能生成原儿茶酸、间苯三酚醛,通过与无菌大鼠对比,经过移植人类肠道微生物的大鼠降解后C3G的代谢产物中3, 4-二羟基苯甲酸、2, 4, 6-三羟基苯甲醛和2, 4, 6-三羟基苯甲酸的含量显著增加[43-44]。
C3G在进入小肠后,在微生物、β-葡萄糖苷酶、UDP葡萄糖醛酸转移酶、儿茶酚-O-甲基转移酶、中性pH环境等因素的作用下发生甲基化[39]、去甲基[30]、去糖基化[17]、葡萄糖醛酸化[23],从而发生降解。虽然C3G在小肠内会发生甲基化、葡萄糖醛酸化,但并未在粪便中检测到C3G的相关代谢产物[13],可见肠道产生的少量产物能被快速吸收或降解。
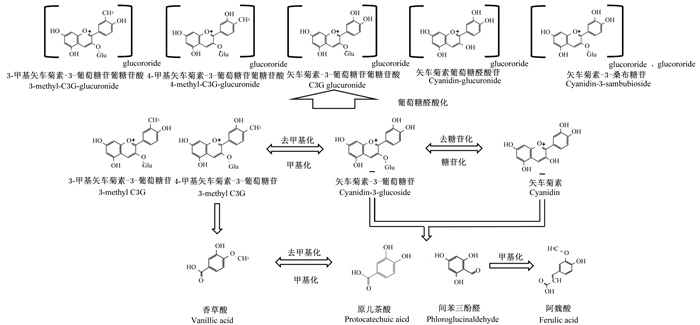
C3G的主要代谢途径如图 2所示。C3G进入体内后在肝脏发生甲基化生成3-甲基-矢车菊素-3-葡萄糖苷和4-甲基-矢车菊素-3-葡萄糖苷,同时也会被去甲基化还原成C3G;C3G在肝脏中去糖苷化生成矢车菊素,同样在去糖苷化过程中也会发生糖苷化还原成C3G。C3G的甲基化、去糖苷化产物均会在肝脏或微生物作用下被葡萄糖醛酸化,形成葡萄糖醛酸化产物。甲基化C3G会在微生物或自发降解生成香草酸,而C3G和去糖苷化矢车菊素则生成原儿茶酸和间苯三酚醛。原儿茶酸与香草酸、间苯三酚醛与阿魏酸会在肝脏甲基化、去甲基化下转换。
|
图 2 矢车菊素-3-葡萄糖苷的主要代谢通路 Fig. 2 Main metabolic pathway of C3G |
C3G能减少机体炎性物质的产生,从而降低代谢性疾病、心血管疾病、非酒精性脂肪肝炎等慢性疾病的风险[45]。大量数据表明C3G能显著升高体内高密度脂蛋白胆固醇(HDL-C)水平,改善机体抗氧化状态,降低炎症反应,改善血管功能[46-49]。体外细胞试验及动物试验均表明,C3G对胃癌细胞(SGC7901和AGS)、乳腺癌细胞(MCF-7)、结肠癌细胞(HCT-116)、肺癌细胞(NCI-H460)、中枢神经系统癌细胞(SF-268)均有抑制作用[50-52]。由于C3G进入体内后被迅速降解,因此真正进入体内发挥生理功能的可能是其代谢产物。已有研究发现,用C3G及其11种代谢产物处理人血管内皮细胞后,C3G对CD40L诱导的白细胞介素-6(IL-6)分泌无显著影响,而其代谢产物如原儿茶酸、香草酸、阿魏酸能显著降低IL-6水平[53]。Krga等[54]研究发现,在使用肿瘤坏死因子-α(TNF-α)诱导的人脐静脉内皮细胞(HUVECs)炎症模型中,原儿茶酸、香草酸、阿魏酸能显著降低细胞间黏附因子-1(ICAM-1)和血管细胞黏附因子-1(VCAM-1)的表达。在由脂多糖(LPS)诱导的单核巨噬细胞THP-1的炎症模型中,C3G的降解产物原儿茶酸和间苯三酚醛均能通过显著降低IL-6的水平来减轻炎症反应[55]。本课题组前期研究表明,以C3G为主要活性成分的蓝靛果多酚能有效缓解高脂饲粮诱导的肝脏炎症及氧化损伤,而其酚类代谢产物原儿茶酸和间苯三酚醛具有类似的保护作用[56]。因此,本文将选取原儿茶酸、间苯三酚醛、香草酸、阿魏酸4种国内外研究中普遍认为在体内发挥C3G主要生理功能的代谢产物进一步阐述。
2.2.1 原儿茶酸原儿茶酸是C3G的代谢产物。大量体外和体内研究表明原儿茶酸具有极高的抗氧化[54]、抗炎[57]、抗菌特性[58],能与一些抗生素产生协同作用[59]。在体外试验中,原儿茶酸能有效清除1, 1-二苯基-2-三硝基苯肼(DPPH)自由基和过氧化氢(H2O2),并降低脂质过氧化水平[60]。原儿茶酸能通过改善线粒体功能、抑制H2O2诱导的人神经元细胞的DNA断裂、减少由H2O2诱导的PC12细胞的乳酸脱氢酶(LDH)释放来减少活性氧(ROS)诱导的细胞凋亡[61]。此外,原儿茶酸还能通过激活核因子E2相关因子2(Nrf2)抗氧化通路促进谷胱甘肽过氧化酶和谷胱甘肽还原酶的表达[62]。在由LPS诱导的RAW264.7细胞的炎症反应中,原儿茶酸能通过调控核因子-κB(NF-κB)和丝裂原活化蛋白激酶(MAPK)通路降低环氧合酶-2(COX-2)、一氧化氮合酶(iNOS)、TNF-α、白细胞介素-1β(IL-1β)的表达,从而缓解细胞的炎症反应[57]。在体外抗菌试验中,原儿茶酸能抑制铜绿假单胞菌的生长,并与磺胺甲唑表现出协同作用[59]。
2.2.2 间苯三酚醛间苯三酚醛在医学上被广泛应用为一种非阿托品、非罂粟碱类平滑肌解痉挛药,通常应用于内脏平滑肌产生的疼痛[63]。但是近来研究发现,间苯三酚醛还具有抗炎[64]、抗氧化及抗细胞凋亡的作用[65-66]。在体外细胞试验中,间苯三酚醛抑制NF-κB诱导激酶(NIK)使得NF-κB失活,抑制MAPK通路降低激活子蛋白-1(AP-1)的活化,减少由LPS诱导的RAW264.7细胞中TNF-α、IL-1β、IL-6和前列腺素E(PGE)等炎症介质的产生[64]。间苯三酚醛通过清除ROS和激活抗氧化系统来减轻氧化应激诱导的细胞损伤[65]。间苯三酚醛能抑制髓过氧化物酶(MPO),增强胃组织过氧化氢酶(CAT)活性缓解由乙醇诱导的大鼠急性胃黏膜损伤[66]。
2.2.3 香草酸香草酸具有很强的抗氧化、抗炎作用,在LPS诱导的小鼠巨噬细胞炎症模型中,香草酸能抑制TNF-α、IL-6的产生,降低COX-2和一氧化氮(NO)的高表达[67]。此外,在由二羟基甲丁酸(DMBA)诱导的仓鼠口腔鳞状细胞癌模型中,香草酸能提高超氧化物歧化酶(SOD)、CAT、谷胱甘肽过氧化物酶(GPH-Px)的活性,并降低脂质过氧化产物丙二醛(MDA)的含量[68]。香草酸还具有保护心脏、降低血压的功能。香草醛具有的抗炎、抗氧化特性能够使其在由异丙肾上腺素诱导的心脏毒性大鼠上发挥保护作用,减轻由NO缺乏引起的大鼠高血压,血脂异常和肝肾损伤[69-70]。香草酸能降低由β-淀粉样蛋白引起的神经炎症、突触缺陷、记忆障碍和神经变性[71]。
2.2.4 阿魏酸阿魏酸具有极强的自由基清除能力[72],表现出极强的抗氧化特性,此外,阿魏酸还具有神经保护功能[73]。阿魏酸能提高机体SOD和CAT活性,提高机体抗氧化能力[74]。阿魏酸通过上调Kelch样ECH相关蛋白1(Keap1)蛋白的表达,调节Nrf2通路,提高血红素氧合酶-1(HO-1)和谷胱甘肽硫转移酶(GST)的基因表达,减轻心肌细胞和肝细胞中由高葡萄糖引起的氧化应激所导致的损伤[75]。通过调节Nrf2/HO-1通路,阿魏酸能缓解由三甲基锡所诱导的人神经母细胞的损伤[73]。
3 C3G在畜禽生产上的应用在畜禽生产中,外界环境、饲料等因素造成的应激常对动物的生产和健康产生不利影响,造成生产性能下降和动物机体损伤,如仔猪早期断奶应激会造成肠道损伤、采食量下降、生长不良[76];热应激会造成家禽生长代谢紊乱、免疫力下降,严重影响其生产性能[77]。C3G及其代谢产物具有强抗氧化和抗炎生理功能,因此可有效缓解各种因素造成的动物应激。例如,阿魏酸能增强肠道抗氧化能力,抑制由热应激诱导的肠道氧化应激损伤[78];减小由于热应激造成的大鼠肠道黏膜闭合蛋白(occludin)、闭锁小带蛋白-1(ZO-1)和钙黏附蛋白-E(E-cadherin)蛋白的表达减少,缓解热应激诱导的肠上皮损伤[79];其抗氧化功能还可保护猪、马精子免受冷冻损伤[80-81]。Li等[82]研究发现,在育肥猪饲粮中添加100 mg/kg的阿魏酸能显著提高肝脏中GSH-Px的活性,降低肌肉中MDA的含量,提高机体抗氧化能力。Macías-Cruz等[83]研究发现,在发情前期母羊饲粮中添加300 mg/d的阿魏酸能显著提高母羊生殖器官重量,显著提高母羊大卵泡比例,阿魏酸具有促进生殖器官和卵巢发育的功能。Yu等[84]研究发现,在饲粮中添加100 mg/kg的阿魏酸能显著提高罗非鱼的饲料转换率,显著降低肝脏和血清中MDA的含量,显著提高肝脏和血清中SOD和CAT的活性,提高其抗氧化能力。原儿茶酸可通过调控促凋亡蛋白Bax和抗凋亡蛋白Bcl-2的表达,加速传染性法氏囊病毒感染细胞的凋亡,有效缓解传染性法氏囊病毒侵袭早期的法氏囊病理变化,在使用20 mg/kg的原儿茶酸治疗组中存活率达到90%以上,并表现出极高的病毒清除率[85]。原儿茶酸可有效缓解传染性法氏囊病毒侵袭早期的法氏囊病理变化,在使用20 mg/kg的原儿茶酸治疗组中存活率达到90%以上,并表现出极高的病毒清除率[86]。本课题组通过LPS诱导的断奶仔猪肠黏膜损伤试验发现,香草酸和原儿茶酸能有效提高仔猪抗氧化能力并降低炎症反应,从而减少肠黏膜损伤,并显著提高断奶仔猪的生长性能。由此可见,C3G及其代谢产物在畜禽生产中可有效发挥促生长、抗炎、提高免疫力等功效。
4 小结在国家大力提倡“绿色养殖”,并限制饲用抗生素使用的大背景下,植物提取物具有天然、低毒性、无污染、无残留的特点,是一种理想的绿色饲料添加剂。但许多天然植物资源有限,难以满足规模化生产。C3G来源广泛,大量存在于植物果实种皮、树皮中,无需人工种植即可满足规模化生产且能实现资源二次开发。明确C3G在体内的吸收代谢途径,找到关键活性代谢产物及其作用靶点,了解代谢产物在体内的相互作用机制,将为实际生产中设计合理的新型配伍饲料添加剂提供理论依据。
| [1] |
WU X L, PRIOR R L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States:fruits and berries[J]. Journal of Agricultural and Food Chemistry, 2005, 53(7): 2589-2599. DOI:10.1021/jf048068b |
| [2] |
杨豆, 张卫波, 赵倩芸, 等. 花青素的生物活性及其在饲料上的应用[J]. 湖南饲料, 2016(3): 25-27. DOI:10.3969/j.issn.1673-7539.2016.03.012 |
| [3] |
MA M M, LI Y, LIU X Y, et al. Cyanidin-3-O-glucoside ameliorates lipopolysaccharide-induced injury both in vivo and in vitro suppression of NF-κB and MAPK pathways[J]. Inflammation, 2015, 38(4): 1669-1682. DOI:10.1007/s10753-015-0144-y |
| [4] |
HE Y, HU Y F, JIANG X W, et al. Cyanidin-3-O-glucoside inhibits the UVB-induced ROS/COX-2 pathway in HaCaT cells[J]. Journal of Photochemistry and Photobiology B:Biology, 2017, 177: 24-31. DOI:10.1016/j.jphotobiol.2017.10.006 |
| [5] |
WALLACE T C. Anthocyanins in cardiovascular disease[J]. Advances in Nutrition, 2011, 2(1): 1-7. |
| [6] |
MIN J Y, YU S W, BAEK S H, et al. Neuroprotective effect of cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral ischemia[J]. Neuroscience Letters, 2011, 500(3): 157-161. DOI:10.1016/j.neulet.2011.05.048 |
| [7] |
GUO H H, GUO J B, JIANG X W, et al. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia:involvement of FoxO1-mediated transcription of adipose triglyceride lipase[J]. Food and Chemical Toxicology, 2012, 50(9): 3040-3047. DOI:10.1016/j.fct.2012.06.015 |
| [8] |
KAY C D, KROON P A, CASSIDY A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products[J]. Molecular Nutrition & Food Research, 2009, 53(S1): S92-S101. |
| [9] |
MIYAZAWA T, NAKAGAWA K, KUDO M, et al. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3, 5-diglucoside, into rats and humans[J]. Journal of Agricultural and Food Chemistry, 1999, 47(3): 1083-1091. DOI:10.1021/jf9809582 |
| [10] |
ICHIYANAGI T, SHIDA Y, RAHMAN M M, et al. Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats[J]. Journal of Agricultural and Food Chemistry, 2006, 54(18): 6578-6587. DOI:10.1021/jf0602370 |
| [11] |
MARCZYLO T H, COOKE D, BROWN K, et al. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice[J]. Cancer Chemotherapy and Pharmacology, 2009, 64(6): 1261-1268. DOI:10.1007/s00280-009-0996-7 |
| [12] |
HANSKE L, ENGST W, LOH G, et al. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats[J]. The British Journal of Nutrition, 2013, 109(8): 1433-1441. DOI:10.1017/S0007114512003376 |
| [13] |
DE FERRARS R M, CZANK C, ZHANG Q, et al. The pharmacokinetics of anthocyanins and their metabolites in humans[J]. British Journal of Pharmacology, 2014, 171(13): 3268-3282. DOI:10.1111/bph.12676 |
| [14] |
KAY C D, MAZZA G J, HOLUB B J. Anthocyanins exist in the circulation primarily as metabolites in adult men[J]. The Journal of Nutrition, 2005, 135(11): 2582-2588. DOI:10.1093/jn/135.11.2582 |
| [15] |
DE FERRARS R M, CASSIDY A, CURTIS P, et al. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women[J]. Molecular Nutrition & Food Research, 2014, 58(3): 490-502. |
| [16] |
CZANK C, CASSIDY A, ZHANG Q Z, et al. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside:a 13C-tracer study[J]. The American Journal of Clinical Nutrition, 2013, 97(5): 995-1003. DOI:10.3945/ajcn.112.049247 |
| [17] |
HASSIMOTTO N M A, GENOVESE M I, LAJOLO F M. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats[J]. Nutrition Research, 2008, 28(3): 198-207. DOI:10.1016/j.nutres.2007.12.012 |
| [18] |
FELGINES C, KRISA S, MAURAY A, et al. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse[J]. British Journal of Nutrition, 2010, 103(12): 1738-1745. DOI:10.1017/S0007114510000061 |
| [19] |
MILBURY P E, KALT W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier[J]. Journal of Agricultural and Food Chemistry, 2010, 58(7): 3950-3956. DOI:10.1021/jf903529m |
| [20] |
KALT W, BLUMBERG J B, MCDONALD J E, et al. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs[J]. Journal of Agricultural and Food Chemistry, 2008, 56(3): 705-712. DOI:10.1021/jf071998l |
| [21] |
FELGINES C, TEXIER O, GARCIN P, et al. Tissue distribution of anthocyanins in rats fed a blackberry anthocyanin-enriched diet[J]. Molecular Nutrition & Food Research, 2009, 53(9): 1098-1103. |
| [22] |
TALAVEÉRA S, FELGINES C, TEXIER O, et al. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats[J]. The Journal of Nutrition, 2003, 133(12): 4178-4182. DOI:10.1093/jn/133.12.4178 |
| [23] |
WOODWARD G M, NEEDS P W, KAY C D. Anthocyanin-derived phenolic acids form glucuronides following simulated gastrointestinal digestion and microsomal glucuronidation[J]. Molecular Nutrition & Food Research, 2011, 55(3): 378-386. |
| [24] |
MILBURY P E, CAO G H, PRIOR R L, et al. Bioavailablility of elderberry anthocyanins[J]. Mechanisms of Ageing and Development, 2002, 123(8): 997-1006. DOI:10.1016/S0047-6374(01)00383-9 |
| [25] |
CAI H, THOMASSET S C, BERRY D P, et al. Determination of anthocyanins in the urine of patients with colorectal liver metastases after administration of bilberry extract[J]. Biomedical Chromatography, 2011, 25(6): 660-663. DOI:10.1002/bmc.v25.6 |
| [26] |
PASSAMONTI S, VRHOVSEK U, VANZO A, et al. The stomach as a site for anthocyanins absorption from food[J]. FEBS Letters, 2003, 544(1/2/3): 210-213. |
| [27] |
TALAVÉRA S, FELGINES C, TEXIER O, et al. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain[J]. Journal of Agricultural and Food Chemistry, 2005, 53(10): 3902-3908. DOI:10.1021/jf050145v |
| [28] |
FANG J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa:extensive Presystemic metabolism reduces apparent bioavailability[J]. Journal of Agricultural and Food Chemistry, 2014, 62(18): 3904-3911. DOI:10.1021/jf405356b |
| [29] |
MATUSCHEK M C, HENDRIKS W H, MCGHIE T K, et al. The jejunum is the main site of absorption for anthocyanins in mice[J]. The Journal of Nutritional Biochemistry, 2006, 17(1): 31-36. DOI:10.1016/j.jnutbio.2005.04.005 |
| [30] |
KEPPLER K, HUMPF H U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora[J]. Bioorganic & Medicinal Chemistry, 2005, 13(17): 5195-5205. |
| [31] |
TALAVEÉRA S, FELGINES C, TEXIER O, et al. Anthocyanins are efficiently absorbed from the small intestine in rats[J]. The Journal of Nutrition, 2004, 134(9): 2275-2279. DOI:10.1093/jn/134.9.2275 |
| [32] |
FELGINES C, TALAVÉRA S, TEXIER O, et al. Absorption and metabolism of red orange juice anthocyanins in rats[J]. British Journal of Nutrition, 2006, 95(5): 898-904. DOI:10.1079/BJN20061728 |
| [33] |
ZOU T B, FENG D, SONG G, et al. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-O-β-glucoside in Caco-2 cells[J]. Nutrients, 2014, 6(10): 4165-4177. DOI:10.3390/nu6104165 |
| [34] |
FELGINES C, TALAVÉRA S, TEXIER O, et al. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans[J]. Journal of Agricultural and Food Chemistry, 2005, 53(20): 7721-7727. DOI:10.1021/jf051092k |
| [35] |
ICHIYANAGI T, SHIDA Y, RAHMAN M M, et al. Metabolic pathway of cyanidin 3-O-β-D-glucopyranoside in rats[J]. Journal of Agricultural and Food Chemistry, 2005, 53(1): 145-150. DOI:10.1021/jf0485943 |
| [36] |
ICHIYANAGI T, SHIDA Y, RAHMAN M M, et al. Extended glucuronidation is another major path of cyanidin 3-O-β-D-glucopyranoside metabolism in rats[J]. Journal of Agricultural and Food Chemistry, 2005, 53(18): 7312-7319. DOI:10.1021/jf051002b |
| [37] |
TSUDA T, HORIO F, OSAWA T. Absorption and metabolism of cyanidin 3-O-β-D-glucoside in rats[J]. FEBS Letters, 1999, 449(2/3): 179-182. |
| [38] |
FLESCHHUT J, KRATZER F, RECHKEMMER G, et al. Stability and biotransformation of various dietary anthocyanins in vitro[J]. European Journal of Nutrition, 2006, 45(1): 7-18. DOI:10.1007/s00394-005-0557-8 |
| [39] |
VITAGLIONE P, DONNARUMMA G, NAPOLITANO A, et al. Protocatechuic acid is the major human metabolite of cyanidin-glucosides[J]. The Journal of Nutrition, 2007, 137(9): 2043-2048. DOI:10.1093/jn/137.9.2043 |
| [40] |
WU X L, PITTMAN Ⅲ H E, PRIOR R L. Pelargonidin is absorbed and metabolized differently than cyanidin after marionberry consumption in pigs[J]. The Journal of Nutrition, 2004, 134(10): 2603-2610. DOI:10.1093/jn/134.10.2603 |
| [41] |
KALT W, LIU Y, MCDONALD J E, et al. Anthocyanin metabolites are abundant and persistent in human urine[J]. Journal of Agricultural and Food Chemistry, 2014, 62(18): 3926-3934. DOI:10.1021/jf500107j |
| [42] |
HIDALGO M, ORUNA-CONCHA M J, KOLIDA S, et al. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth[J]. Journal of Agricultural and Food Chemistry, 2012, 60(15): 3882-3890. DOI:10.1021/jf3002153 |
| [43] |
WILLIAMSON G, CLIFFORD M N. Colonic metabolites of berry polyphenols:the missing link to biological activity?[J]. British Journal of Nutrition, 2010, 104(Suppl.3): S48-S66. |
| [44] |
GONZALEZ-BARRIO R, EDWARDS C A, CROZIER A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins:in vivo and in vitro studies[J]. Drug Metabolism and Disposition, 2011, 39(9): 1680-1688. DOI:10.1124/dmd.111.039651 |
| [45] |
CASSIDY A, ROGERS G, PETERSON J J, et al. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults[J]. The American Journal of Clinical Nutrition, 2015, 102(1): 172-181. DOI:10.3945/ajcn.115.108555 |
| [46] |
EDIRISINGHE I, BANASZEWSKI K, CAPPOZZO J, et al. Strawberry anthocyanin and its association with postprandial inflammation and insulin[J]. British Journal of Nutrition, 2011, 106(6): 913-922. DOI:10.1017/S0007114511001176 |
| [47] |
ZHU Y, LING W, GUO H, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia:a randomized controlled trial[J]. Nutrition, Metabolism and Cardiovascular Diseases, 2013, 23(9): 843-849. DOI:10.1016/j.numecd.2012.06.005 |
| [48] |
RODRIGUEZ-MATEOS A, RENDEIRO C, BERGILLOS-MECA T, et al. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function:a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity[J]. The American Journal of Clinical Nutrition, 2013, 98(5): 1179-1191. DOI:10.3945/ajcn.113.066639 |
| [49] |
HASSELLUND S S, FLAA A, KJELDSEN S E, et al. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men:a double-blind randomized placebo-controlled crossover study[J]. Journal of Human Hypertension, 2013, 27(2): 100-106. |
| [50] |
SUN C D, ZHENG Y X, CHEN Q J, et al. Purification and anti-tumour activity of cyanidin-3-O-glucoside from Chinese bayberry fruit[J]. Food Chemistry, 2012, 131(4): 1287-1294. DOI:10.1016/j.foodchem.2011.09.121 |
| [51] |
SHIH P H, YEH C T, YEN G C. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells[J]. Food and Chemical Toxicology, 2005, 43(10): 1557-1566. DOI:10.1016/j.fct.2005.05.001 |
| [52] |
REDDY M K, ALEXANDER-LINDO R L, NAIR M G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors[J]. Journal of Agricultural and Food Chemistry, 2005, 53(23): 9268-9273. DOI:10.1021/jf051399j |
| [53] |
AMIN H P, CZANK C, RAHEEM S, et al. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells[J]. Molecular Nutrition & Food Research, 2015, 59(6): 1095-1106. |
| [54] |
KRGA I, MONFOULET L E, KONIC-RISTIC A, et al. Anthocyanins and their gut metabolites reduce the adhesion of monocyte to TNFα-activated endothelial cells at physiologically relevant concentrations[J]. Archives of Biochemistry and Biophysics, 2016, 599: 51-59. DOI:10.1016/j.abb.2016.02.006 |
| [55] |
AMINI A M, SPENCER J P E, YAQOOB P. Effects of pelargonidin-3-O-glucoside and its metabolites on lipopolysaccharide-stimulated cytokine production by THP-1 monocytes and macrophages[J]. Cytokine, 2018, 103: 29-33. DOI:10.1016/j.cyto.2017.12.031 |
| [56] |
WU S S, YANO S, HISANAGA A, et al. Polyphenols from Lonicera caerulea L.berry attenuate experimental nonalcoholic steatohepatitis by inhibiting proinflammatory cytokines productions and lipid peroxidation[J]. Molecular Nutrition & Food Research, 2017, 61(4): 1600858. |
| [57] |
MIN S W, RYU S N, KIM D H. Anti-inflammatory effects of black rice, cyanidin-3-O-β-D-glycoside, and its metabolites, cyanidin and protocatechuic acid[J]. International Immunopharmacology, 2010, 10(8): 959-966. DOI:10.1016/j.intimp.2010.05.009 |
| [58] |
SEMAMING Y, PANNENGPETCH P, CHATTIPAKORN S C, et al. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine[J]. Evidence-Based Complementary and Alternative Medicine, 2015, 2015: 593902. |
| [59] |
JAYARAMAN P, SAKHARKAR M K, LIM C S, et al. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro[J]. International Journal of Biological Sciences, 2010, 6(6): 556-568. |
| [60] |
SROKA Z, CISOWSKI W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids[J]. Food and Chemical Toxicology, 2003, 41(6): 753-758. DOI:10.1016/S0278-6915(02)00329-0 |
| [61] |
SHI G F, AN L J, JIANG B, et al. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo[J]. Neuroscience Letters, 2006, 403(3): 206-210. DOI:10.1016/j.neulet.2006.02.057 |
| [62] |
VARÌ R, D'ARCHIVIO M, FILESI C, et al. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages[J]. Journal of Nutritional Biochemistry, 2011, 22(5): 409-417. DOI:10.1016/j.jnutbio.2010.03.008 |
| [63] |
BLANCHARD C, POUCHAIN D, VANDERKAM P, et al. Efficacy of phloroglucinol for treatment of abdominal pain:a systematic review of literature and meta-analysis of randomised controlled trials versus placebo[J]. European Journal of Clinical Pharmacology, 2018, 74(5): 541-548. DOI:10.1007/s00228-018-2416-6 |
| [64] |
KIM M M, KIM S K. Effect of phloroglucinol on oxidative stress and inflammation[J]. Food and Chemical Toxicology, 2010, 48(10): 2925-2933. DOI:10.1016/j.fct.2010.07.029 |
| [65] |
KANG K A, LEE K H, CHAE S, et al. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation[J]. Journal of Cellular Biochemistry, 2006, 97(3): 609-620. DOI:10.1002/(ISSN)1097-4644 |
| [66] |
LI N S, LUO X J, ZHANG Y S, et al. Phloroglucinol protects gastric mucosa against ethanol-induced injury through regulating myeloperoxidase and catalase activities[J]. Fundamental & Clinical Pharmacology, 2011, 25(4): 462-468. |
| [67] |
KIM M C, KIM S J, KIM D S, et al. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages[J]. Immunopharmacology and Immunotoxicology, 2011, 33(3): 525-532. DOI:10.3109/08923973.2010.547500 |
| [68] |
ANBALAGAN V, RAJU K, SHANMUGAM M. Assessment of lipid peroxidation and antioxidant status in vanillic acid treated 7, 12-dimethylbenz[J]. Journal of Clinical and Diagnostic Research, 2017, 11(3): BF01-BF04. |
| [69] |
PRINCE P S M, RAJAKUMAR S, DHANASEKAR K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats[J]. European Journal of Pharmacology, 2011, 668(1/2): 233-240. |
| [70] |
KUMAR S, PRAHALATHAN P, SARAVANAKUMAR M, et al. Vanillic acid prevents the deregulation of lipid metabolism, endothelin 1 and up regulation of endothelial nitric oxide synthase in nitric oxide deficient hypertensive rats[J]. European Journal of Pharmacology, 2014, 743: 117-125. DOI:10.1016/j.ejphar.2014.09.010 |
| [71] |
AMIN F U, SHAH S A, KIM M O. Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice[J]. Scientific Reports, 2017, 7: 40753. DOI:10.1038/srep40753 |
| [72] |
WOLSZLEGER M, STAN C D, APOTROSOAEI M, et al. New hydrazones of ferulic acid:synthesis, characterization and biological activity[J]. Revista Medico-Chirurgicalǎ A Societǎtii De Medici Şi Naturalişti Din Iaşi, 2014, 118(4): 1150-1156. |
| [73] |
CATINO S, PACIELLO F, MICELI F, et al. Ferulic acid regulates the Nrf2/heme oxygenase-1 system and counteracts trimethyltin-induced neuronal damage in the human neuroblastoma cell line SH-SY5Y[J]. Frontiers in Pharmacology, 2015, 6: 305. |
| [74] |
ALAM M A, SERNIA C, BROWN L. Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats[J]. Journal of Cardiovascular Pharmacology, 2013, 61(3): 240-249. DOI:10.1097/FJC.0b013e31827cb600 |
| [75] |
SONG Y, WEN L N, SUN J X, et al. Cytoprotective mechanism of ferulic acid against high glucose-induced oxidative stress in cardiomyocytes and hepatocytes[J]. Food & Nutrition Research, 2016, 60: 30323. |
| [76] |
YIN J, WU M M, XIAO H, et al. Development of an antioxidant system after early weaning in piglets[J]. Journal of Animal Science, 2014, 92(2): 612-619. DOI:10.2527/jas.2013-6986 |
| [77] |
LIU L L, HE J H, XIE H B, et al. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens[J]. Poultry Science, 2014, 93(1): 54-62. DOI:10.3382/ps.2013-03423 |
| [78] |
HE S S, GUO Y H, ZHAO J X, et al. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway[J]. International Journal of Hyperthermia, 2018, 1-10. DOI:10.1080/02656736.2018.1483534 |
| [79] |
HE S S, LIU F H, XU L, et al. Protective effects of Ferulic acid against heat stress-induced intestinal epithelial barrier dysfunction in vitro and in vivo[J]. PLoS One, 2016, 11(2): e0145236. DOI:10.1371/journal.pone.0145236 |
| [80] |
PEI Y F, YANG L, WU L, et al. Combined effect of apigenin and ferulic acid on frozen-thawed boar sperm quality[J]. Animal Science Journal, 2018, 89(7): 956-965. DOI:10.1111/asj.2018.89.issue-7 |
| [81] |
AFFONSO F J, CARVALHO H F, LANÇONI R, et al. Addition of antioxidants myoinositol, ferulic acid, and melatonin and their effects on sperm motility, membrane integrity, and reactive oxygen species production in cooled equine semen[J]. Journal of Equine Veterinary Science, 2017, 59: 57-63. DOI:10.1016/j.jevs.2017.09.006 |
| [82] |
LI Y J, LI L Y, LI J L, et al. Effects of dietary supplementation with ferulic acid or vitamin E individually or in combination on meat quality and antioxidant capacity of finishing pigs[J]. Asian-Australasian Journal of Animal Sciences, 2015, 28(3): 374-381. DOI:10.5713/ajas.14.0432 |
| [83] |
MACÍAS-CRUZ U, VICENTE-PÉREZ R, LÓPEZ-BACA M A, et al. Effects of dietary ferulic acid on reproductive function and metabolism of pre-pubertal hairbreed ewes during the anestrous season[J]. Theriogenology, 2018, 119: 220-224. DOI:10.1016/j.theriogenology.2018.07.012 |
| [84] |
YU L J, WU F, JIANG M, et al. Ferulic acid:a natural compound as an efficient feed additive for GIFT (Oreochromis niloticus)[J]. Aquaculture Nutrition, 2018, 24(1): 27-35. DOI:10.1111/anu.12529 |
| [85] |
VAN DEN BERG T P. Acute infectious bursal disease in poultry:a review[J]. Avian Pathology, 2000, 29(3): 175-194. DOI:10.1080/03079450050045431 |
| [86] |
OU C B, PANG Q, CHEN X, et al. Protocatechuic acid, a new active substance against the challenge of avian infectious bursal disease virus[J]. Poultry Science, 2012, 91(7): 1604-1609. DOI:10.3382/ps.2011-02069 |