共轭亚油酸(conjugated linoleic acid, CLA)是一类含有共轭双键亚油酸的位置、几何异构体的统称,属于长链多不饱和脂肪酸。目前CLA家族中已鉴定出有28种同分异构体,其中,以顺9, 反11-CLA(cis9, trans11-CLA,c9, t11-CLA)和反10, 顺12-CLA(trans10, cis12-CLA, t10, c12-CLA)为发挥生理调控功能的最主要的2种异构体。CLA具有抗癌、抗动脉粥样硬化、抗氧化、提高免疫等多种功能[1]。因在降低体脂沉积中的生物学作用,CLA被作为一类重要的生物活性物质,得到了广泛研究。研究显示,饲粮添加CLA能显著降低猪皮下脂肪的沉积[2],增加肌内脂肪的沉积[3]。此外,在育肥期母猪的饲粮中添加CLA,对于改善仔猪体况也有积极作用[4]。饲粮来源的CLA可以沉积到肉鸡的脂肪组织中,并通过抑制成脂分化关键调控因子的活性,减小脂肪细胞体积,从而导致肉鸡腹脂沉积降低[5]。在2005年,Cherian等[6]就发现,在肉种鸡饲粮中添加CLA降低了子代雏鸡胴体的体脂含量,表明母源性CLA也可以调控子代肉鸡脂肪代谢过程。
脂肪组织不仅是机体的能量贮存场所,也是重要的内分泌器官,且代谢功能活跃。脂肪代谢过程受多种因素影响,从而导致其调控机制的复杂性。深入探究CLA在降低脂肪沉积中的分子机制,对于生产中降低脂肪的过量沉积、减少饲料损失、提高生产效益,以及临床上肥胖症、糖尿病等代谢紊乱相关疾病的治疗,均具有重要意义。本文将就CLA调控脂肪代谢的相关机制的研究进展进行综述。
1 CLA在机体内的代谢途径瘤胃微生物可通过发酵作用利用亚油酸、亚麻酸等合成CLA,并沉积入反刍动物的乳脂及肉制品中,成为天然CLA的主要来源[7]。而饲粮来源的CLA可被胰脂酶水解为游离态CLA,以混合乳糜微粒的形式运送至肠绒毛处,经易化作用吸收入肠黏膜细胞,再以乳糜微粒的形式逸出,经乳糜管、淋巴系统、胸导管、血液循环等途径运输至各组织进行代谢或沉积[8]。研究显示,CLA可沉积入大脑、肝脏、肾脏、心脏、脾脏、骨骼肌和脂肪组织中[9-12],而其沉积量也与细胞的类型有关[13]。由于CLA具有抗肥胖的功能,其在脂肪组织中的沉积和对脂肪代谢的调控机制,得到了广泛的研究,而CLA抗脂肪沉积的具体分子机制尚不明确。研究显示,在主要的2种CLA异构体中,t10, c12-CLA是发挥抗肥胖功能的主要异构体[14]。
2 CLA降低脂肪沉积的分子机制 2.1 CLA抑制成脂分化进程对成脂分化进程和脂肪合成的抑制,是CLA降低脂肪沉积的重要机制。饲喂CLA可引起脂肪组织中与成脂分化、脂质合成相关的基因表达或活性的下调。研究显示,t10, c12-CLA沉积入脂肪组织中进行β-氧化[15],通过下调脂肪细胞决定和分化因子1、过氧化物酶增殖子激活受体(peroxisome proliferator activated receptor, PPAR)γ活性而促进硬脂酰辅酶A去饱和酶-1(stearoyl-CoA desaturase-1, SCD-1)的表达,抑制成脂和生脂过程[16-17],且t10, c12-CLA对PPARγ活性的抑制呈剂量依赖性[18]。在小鼠和人的脂肪组织中,t10, c12-CLA通过上调肉毒碱棕榈酰基转移酶-1(carnitine palmityl transferase-1, CPT-1)、PPARα、PPARδ等基因的表达,促进脂肪酸氧化,降低脂肪沉积[19]。在牛原代脂肪细胞中的研究显示,t10, c12-CLA抑制了SCD-1、乙酰辅酶A羧化酶1和脂肪酸合成酶的表达,增加了激素敏感脂酶受体和CPT-1基因的表达,促进了脂肪分解和氧化,减少了脂肪沉积[20]。
已知,成脂分化过程受高度复杂且精密的转录级联网络调控,其中最为核心的调控因子是PPARγ。核受体PPARγ是脂肪细胞成脂分化的关键且必需的转录调控因子,可刺激下游脂蛋白酯酶、围脂滴蛋白、脂肪酸结合蛋白4、脂肪细胞脂肪酸结合蛋白2及胰岛素介导的葡萄糖转运蛋白4等一系列基因的表达,提高细胞的成脂分化能力,加速脂滴的沉积[21]。而PPARγ的缺失将导致细胞成脂分化的终止[22-23]。大量研究均显示,CLA抑制脂肪沉积功能的发挥与PPARγ基因的表达或活性的降低有关[5, 24-26]。而PPARγ的转录活性也受上游多种激酶、磷酸酶的直接调控,因而也导致了CLA调控脂肪代谢的分子机制具有复杂性。
2.2 CLA促进能量代谢根据脂肪细胞结构和功能的差异,通常将脂肪组织分为以储能为主的白色脂肪组织(white adipose tissue, WAT)和以产热为主的棕色脂肪组织(brown adipose tissue, BAT)。BAT细胞中含有丰富的线粒体,并可特异性表达解偶联蛋白(uncoupling protein, UCP),参与能量代谢[27]。米色脂肪细胞是WAT受到外界刺激后产生的一种具有类似BAT功能的细胞,用以提高脂肪组织的散热能力。WAT棕化就是WAT中米色脂肪细胞数量增加的结果[28-29]。
CLA抗肥胖效应的发挥与组织的产热或能量消耗增加有关。研究显示,CLA处理显著促进了小鼠WAT中UCP-1、CPT-1b、环氧合酶-2(cyclooxygenase-2, COX-2)等基因的表达,诱导了WAT棕化[12]。激活BAT中自适应性产热、促进脂肪酸氧化也是t10, c12-CLA降低体脂沉积的一种机制[12, 30-31]。研究发现,饲粮中添加0.5%的CLA上调了小鼠腹膜后脂肪中UCP-1和UCP-2基因的表达,引起机体能量代谢的增加[32]。t10, c12-CLA促进了人脂肪细胞中COX-2基因的表达和前列腺素(prostaglandin, PG)的合成,并诱导了WAT中棕色样脂肪细胞的产生,表明CLA介导的WAT棕化与炎症反应有关[33-35]。但Shen等[31]的研究发现,在肥胖小鼠的WAT中,抑制COX-2基因表达后,仅影响了t10, c12-CLA刺激的炎症基因表达的上调,而对t10, c12-CLA介导的脂肪代谢和棕化标志基因的表达没有显著影响,表明t10, c12-CLA对能量代谢的调控作用不依赖于炎症信号通路。
而CLA诱导WAT适应性产热,与BAT减少导致机体的体温防御机制受到损伤有关。研究显示,饲喂CLA混合物(含0.12% t10, c12-CLA和0.61% c9, t11-CLA)引起了小鼠机体BAT沉积和氧消耗量的减少[12, 36]。给肥胖的雄性小鼠连续3周饲喂含t10, c12-CLA(0.1%)饲粮,损害了机体的体温防御能力[12, 36],表明t10, c12-CLA对体脂沉积特别是BAT沉积的降低引起了WAT的棕化,用以弥补BAT减少引起的能量供应不足[25]。
2.3 CLA激活炎症信号通路脂肪组织不仅是机体的储能器官,也是重要的内分泌器官。除分泌脂联素、瘦素等脂肪细胞因子外,脂肪组织在应激下可分泌白细胞介素6(interleukin-6, IL-6)、肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)、干扰素-γ(interferon-γ, IFN-γ)等多种促炎因子,调控机体的炎症反应,并在抑制细胞成脂分化、介导脂肪细胞去脂化中发挥作用[37-38]。研究显示,饲粮添加0.2%或0.6% CLA异构体混合剂可促进小鼠WAT中单核细胞趋化蛋白-1(monocyte chemoattractant protein-1, MCP-1)、IL-6、TNF-α等炎症标志基因的表达,且该效应与t10, c12-CLA的添加比例有关[12]。膳食中补充t10, c12-CLA可升高人炎症性前列腺素的水平,且上调了新分化的人脂肪细胞中COX-2(与PG合成有关的酶)基因的表达,并促进前列腺素F2α(prostaglandin F2α,PGF2α)的分泌[14, 39-40]。PGF2α可以通过上调丝裂原活化蛋白激酶(mitogen activated protein kinases,MAPKs)通路活性而介导PPARγ磷酸化,也能诱导促炎转录因子(如核转录因子-κB)表达而抑制PPARγ的活性,从而干扰细胞成脂分化进程。此外,PGF2α激活缺氧诱导因子-1而降低PPARγ、CCAAT增强子结合蛋白α的表达[41]。相反,c9, t11-CLA具有抗炎和生脂的效应,并可提高小鼠的胰岛素敏感性[42-43]。
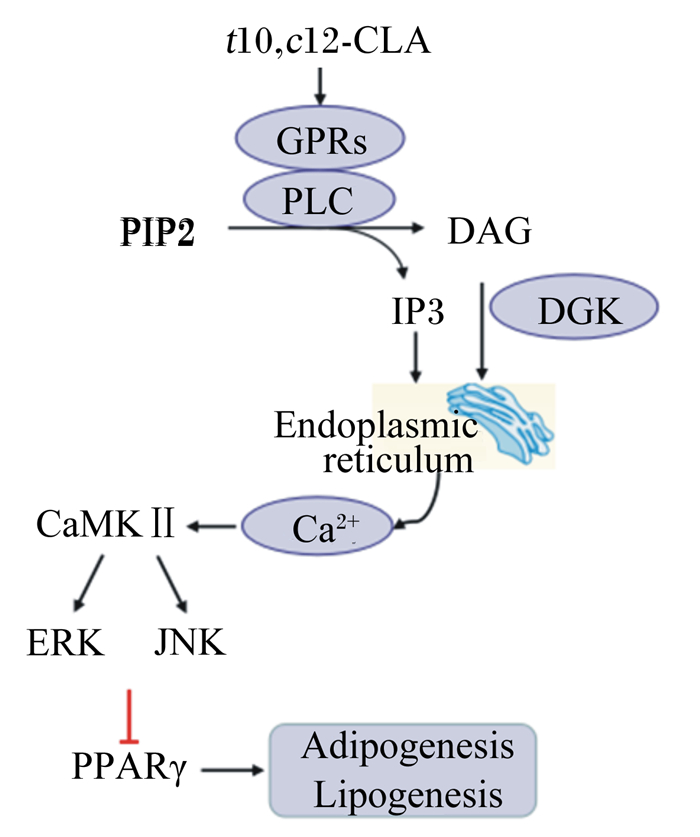
2.4 CLA激活钙离子通路胞内钙离子([Ca2+]i)平衡是活细胞重要的生理基础,也是激活细胞增殖、分化和代谢过程重要的第二信使[44]。胞内[Ca2+]i平衡受多种激酶的调控,并通过介导相关激酶的活性,参与多种细胞代谢过程。研究显示,CLA降低脂肪沉积的过程伴随胞内内质网释放钙离子的增加。MAPKs通路活性受胞内[Ca2+]i及其上游的磷脂酶C(phospholipase C, PLC)、二酰甘油激酶(diacylglycerol kinase, DGK)的调控。在人脂肪细胞中,胞内[Ca2+]i信号通路激活后,通过上调细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)1/2、c-Jun氨基端激酶磷酸化蛋白活性而抑制PPARγ的表达,促进脂解和脂肪酸氧化进程,介导t10, c12-CLA去脂化[15, 45-46]。但t10, c12-CLA的这种去脂化机制,在PLC、DGK抑制剂处理后发生了逆转[47-48],表明t10, c12-CLA通过胞内[Ca2+]i通路调控脂肪代谢的过程受PLC、DGK活性的影响。
2.5 CLA诱导细胞凋亡细胞凋亡是指为了维持细胞稳态,细胞主动结束生命进程的过程,受多种基因的激活、表达的调控。以小鼠或3T3-L1细胞为模型的研究显示,t10, c12-CLA或CLA的异构体混合剂均可诱导脂肪细胞发生凋亡[32, 49]。在高脂饲粮中添加1.5% CLA提高了小鼠Bcl-2相关X蛋白(Bcl-2 associated protein, Bax)与B细胞淋巴瘤基因-2(B cell lymphoma 2, Bcl-2)的比率(分别为线粒体凋亡通路中的诱导因子和抑制因子)[50]。TNF-α是有效的细胞凋亡诱导因子,也在脂肪细胞功能的发挥中起关键作用[51]。在饲粮中添加1%CLA混合物饲喂C57BL/6J小鼠后上调了WAT中TNF-α的表达,诱导了细胞凋亡[52]。t10, c12-CLA处理促进了小鼠TNF-α的表达和分泌[53-54]。此外,综合应激反应(integrated stress response, ISR)的激活也可诱导细胞凋亡[41]。研究显示,t10, c12-CLA促进了小鼠和3T3-L1细胞中ISR有关基因的表达,如转录因子3、C/EP同源蛋白(C/EBP homologous protein, CHOP)以及生长停滞和DNA损伤诱导蛋白34等,而在CLA诱导脂肪细胞ISR活化之前,会先促进IL-6、白细胞介素-8(interleukin-8, IL-8)等炎症基因的表达[55]。在小鼠乳腺癌细胞中,t10, c12-CLA通过诱导CHOP的表达和内质网应激而促进凋亡进程[56-57]。因此,CLA可通过内质网应激和ISR反应诱导细胞凋亡,具体的作用机制则受CLA的处理剂量、异构体的添加种类或剂量不同的影响[41, 57]。研究显示,t10, c12-CLA调控脂肪细胞凋亡的分子机制与ω-3亚油酸、二十二碳六烯酸(DHA)相似[58]。
2.6 CLA激活G蛋白偶联受体(G protein-coupled receptors, GPRs)信号通路GPRs家族基因的表达与细胞代谢相关调控通路的活性紧密相关。激活GPRs可刺激下游一系列信号通路的表达,如激活PLC和DGK,产生二酰基甘油和三磷酸肌醇,促进内质网释放[Ca2+]i;此外,还可激活环磷酸腺苷介导的脂肪分解,诱导炎症反应的发生[25]。在GPRs家族中,GPR41和GPR120可在WAT中表达,而GPR43和GPR84在脂肪细胞中表达丰富[59]。研究显示,百日咳毒素(GPR-Gi/o偶联抑制剂)处理抑制了t10, c12-CLA介导的丝裂原活化蛋白激酶和ERK1/2的磷酸化,抑制了葡萄糖的摄取[60]。t10, c12-CLA处理的人原代脂肪细胞中,GPRC5A、GPR56的表达量分别升高了5倍、4倍,而GPR120的表达量降低了60%[61],与GPRs信号通路有关的膜蛋白(如PLCγ1、PLCδ4、DGKδ、DGKγ等)的表达量也显著升高[47-48, 61]。此外,CLA异构体混合剂通过激活特异的GPRs而降低啮齿动物WAT内的脂肪酸转运[62]。但是目前,有关CLA经GPRs信号通路调控脂肪代谢的具体分子机制还不明确,仍需深入研究。
t10, c12-CLA降低体脂沉积的潜在分子机制如图 1所示。
|
t10, c12-CLA:反10, 顺12-CLA trans10, cis12-CLA;GPRs:G蛋白偶联受体G protein-coupled receptors;PLC:磷脂酶C phospholipase C;PIP2:磷脂酰肌醇-4, 5-二磷酸phosphatidylinositol 4, 5-biphosphate;DAG:二酰甘油diglyceride;IP3:三磷酸肌醇inositol 1,4,5-triphosphate;DGK:二酰甘油激酶diacylglycerol kinase;CaMKⅡ:钙/钙调素依赖性蛋白激酶Ⅱ Ca2+/calmodulin-dependent protein kinase Ⅱ;ERK:细胞外信号调节激酶extracellular signal-regulated kinase;JNK:c-Jun氨基末端激酶c-Jun N-terminal kinase;PPARγ:过氧化物酶增殖子激活受体γ peroxisome proliferator activated receptor γ;endoplasmic reticulum:内质网;Adipogenesis:脂肪形成;Lipogenesis:脂肪生成。 图 1 t10, c12-CLA降低体脂沉积的潜在分子机制 Fig. 1 Potential molecular mechanisms of t10, c12-CLA reducing body fat deposition |
过量的脂肪沉积,不仅造成饲料资源的浪费和动物生产效益的降低,还会增加畜禽、人等患代谢紊乱相关疾病的风险。CLA在降低体脂沉积、抗肥胖中发挥着重要的生理调控作用,研究CLA发挥该生理作用的分子机制,对于促进CLA在医学、畜牧业中的合理应用,改善人体健康以及畜牧养殖效益等都具有深远的意义。近年来,许多学者均对CLA降低体脂沉积的分子机制进行了研究,但由于动物模型、细胞类型或者状态的不同,不同研究所得结论并不一致,也使得我们对该机制的认识不明确。充分利用分子生物学技术,深入揭示CLA调控脂肪代谢的规律及作用机理,依然是今后的研究热点。
| [1] |
王美艳. 共轭亚油酸在畜牧生产中的研究和应用[J]. 广东饲料, 2019(1): 29-32. DOI:10.3969/j.issn.1005-8613.2019.01.008 |
| [2] |
MEADUS W J, MACINNIS R, DUGAN M E R. Prolonged dietary treatment with conjugated linoleic acid stimulates porcine muscle peroxisome proliferator activated receptor gamma and glutamine-fructose aminotransferase gene expression in vivo[J]. Journal of Molecular Endocrinology, 2002, 28(2): 79-86. |
| [3] |
CORDERO G, ISABEL B, MENOYO D, et al. Dietary CLA alters intramuscular fat and fatty acid composition of pig skeletal muscle and subcutaneous adipose tissue[J]. Meat Science, 2010, 85(2): 235-239. DOI:10.1016/j.meatsci.2010.01.004 |
| [4] |
BEE G. Dietary conjugated linoleic acid consumption during pregnancy and lactation influences growth and tissue composition in weaned pigs[J]. The Journal of Nutrition, 2000, 130(12): 2981-2989. DOI:10.1093/jn/130.12.2981 |
| [5] |
RAMIAH S K, MENG G Y, SHEAU WEI T, et al. Dietary conjugated linoleic acid supplementation leads to downregulation of PPAR transcription in broiler chickens and reduction of adipocyte cellularity[J]. PPAR Research, 2014, 2014: 137652. |
| [6] |
CHERIAN G, AI W, GOEGER M P. Maternal dietary conjugated linoleic acid alters hepatic triacylglycerol and tissue fatty acids in hatched chicks[J]. Lipids, 2005, 40(2): 131-136. DOI:10.1007/s11745-005-1367-3 |
| [7] |
BU D P, WANG J Q, DHIMAN T R, et al. Effectiveness of oils rich in linoleic and linolenic acids to enhance conjugated linoleic acid in milk from dairy cows[J]. Journal of Dairy Science, 2007, 90(2): 998-1007. DOI:10.3168/jds.S0022-0302(07)71585-0 |
| [8] |
杨凤. 动物营养学[M]. 2版. 北京: 中国农业出版社, 2001.
|
| [9] |
SARTORIUS T, DRESCHER A, PANSE M, et al. Mice lacking free fatty acid receptor 1(GPR40/FFAR1) are protected against conjugated linoleic acid-induced fatty liver but develop inflammation and insulin resistance in the brain[J]. Cellular Physiology and Biochemistry, 2015, 35(6): 2272-2284. DOI:10.1159/000374031 |
| [10] |
YUAN G F, SINCLAIR A J, SUN H Y, et al. Fatty acid composition in tissues of mice fed diets containing conjugated linolenic acid and conjugated linoleic acid[J]. Journal of Food Lipids, 2009, 16(2): 148-163. DOI:10.1111/j.1745-4522.2009.01138.x |
| [11] |
CZAUDERNA M, KOWALCZYK J, KORNILUK K. Effect of dietary conjugated linoleic acid and selenized yeast on the concentration of fatty acids and minerals in rats[J]. Archives of Animal Nutrition, 2007, 61(2): 135-150. DOI:10.1080/17450390701204004 |
| [12] |
SHEN W, CHUANG C C, MARTINEZ K, et al. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice[J]. Journal of Lipid Research, 2013, 54(4): 909-922. DOI:10.1194/jlr.M030924 |
| [13] |
SUBBAIAH P V, SIRCAR D, AIZEZI B, et al. Differential effects of conjugated linoleic acid isomers on the biophysical and biochemical properties of model membranes[J]. Biochimica et Biophysica Acta:Biomembranes, 2010, 1798(3): 506-514. DOI:10.1016/j.bbamem.2009.11.020 |
| [14] |
KENNEDY A, OVERMAN A, LAPOINT K, et al. Conjugated linoleic acid-mediated inflammation and insulin resistance in human adipocytes are attenuated by resveratrol[J]. Journal of Lipid Research, 2009, 50(2): 225-232. DOI:10.1194/jlr.M800258-JLR200 |
| [15] |
MARTIN J C, GRÉGOIRE S, SIESS M H, et al. Effects of conjugated linoleic acid isomers on lipid-metabolizing enzymes in male rats[J]. Lipids, 2000, 35(1): 91-98. DOI:10.1007/s11745-000-0499-9 |
| [16] |
KENNEDY A, CHUNG S, LAPOINT K, et al. Trans-10, cis-12 conjugated linoleic acid antagonizes ligand-dependent PPARγ activity in primary cultures of human adipocytes[J]. The Journal of Nutrition, 2008, 138(3): 455-461. DOI:10.1093/jn/138.3.455 |
| [17] |
OBSEN T, FAERGEMAN N J, CHUNG S, et al. Trans-10, cis-12 conjugated linoleic acid decreases de novo lipid synthesis in human adipocytes[J]. The Journal of nutritional biochemistry, 2012, 23(6): 580-590. DOI:10.1016/j.jnutbio.2011.02.014 |
| [18] |
BRANDEBOURG T D, HU C Y. Isomer-specific regulation of differentiating pig preadipocytes by conjugated linoleic acids[J]. Journal of Animal Science, 2005, 83(9): 2096-2105. DOI:10.2527/2005.8392096x |
| [19] |
DEN HARTIGH L J, WANG S R, GOODSPEED L, et al. Metabolically distinct weight loss by 10, 12 CLA and caloric restriction highlight the importance of subcutaneous white adipose tissue for glucose homeostasis in mice[J]. PLoS One, 2017, 12(2): e0172912. DOI:10.1371/journal.pone.0172912 |
| [20] |
KADEGOWDA A K G, BURNS T A, PRATT S L, et al. Inhibition of stearoyl-CoA desaturase 1 reduces lipogenesis in primary bovine adipocytes[J]. Lipids, 2013, 48(10): 967-976. DOI:10.1007/s11745-013-3823-1 |
| [21] |
陈犹白, 陈聪慧, ZHANG Q X, 等. 脂肪干细胞分离、纯化和保存:研究进展与未来方向[J]. 中国组织工程研究, 2016, 20(10): 1508-1520. DOI:10.3969/j.issn.2095-4344.2016.10.020 |
| [22] |
ONG W K, SUGⅡ S. Adipose-derived stem cells:fatty potentials for therapy[J]. The International Journal of Biochemistry & Cell Biology, 2013, 45(6): 1083-1086. |
| [23] |
CHOI S K, PARK S, JANG S, et al. Cascade regulation of PPARγ2 and C/EBPα signaling pathways by celastrol impairs adipocyte differentiation and stimulates lipolysis in 3T3-L1 adipocytes[J]. Metabolism, 2016, 65(5): 646-654. DOI:10.1016/j.metabol.2016.01.009 |
| [24] |
MILLER J R, SIRIPURKPONG P, HAWES J, et al. The trans-10, cis-12 isomer of conjugated linoleic acid decreases adiponectin assembly by PPARγ-dependent and PPARγ-independent mechanisms[J]. Journal of Lipid Research, 2008, 49(3): 550-562. DOI:10.1194/jlr.M700275-JLR200 |
| [25] |
SHEN W, MCINTOSH M K. Nutrient regulation:conjugated linoleic acid's inflammatory and browning properties in adipose tissue[J]. Annual Review of Nutrition, 2016, 36: 183-210. DOI:10.1146/annurev-nutr-071715-050924 |
| [26] |
LAVANDERA J, GERSTNER C D, SAÍN J, et al. Maternal conjugated linoleic acid modulates TAG metabolism in adult rat offspring[J]. British Journal of Nutrition, 2017, 118(11): 906-913. DOI:10.1017/S0007114517003002 |
| [27] |
BUSIELLO R A, SAVARESE S, LOMBARDI A. Mitochondrial uncoupling proteins and energy metabolism[J]. Frontiers in Physiology, 2015, 6: 36. |
| [28] |
WU J, BOSTRÖM P, SPARKS L M, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human[J]. Cell, 2012, 150(2): 366-376. DOI:10.1016/j.cell.2012.05.016 |
| [29] |
WALDÉN T B, HANSEN I R, TIMMONS J A, et al. Recruited vs.nonrecruited molecular signatures of brown, "brite, "and white adipose tissues[J]. American Journal of Physiology:Endocrinology and Metabolism, 2012, 302(1): E19-E31. DOI:10.1152/ajpendo.00249.2011 |
| [30] |
WENDEL A A, PURUSHOTHAM A, LIU L F, et al. Conjugated linoleic acid induces uncoupling protein 1 in white adipose tissue of ob/ob mice[J]. Lipids, 2009, 44(11): 975-982. DOI:10.1007/s11745-009-3348-9 |
| [31] |
SHEN W, BALDWIN J, COLLINS B, et al. Low level of trans-10, cis-12 conjugated linoleic acid decreases adiposity and increases browning independent of inflammatory signaling in overweight Sv129 mice[J]. The Journal of Nutritional Biochemistry, 2015, 26(6): 616-625. DOI:10.1016/j.jnutbio.2014.12.016 |
| [32] |
LAROSA P C, MINER J, XIA Y N, et al. Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice:a microarray and histological analysis[J]. Physiological Genomics, 2006, 27(3): 282-294. DOI:10.1152/physiolgenomics.00076.2006 |
| [33] |
MARTINEZ K, KENNEDY A, WEST T, et al. Trans-10, cis-12-conjugated linoleic acid instigates inflammation in human adipocytes compared with preadipocytes[J]. Journal of Biological Chemistry, 2010, 285(23): 17701-17712. DOI:10.1074/jbc.M109.043976 |
| [34] |
VEGIOPOULOS A, MÜLLER-DECKER K, STRZODA D, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes[J]. Science, 2010, 328(5982): 1158-1161. DOI:10.1126/science.1186034 |
| [35] |
MADSEN L, PEDERSEN L M, LILLEFOSSE H H, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity[J]. PLoS One, 2010, 5(6): e11391. DOI:10.1371/journal.pone.0011391 |
| [36] |
STOUT M B.Differential metabolic effects in white and brown adipose tissue by conjugated linoleic acid elicit lipodystrophy-associated hepatic insulin resistance[D]. Ph.D Thesis.Ohio: The Ohio State University, 2011.
|
| [37] |
SIMONS P J, VAN DEN PANGAART P S, AERTS J M F G, et al. Pro-inflammatory delipidizing cytokines reduce adiponectin secretion from human adipocytes without affecting adiponectin oligomerization[J]. Journal of Endocrinology, 2007, 192(2): 289-299. DOI:10.1677/JOE-06-0047 |
| [38] |
POULOS S P, HAUSMAN D B, HAUSMAN G J. The development and endocrine functions of adipose tissue[J]. Molecular and Cellular Endocrinology, 2010, 323(1): 20-34. DOI:10.1016/j.mce.2009.12.011 |
| [39] |
KANG K, LIU W, ALBRIGHT K J, et al. Trans-10, cis-12 CLA inhibits differentiation of 3T3-L1 adipocytes and decreases PPARγ expression[J]. Biochemical and Biophysical Research Communications, 2003, 303(3): 795-799. DOI:10.1016/S0006-291X(03)00413-3 |
| [40] |
STECK S E, CHALECKI A M, MILLER P, et al. Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans[J]. The Journal of Nutrition, 2007, 137(5): 1188-1193. DOI:10.1093/jn/137.5.1188 |
| [41] |
KENNEDY A, MARTINEZ K, SCHMIDT S, et al. Antiobesity mechanisms of action of conjugated linoleic acid[J]. The Journal of Nutritional Biochemistry, 2010, 21(3): 171-179. DOI:10.1016/j.jnutbio.2009.08.003 |
| [42] |
MOLONEY F, TOOMEY S, NOONE E, et al. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue[J]. Diabetes, 2007, 56(3): 574-582. DOI:10.2337/db06-0384 |
| [43] |
HALADE G V, RAHMAN M M, FERNANDES G. Differential effects of conjugated linoleic acid isomers in insulin-resistant female C57Bl/6J mice[J]. The Journal of Nutritional Biochemistry, 2010, 21(4): 332-337. DOI:10.1016/j.jnutbio.2009.01.006 |
| [44] |
BRAVO-SAGUA R, MATTAR P, DÍAZ X, et al. Calcium sensing receptor as a novel mediator of adipose tissue dysfunction:mechanisms and potential clinical implications[J]. Frontiers in Physiology, 2016, 7: 395. |
| [45] |
BROWN J M, SANDBERG BOYSEN M, JENSEN S S, et al. Isomer-specific regulation of metabolism and PPARγ signaling by CLA in human preadipocytes[J]. Journal of Lipid Research, 2003, 44(7): 1287-1300. DOI:10.1194/jlr.M300001-JLR200 |
| [46] |
CHUNG S, BROWN J M, PROVO J N, et al. Conjugated linoleic acid promotes human adipocyte insulin resistance through NFκB-dependent cytokine production[J]. Journal of Biological Chemistry, 2005, 280(46): 38445-38456. DOI:10.1074/jbc.M508159200 |
| [47] |
SHEN W, MARTINEZ K, CHUANG C C, et al. The phospholipase C inhibitor U73122 attenuates trans-10, cis-12 conjugated linoleic acid-mediated inflammatory signaling and insulin resistance in human adipocytes[J]. The Journal of Nutrition, 2013, 143(5): 584-590. DOI:10.3945/jn.112.173161 |
| [48] |
MARTINEZ K, SHYAMASUNDAR S, KENNEDY A, et al. Diacylglycerol kinase inhibitor R59022 attenuates conjugated linoleic acid-mediated inflammation in human adipocytes[J]. Journal of Lipid Research, 2013, 54(3): 662-670. DOI:10.1194/jlr.M031211 |
| [49] |
MOON H S, LEE H G, SEO J H, et al. Down-regulation of PPARγ2-induced adipogenesis by PEGylated conjugated linoleic acid as the pro-drug:attenuation of lipid accumulation and reduction of apoptosis[J]. Archives of Biochemistry and Biophysics, 2006, 456(1): 19-29. DOI:10.1016/j.abb.2006.10.002 |
| [50] |
LIU L F, PURUSHOTHAM A, WENDEL A A, et al. Combined effects of rosiglitazone and conjugated linoleic acid on adiposity, insulin sensitivity, and hepatic steatosis in high-fat-fed mice[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2007, 292(6): G1671-G1682. DOI:10.1152/ajpgi.00523.2006 |
| [51] |
CAWTHORN W P, HEYD F, HEGYI K, et al. Tumour necrosis factor-α inhibits adipogenesis via a β-catenin/TCF4(TCF7L2)-dependent pathway[J]. Cell Death and Differentiation, 2007, 14(7): 1361-1373. DOI:10.1038/sj.cdd.4402127 |
| [52] |
TSUBOYAMA-KASAOKA N, TAKAHASHI M, TANEMURA K, et al. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice[J]. Diabetes, 2000, 49(9): 1534-1542. DOI:10.2337/diabetes.49.9.1534 |
| [53] |
HOUSE R L, CASSADY J P, EISEN E J, et al. Conjugated linoleic acid evokes de-lipidation through the regulation of genes controlling lipid metabolism in adipose and liver tissue[J]. Obesity Reviews, 2005, 6(3): 247-258. DOI:10.1111/j.1467-789X.2005.00198.x |
| [54] |
POIRIER H, NIOT I, CLÉMENT L, et al. Development of conjugated linoleic acid (CLA)-mediated lipoatrophic syndrome in the mouse[J]. Biochimie, 2005, 87(1): 73-79. DOI:10.1016/j.biochi.2004.11.006 |
| [55] |
LAROSA P C, RIETHOVEN J J M, CHEN H, et al. Trans-10, cis-12 conjugated linoleic acid activates the integrated stress response pathway in adipocytes[J]. Physiological Genomics, 2007, 31(3): 544-553. DOI:10.1152/physiolgenomics.00156.2007 |
| [56] |
OU L H, WU Y, IP C, et al. Apoptosis induced by t10, c12-conjugated linoleic acid is mediated by an atypical endoplasmic reticulum stress response[J]. Journal of Lipid Research, 2008, 49(5): 985-994. DOI:10.1194/jlr.M700465-JLR200 |
| [57] |
IP M M, MASSO-WELCH P A, SHOEMAKER S F, et al. Conjugated linoleic acid inhibits proliferation and induces apoptosis of normal rat mammary epithelial cells in primary culture[J]. Experimental Cell Research, 1999, 250(1): 22-34. DOI:10.1006/excr.1999.4499 |
| [58] |
MEADUS J, VAHMANI P, DUFF P, et al. CLA isomer t10, c12 induce oxidation and apoptosis in 3t3 adipocyte cells in a similar effect as omega-3 linolenic acid and DHA[J]. Functional Foods in Health & Disease, 2017, 7(2): 149-167. |
| [59] |
MILLIGAN G, ULVEN T, MURDOCH H, et al. G-protein-coupled receptors for free fatty acids:nutritional and therapeutic targets[J]. British Journal of Nutrition, 2014, 111(Suppl.1): S3-S7. |
| [60] |
BROWN J M, BOYSEN M S, CHUNG S, et al. Conjugated linoleic acid induces human adipocyte delipidation:autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines[J]. Journal of Biological Chemistry, 2004, 279(25): 26735-26747. DOI:10.1074/jbc.M401766200 |
| [61] |
REARDON M, GOBERN S, MARTINEZ K, et al. Oleic acid attenuates trans-10, cis-12 conjugated linoleic acid-mediated inflammatory gene expression in human adipocytes[J]. Lipids, 2012, 47(11): 1043-1051. DOI:10.1007/s11745-012-3711-0 |
| [62] |
SAUER L A, DAUCHY R T, BLASK D E, et al. Conjugated linoleic acid isomers and trans fatty acids inhibit fatty acid transport in hepatoma 7288CTC and inguinal fat pads in Buffalo rats[J]. The Journal of Nutrition, 2004, 134(8): 1989-1997. DOI:10.1093/jn/134.8.1989 |




