2. 河南牧业经济学院动物科技学院, 郑州 450046;
3. 农业部饲料生物技术重点实验室, 北京 100081;
4. 中国农业科学院北京畜牧兽医研究所, 北京 100081
2. College of Animal and Technology, Henan University of Animal Husbandry and Economy, Zhengzhou 450046, China;
3. Key Laboratory of Feed Biotechnology of Ministry of Agriculture, Beijing 100081, China;
4. Institute of Animal Science, Chinese Academy of Agriculture Sciences, Beijing 100081, China
维生素是机体为维持正常生理功能而必须从食物中获得的一类微量有机物质,在生长、代谢、发育过程中发挥着重要的作用[1-4]。饲粮中常添加维生素以满足动物营养需要,然而,目前饲料加工过程常为湿热加工处理,饲粮配方中的热敏性饲料成分在饲料加工过程中其生物学活性将受到影响,目前往往采用过量添加的方式来弥补饲料加工过程中的损失,以期达到预期的效果,但这种方式成本高、浪费大。采用高效调质冷却后再低温制粒的畜禽饲料生产新工艺可克服这个难题,最大程度保留热敏性物质的活性。
段海涛等[5]研究了饲料加工工艺及维生素添加量对肉鸡生长性能及屠宰性能的影响,结果发现肉鸡饲粮采用高效调质低温制粒工艺,颗粒饲料的加工质量优于普通饲料加工工艺,且配方中减少维生素添加量对肉鸡生长性能和屠宰性能的影响与普通饲料加工工艺无显著差异,即高效调质低温制粒工艺可节约维生素使用量。
然而,饲料加工工艺与维生素添加量对猪生长性能影响的研究却较少,且已有研究液较少考虑到饲料加工工艺对维生素含量的影响。因此,本试验综合考虑饲料加工工艺类型及维生素添加量对猪生长性能的影响,对照组采用普通畜禽饲料加工工艺,配方中添加正常剂量复合维生素,试验组采用高效调质低温制粒工艺,减少配方中复合维生素添加量,对试验组与对照组猪生长性能、血液指标及营养物质表观消化率进行对比分析,旨在证明采用高效调质低温制粒工艺加工饲料可以减少维生素的添加量且不影响猪的生长性能,可达到节约维生素等热敏性饲料原料使用量的目的。
1 材料与方法 1.1 试验设计试验共设4个组,对照组饲粮采用普通加工工艺生产,调质时间约为30 s,调质温度为80 ℃;3个试验组饲粮采用高效调质低温制粒工艺生产,调质时间约为30 s,高温调质温度为80 ℃,熟化粉状料冷却后添加预混料等热敏性饲料添加剂低温制粒,调质时间约为30 s,低温调质温度为60 ℃。对照组饲粮添加正常剂量的复合维生素(生长期350 mg/kg、育肥期200 mg/kg,作为复合维生素添加量A组),试验1组饲粮复合维生素添加量与对照组相同,同时作为复合维生素添加量A组,试验2组和试验3组饲粮复合维生素添加量分别较对照组降低20%和40%(试验2组:生长期280 mg/kg、育肥期160 mg/kg,作为复合维生素添加量B组;试验3组:生长期210 mg/kg、育肥期120 mg/kg,作为复合维生素添加量C组)。试验所用基础饲粮组成及营养水平见表 1,复合维生素组成见表 2。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of basal diets (air-dry basis) |
|
|
表 2 复合维生素组成 Table 2 Multivitamin composition |
试验选用胎次一致且体重(30 kg)相近的“杜×长×大”三元杂交试验猪,按照体重相近和性别比例分为4个组,每组5个重复,每个重复4头猪,进行14周的饲养试验(生长期6周,育肥期8周)。饲养试验在中国农业科学院南口养殖基地进行。试验期间猪只自由采食、自由饮水,保持猪舍清洁和通风,并定期消毒。
1.3 饲粮样品采集对照组在制粒工段调质前和制粒机出料口各取样3次,试验组在大料混合料调质后,低温制粒调质前和制粒机出料口各取样3次,湿热粉料、颗粒料摊开变凉后采用“四分法”逐渐缩减至2 kg,装入自封袋中于4 ℃冰箱保存待测。
1.4 检测指标与方法 1.4.1 淀粉糊化度样品的淀粉糊化度参照熊易强[6]介绍的简易酶法测定。
1.4.2 颗粒硬度颗粒硬度参照《饲料检验化验员》[7]中颗粒饲料硬度的测定方法检测。
1.4.3 颗粒耐久性指数(PDI)PDI参照Thomas等[8]的方法测定,具体如下:取500 g筛分后的颗粒饲料装入回转箱内,以50 r/min回转10 min,停止后取出样品,称取颗粒饲料重量(m1)。
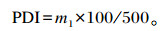
|
分别于试验第6周与第14周结束前1天晚上开始控料,自由饮水,使试验猪空腹24 h,于第6周与第14周末早上逐只称重,以重复为单位计算各组试验猪的平均体重。准确记录每天的耗料量,出现死猪时截料称重,计算各阶段总耗料量。
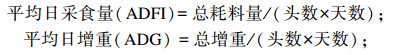
|
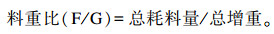
|
于试验第14周末,每组随机抽取4头猪,颈静脉空腹采集血液5~10 mL,分别存放于抗凝管(用于制备血浆)及促凝管(用于制备血清)中,3 000 r/min离心5 min,制备血清与血浆,分别测定血清中总蛋白(TP)、白蛋白(ALB)、球蛋白(GLB)、免疫球蛋白G(IgG)、免疫球蛋白M(IgM)、免疫球蛋白A(IgA)及葡萄糖(GLU)含量与谷丙转氨酶(ALT)、谷草转氨酶(AST)活性,以及血浆尿素氮(UN)含量,所用仪器为科华ZYKHB-1280全自动生化仪。
1.4.6 营养物质表观消化率分别在试验第6周与第14周结束前的最后3 d采集新鲜的粪样,将采集的粪样混合均匀,加入10%的盐酸固氮处理,然后于65 ℃的烘箱内干燥72 h,室内回潮24 h制成风干样,将风干样粉碎后过40目筛,保存待测。
营养物质表观消化率采用内源指示剂法测定,用4 mol/L盐酸不溶灰分作为内源指示剂。
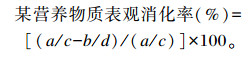
|
式中:a为饲粮中该营养物质含量(%);b为粪中该营养物质含量(%);c为饲粮中盐酸不溶灰分含量(%);d为粪中盐酸不溶灰分含量(%)。
盐酸不溶灰分含量按照盐酸消煮法测定,粗蛋白质含量参照GB/T 6432—1994通过凯氏定氮法测定,干物质含量参照GB/T 6435—2006测定。
1.5 数据处理与分析试验数据以平均值±标准差形式表示。采用软件SAS 9.2进行2×3双因子试验统计分析,加工工艺对试验结果的影响采用配对法t检验,维生素添加量对试验结果的影响采用单因素方差分析,用Duncan氏法多重比较检验组间差异显著性,显著性水平为P<0.05。
2 结果 2.1 饲料加工工艺对生长育肥猪颗粒饲料加工质量的影响表 3为饲料加工工艺对生长猪颗粒饲料加工质量的影响,由表中数据可知,普通加工工艺组颗粒硬度及淀粉糊化度显著低于高效调质低温制粒工艺组(P<0.05)。
|
|
表 3 饲料加工工艺对生长猪颗粒饲料加工质量的影响 Table 3 Effects of feed processing technology on pellet feed processing quality of growing pigs |
表 4为饲料加工工艺对育肥猪颗粒饲料加工质量的影响,由表中数据可知,普通加工工艺组颗粒硬度显著低于高效调质低温制粒工艺组(P<0.05)。
|
|
表 4 饲料加工工艺对育肥猪颗粒饲料加工质量的影响 Table 4 Effects of feed processing technology on pellet feed processing quality of finishing pigs |
表 5为饲料加工工艺与复合维生素添加量对生长猪生长性能的影响,由表中数据可知,高效调质低温制粒工艺组生长猪末重高于普通加工工艺组,料重比略低于普通加工工艺组,但2组间差异均不显著(P>0.05);生长猪的各生长性能指标在不同复合维生素添加量组间均差异不显著(P>0.05),但B组生长性能略好于其余2组。
|
|
表 5 饲料加工工艺与复合维生素添加量对生长猪生长性能的影响 Table 5 Effects of feed processing technology and multivitamin additive amount on growth performance of growing pigs |
表 6为饲料加工工艺与复合维生素添加量对育肥猪生长性能的影响,由表中数据可知,高效调质低温制粒工艺组育肥猪末重显著高于普通加工工艺组(P<0.05);育肥猪的各生长性能指标在不同复合维生素添加量组间均差异不显著(P>0.05),但B组的末重高于其余2组,料重比略低于其余2组。
|
|
表 6 饲料加工工艺与复合维生素添加量对育肥猪生长性能的影响 Table 6 Effects of feed processing technology and multivitamin additive amount on growth performance of finishing pigs |
表 7为饲料加工工艺与复合维生素添加量对生长育肥猪生长性能的影响,由表中数据可知,高效调质低温制粒工艺组生长育肥猪末重显著高于普通加工工艺组(P<0.05),料重比低于普通加工工艺组,但差异不显著(P>0.05);不同复合维生素添加量组之间,B组末重高于其余2组,料重比略低于其余2组,但差异均不显著(P>0.05)。
|
|
表 7 饲料加工工艺与复合维生素添加量对生长育肥猪生长性能的影响 Table 7 Effects of feed processing technology and multivitamin additive amount on growth performance of growing and finishing pigs |
表 8为饲料加工工艺与复合维生素添加量对生长猪营养物质表观消化率的影响,由表中数据可知,高效调质低温制粒工艺组的粗蛋白质、干物质表观消化率均显著高于普通加工工艺组(P<0.05);不同复合维生素添加量组间,B组的粗蛋白质、干物质表观消化率显著高于A组及C组(P<0.05)。
|
|
表 8 饲料加工工艺与复合维生素添加量对生长猪营养物质表观消化率的影响 Table 8 Effects of feed processing technology and multivitamin additive amount on nutrient apparent digestibility of growing pigs |
表 9为饲料加工工艺与复合维生素添加量对育肥猪营养物质表观消化率的影响,由表中数据可知,高效调质低温制粒工艺组的粗蛋白质、干物质表观消化率与普通加工工艺组无显著差异(P>0.05);不同复合维生素添加量组间,B组的干物质表观消化率显著高于A组及C组(P<0.05),A组、B组及C组间粗蛋白质表观消化率差异不显著(P>0.05)。
|
|
表 9 饲料加工工艺与复合维生素添加量对育肥猪营养物质表观消化率的影响 Table 9 Effects of feed processing technology and multivitamin additive amount on nutrient apparent digestibility of finishing pigs |
表 10和表 11为饲料加工工艺与复合维生素添加量对育肥猪血液指标的影响,由表中数据可知,普通加工工艺组血清IgA、GLU含量与ALT、AST活性显著高于高效调质低温制粒工艺组(P<0.05),其余指标2组间差异不显著(P>0.05);不同复合维生素添加量组间,B组血清IgM含量显著高于C组(P<0.05),A组血清ALT、AST活性显著高于其余2组(P<0.05)。
|
|
表 10 饲料加工工艺与复合维生素添加量对育肥猪血清TP、ALB、GLB、IgG、IgM和IgA含量的影响 Table 10 Effects of feed processing technology and multivitamin additive amount on serum TP, ALB, GLB, IgG, IgM and IgA contents of finishing pigs |
|
|
表 11 饲料加工工艺与复合维生素添加量对育肥猪血清ALT、AST活性与GLU含量及血浆UN含量的影响 Table 11 Effects of feed processing technology and multivitamin additive amount on serum ALT, AST activities and GLU content and plasma UN content of finishing pigs |
合理的配方、优质的饲料原料,只有在性能可靠的加工设备和科学的工艺流程下才能生产出优质的饲料,一旦配方确定,加工工艺是影响颗粒饲料加工质量的重要因素[9-10]。目前,畜禽饲料加工工艺主要采用普通畜禽饲料加工工艺、大料膨胀(膨化)低温制粒工艺、二次制粒工艺及清洁粉状料加工工艺,其中大料膨胀(膨化)低温制粒工艺及二次制粒工艺常应用于乳猪料生产,孙杰[11]曾系统地对比分析了断奶仔猪料加工工艺对颗粒饲料加工质量及断奶仔猪生长性能的影响,研究发现,饲料加工工艺的不同对颗粒饲料加工质量及断奶仔猪生长性能具有显著影响。清洁粉状料加工工艺常应用于蛋鸡料生产,杨德川等[12]介绍,在畜禽饲料生产中清洁粉状料加工工艺逐渐得到推广使用,采用该工艺可生产优质蛋产品。本试验中,对照组饲粮采用普通畜禽饲料加工工艺生产,试验组饲粮采用大料高效调质低温制粒工艺生产,结果发现高效调质低温制粒工艺组的淀粉糊化度显著高于普通加工工艺组。影响颗粒饲料加工质量的核心因素在于淀粉的凝胶化和蛋白质的黏结性[13-14],由粗蛋白质表观消化率可知,高效调质低温制粒工艺组蛋白质变性程度较大,淀粉糊化度较高,因此该组颗粒饲料加工质量优于普通加工工艺组。
3.2 饲料加工工艺与复合维生素添加量对生长育肥猪生长性能的影响维生素在机体生长、代谢、发育过程中发挥重要作用,参与机体代谢及免疫[15-17]。单胃动物体内一般不能合成维生素,在生产过程中需要从饲料中摄取。饲料加工虽然可以提高原料的利用率,但也将对维生素造成较大程度的破坏[18]。Lewis等[19]曾研究了调质温度及调质时间对维生素保留率的影响,结果发现,调质温度对维生素的保留率呈弱显著性,调质温度为88 ℃时维生素保留率弱显著低于调质温度为77 ℃。不仅调质温度、调质时间对维生素保留率具有显著影响,饲料加工工艺对维生素保留率同样具有显著影响。严芳芳[20]曾系统研究了不同加工工艺及加工工段对鱼类饲料维生素保留率的影响,结果发现,制粒工艺中维生素C晶体损失率达71%,其中调质工段维生素损失率最高。石永峰[21]报道,一般情况下,饲料调质稳定达到90 ℃时,就会导致大多数维生素的活性降低10%。本试验中,A组复合维生素添加量为厂家推荐添加剂量,采用普通畜禽饲料加工工艺,复合维生素添加量最低组为C组,采用高效调质低温制粒工艺。由试验结果可知,高效调质低温制粒工艺组生长育肥猪的到末重高于普通加工工艺组;复合维生素添加量B组生长育肥猪的末重高于其余2组,这可能是因为高效调质低温制粒工艺生产的颗粒饲料加工质量优于普通畜禽饲料加工工艺,同时,该工艺减少了热敏性饲料原料损失率,但复合维生素添加量过少可能引起生长性能下降[18, 22-24],这与试验结果中颗粒饲料加工质量及营养物质表观消化率相对应。由此可知,采用高效调质低温制粒工艺一定程度上可以减少维生素预混料的使用量。
3.3 饲料加工工艺与复合维生素添加量对育肥猪血液指标的影响动物血液指标与机体代谢、营养状况及疾病有密切关系,当发生生理或病理变化时,能够第一时间从血液指标中反映出来[25-26]。TP的主要成分是ALB和GLB,血清TP含量是机体蛋白质代谢旺盛与否的重要依据,主要反映肝脏合成功能的高低[27-28]。本试验中,血清TP、ALB及GLB含量2种加工工艺组间以及3个复合维生素添加量组间差异均不显著,表明饲料加工工艺与复合维生素添加量均未对育肥猪肝脏合成功能产生影响。ALT和AST是动物体内参与转氨基作用活性最高的2种酶类,其活性的改变往往是肝脏受损的一种表现[29]。本试验中高效调质低温制粒工艺组血清ALT和AST活性均显著低于普通加工工艺组,表明高效调质低温制粒工艺生成的颗粒饲料对肝脏具有保护作用。血清中IgG、IgA及IgM含量是机体体液免疫能力的体现,IgG是动物血清中含量最高的免疫球蛋白,在初级免疫反应中是最重要的抗体;IgM主要在感染初期发挥免疫作用[30]。本试验中高效调质低温制粒工艺组血清IgA含量显著低于普通加工工艺组,表明普通畜禽饲料加工工艺生成的颗粒饲料引起机体的免疫反应,其颗粒饲料加工质量低于高效调质低温制粒工艺生成的颗粒饲料。血浆UN含量的高低代表机体利用蛋白质效率的高低,血清GLU含量的高低代表机体利用糖效率的高低[31]。本试验中高效调质低温制粒工艺组血清GLU含量低于普通加工工艺组,但2种加工工艺组血清GLU含量均处于正常范围(3.9~6.1 mmol/L)内,表明机体对2种加工工艺生成的颗粒饲料中糖和蛋白质的利用率无显著差异,机体组织器官未表现受损症状。
4 结论① 与普通畜禽饲料加工工艺相比,采用高效调质低温制粒工艺生产生长育肥猪饲粮可节约维生素的使用量,且颗粒饲料加工质量及营养物质表观消化率得到提高,同时生长育肥猪的生长性能无显著差异。
② 从营养物质表观消化率及生长性能方面看,采用高效调质低温制粒工艺后复合维生素添加量可降低20%,即复合维生素添加量由普通畜禽饲料加工工艺的生长期350 mg/kg、育肥期200 mg/kg降低为高效调质低温制粒工艺的生长期280 mg/kg、育肥期160 mg/kg。
| [1] |
王效京, 杨光, 徐凯, 等. 不同剂型维生素对猪生产性能和胴体品质的影响[J]. 畜牧与饲料科学, 2014, 35(12): 37-38, 39. DOI:10.3969/j.issn.1672-5190.2014.12.015 |
| [2] |
唐现文, 张响英. 维生素E在畜禽生产上的应用[J]. 畜禽业, 2001(4): 40-41. DOI:10.3969/j.issn.1008-0414.2001.04.025 |
| [3] |
苏基双. 畜禽维生素的需要量及最适维生素供给量[J]. 中国饲料, 1997(15): 24-26. |
| [4] |
石凤霞, 王继强, 龙强, 等. 维生素C的生理功能及其在畜禽生产中的应用[J]. 中国饲料, 2016(4): 36-38, 44. |
| [5] |
段海涛, 李军国, 葛春雨, 等. 不同饲料加工工艺及维生素添加量对肉仔鸡生长性能和屠宰性能的影响[J]. 动物营养学报, 2018, 30(6): 2128-2135. DOI:10.3969/j.issn.1006-267x.2018.06.015 |
| [6] |
熊易强. 饲料淀粉糊化度(熟化度)的测定[J]. 饲料工业, 2000, 21(3): 30-31. |
| [7] |
顾君华. 饲料检验化验员[M]. 北京: 中国农业出版社, 2010: 488.
|
| [8] |
THOMAS M, VAN VLIET T, VAN DER POEL A F B. Physical quality of pelleted animal feed 3.Contribution of feedstuff components[J]. Animal Feed Science and Technology, 1998, 70(1/2): 59-78. |
| [9] |
TUMULURU J S, CONNER C C, HOOVER A N. Method to produce durable pellets at lower energy consumption using high moisture corn stover and a corn starch binder in a flat die pellet mill[J]. Journal of Visualized Experiments, 2016(112): 54092. |
| [10] |
VON BORRIES-MEDRANO E, JAIME-FONSECA M R, AGUILAR-MÉNDEZ M A. Starch-guar gum extrudates:microstructure, physicochemical properties and in vitro digestion[J]. Food Chemistry, 2016, 194: 891-899. DOI:10.1016/j.foodchem.2015.08.085 |
| [11] |
孙杰.断奶仔猪颗粒料加工工艺比较研究[D].硕士学位论文.北京: 中国农业科学院, 2014: 24-27.
|
| [12] |
杨德川, 韩俊巍, 孟展鹏. 清洁粉状饲料生产工艺及关键设备[J]. 饲料工业, 2011, 32(7): 4-7. |
| [13] |
BENCZDI D, TOMKA I, ESCHER F. Thermodynamics of amorphous starch-water systems.2.Concentration fluctuations[J]. Macromolecules, 1998, 31(9): 3062-3074. DOI:10.1021/ma960950y |
| [14] |
THOMAS M, VAN ZUILICHEM D J, VAN DER POEL A B F. Physical quality of pelleted animal feed.2.Contribution of processes and its conditions[J]. Animal Feed Science and Technology, 1997, 64(2/3/4): 173-792. |
| [15] |
PINKAEW S, WEGMULLER R, HURRELL R. Vitamin A stability in triple fortified extruded, artificial rice grains containing iron, zinc and vitamin A[J]. International Journal of Food Science And Technology, 2012, 47(10): 2212-2220. DOI:10.1111/ijfs.2012.47.issue-10 |
| [16] |
CANNON J E, MORGAN J B, SCHMIDT G R, et al. Growth and fresh meat quality characteristics of pigs supplemented with vitamin E[J]. Journal of Animal Science, 1996, 74(1): 98-105. DOI:10.2527/1996.74198x |
| [17] |
MARCHETTI M, TOSSANI N, MARCHETTI S, et al. Stability of crystalline and coated vitamins during manufacture and storage of fish feeds[J]. Aquaculture Nutrition, 1999, 5(2): 115-120. DOI:10.1046/j.1365-2095.1999.00094.x |
| [18] |
BESWA D, DLAMINI N R, SIWELA M, et al. Effect of Amaranth addition on the nutritional composition and consumer acceptability of extruded provitamin A-biofortified maize snacks[J]. Food Science and Technology, 2016, 36(1): 30-39. |
| [19] |
LEWIS L L, STARK C R, FAHRENHOLZ A C, et al. Evaluation of conditioning time and temperature on gelatinized starch and vitamin retention in a pelleted swine diet[J]. Journal of Animal Science, 2015, 93(2): 615-619. DOI:10.2527/jas.2014-8074 |
| [20] |
严芳芳.不同加工工艺对鱼饲料维生素保留率的影响[D].硕士学位论文.青岛: 中国海洋大学, 2013: 34-37.
|
| [21] |
石永峰. 饲料调质对维生素稳定性的影响[J]. 国外畜牧学(猪与禽), 2007, 27(4): 73-74, 77. |
| [22] |
ANDERSON J S, SUNDERLAND R. Effect of extruder moisture and dryer processing temperature on vitamin C and E and astaxanthin stability[J]. Aquaculture, 2002, 207(1/2): 137-149. |
| [23] |
GUIMARÄES I G, PEZZATO L E, SANTOS V G, et al. Vitamin A affects haematology, growth and immune response of Nile tilapia (Oreochromis niloticus L.), but has no protective effect against bacterial challenge or cold-induced stress[J]. Aquaculture Research, 2016, 47(6): 2004-2018. DOI:10.1111/are.2016.47.issue-6 |
| [24] |
HASHIZAWA Y, KUBOTA M, KADOWAKI M, et al. Effect of dietary vitamin E on broiler meat qualities, color, water-holding capacity and shear force value, under heat stress conditions[J]. Animal Science Journal, 2013, 84(11): 732-736. DOI:10.1111/asj.2013.84.issue-11 |
| [25] |
CHEN Y J, SON K S, MIN B J, et al. Effects of dietary probiotic on growth performance, nutrients digestibility, blood characteristics and fecal noxious gas content in growing pigs[J]. Asian-Australasian Journal of Animal Sciences, 2005, 18(10): 1464-1468. DOI:10.5713/ajas.2005.1464 |
| [26] |
HUANG C, ZANG J J, SONG P X, et al. Effects of particle size and drying methods of corn on growth performance, digestibility and haematological and immunological characteristics of weaned piglets[J]. Archives of Animal Nutrition, 2015, 69(1): 30-45. DOI:10.1080/1745039X.2014.1002673 |
| [27] |
GREWAL R S, SINGLA M, LAMBA J S, et al. Effect of diets varying in energy source on the growth and blood biochemical profile of Beetal kids under stall fed conditions[J]. The Indian Journal of Animal Sciences, 2013, 83(3): 290-294. |
| [28] |
MENG Q W, YAN L, AO X, et al. Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs[J]. Journal of Animal Science, 2010, 88(10): 3320-3326. DOI:10.2527/jas.2009-2308 |
| [29] |
YAN L, KIM I H. Effect of probiotics supplementation in diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, faecal microbial population and faecal noxious gas content in growing pigs[J]. Journal of Applied Animal Research, 2013, 41(1): 23-28. DOI:10.1080/09712119.2012.739092 |
| [30] |
YAN L, WANG J P, KIM H J, et al. Influence of essential oil supplementation and diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, meat quality and fecal noxious gas content in grower-finisher pigs[J]. Livestock Science, 2010, 128(1/2/3): 115-122. |
| [31] |
CHO J H, CHEN Y J, MIN B J, et al. Effects of reducing dietary crude protein on growth performance, odor gas emission from manure and blood urea nitrogen and IGF-1 concentrations of serum in nursery pigs[J]. Animal Science Journal, 2008, 79(4): 453-459. DOI:10.1111/asj.2008.79.issue-4 |




