2. 华中农业大学动物医学院, 武汉 430070
2. College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, China
“肠-肝轴”一词最早由Volta等[1]于1978年提出,指动物机体的肠道与肝脏之间通过肝门静脉及肠系膜静脉连接,形成肠-肝轴。肠道中的化合物及细菌代谢产物可通过肠肝循环到达肝脏,可以说肝脏是肠道化合物及一些物质的大型收集基地。胆汁酸是肝脏中胆固醇代谢后的终产物之一,其在肝脏中合成,并以相应形式分泌到肠道中,再通过肠道中的胆汁酸转运体重吸收进入肝门静脉,进而重新返回肝脏,这一过程被称为胆汁酸的肠肝循环。胆汁酸在肠肝循环中不仅参与机体营养物质的消化吸收,调节机体健康,最重要的是与胆汁酸受体结合后调节胆汁酸代谢及糖脂代谢。本文针对胆汁酸受体在肠肝循环中的作用及其对糖脂代谢的调节机制进行综述,以期为今后探究胆汁酸及其受体在代谢相关疾病防治中的作用提供参考。
1 胆汁酸的肠肝循环 1.1 胆汁酸合成在肝周细胞中,胆固醇在约15种酶的作用下合成胆汁酸,其主要合成途径有2条[2]:75%的胆汁酸是由限速酶胆固醇7α-羟化酶(CYP7A1)启动的经典途径产生,在此过程中,胆固醇在CYP7A1[3]的作用下生成7α-羟固醇,再通过甾醇12α羟化酶(CYP8B1)作用形成胆酸(CA);其余的胆汁酸则是由甾醇-27羟化酶(CYP27A1)和氧固醇7α羟化酶(CYP7B1)限速催化启动的替代途径产生[4-5]。其中,CYP27A1是一种广泛分布在巨噬细胞和各种组织中的线粒体酶,由其催化的反应最终产物为25-羟基胆固醇和27-羟基胆固醇,再通过替代途径在CYP8B1的作用下合成鹅去氧胆酸(CDCA)。通过上述2种途径合成的胆汁酸均被称为初级胆汁酸,也称为游离型胆汁酸。但最新研究发现,在啮齿类动物中,在另外一种细胞色素P450 2C家族酶(CYP2C70)的作用下,可将CDCA和熊去氧胆酸(UDCA)转化成α-鼠胆酸(α-MCA)和β-鼠胆酸(β-MCA)[6],所以啮齿类动物的初级胆汁酸以CA及鼠胆酸(MCA)为主[7]。
在肝细胞中,未结合的初级胆汁酸在胆汁酸辅酶A合成酶(BACS)和胆汁酸辅酶A氨基酸N-乙酰基转移酶(BAAT)的作用下,在C24位置与甘氨酸(主要在猪中)[8-9]或牛磺酸(主要在鼠和鸡中)结合,形成结合型胆汁酸。未结合的胆汁酸和结合后的胆汁酸有一部分可以被磺基转移酶家族2A1(SULT2A1)硫酸化,鼠中则是被磺基转移酶家族22 A9(SULT22A9)硫酸化,另一部分胆汁酸会被尿苷二磷酸(UDP)-葡萄糖醛酸基转移酶(UGT)如UGT2B4、UGT2B7和UGT1A3糖酯化[2],还有一部分被细胞色素P450 3A4(CYP3A4)和细胞色素P450 3A11(CYP3A11)羟基化。在这些酶的作用下,肝脏可免受胆汁酸的毒性作用侵害[10],这些酶的缺失或者突变将导致胆汁淤积[11-12]。
1.2 胆汁酸的分泌与转化胆汁酸会通过胆盐输出泵(BSEP,也称为ABCB11)、多药耐药蛋白2(MRP2,也称为ABCC2)、多药耐药蛋白3(MDR3,也称ABCB4)和多药耐药蛋白1(MDR1,也称ABAB1)等转运蛋白分泌到胆小管中[2]。BSEP是胆汁酸的主要流出通道,大多数的结合型胆汁酸都是通过此通道输送到胆管;MRP2属于ABC转运蛋白的超级家族,可将有机阴离子从肝细胞输送到胆管[13];MDR3主要将磷脂等运送到胆管;MDR1将有机阳离子、细胞毒素和其他外源性生物排泄入胆汁。胆汁在胆囊中储存浓缩,进食后,肠L细胞刺激胆囊收缩素(CCK)的分泌,使胆囊收缩,将胆汁释放到十二指肠中[2]。因大鼠在解剖学上不存在胆囊,所以肝脏合成的胆汁酸会直接分泌到肠道中,不经过胆囊储存的过程。胆汁酸进入肠道后,在肠道细菌作用下初级胆汁酸经去结合作用(水解结合型初级胆汁酸)、差向异构作用和脱羟基作用被转化为次级胆汁酸,其中CA转化为去氧胆酸(DCA),CDCA转化为石胆酸(LCA),鼠体内的α-MCA、β-MCA转化为ω-鼠胆酸(ω-MCA)、猪胆酸(HCA)、猪去氧胆酸(HDCA)等[14]。胆汁酸的结构与组成如图 1所示。
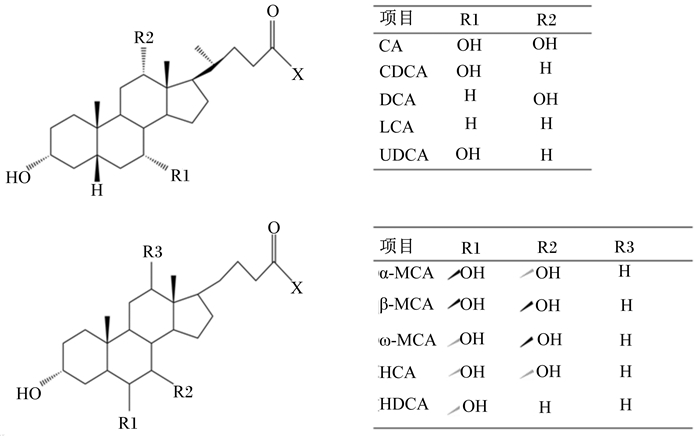
|
游离型胆汁酸:X=OH;结合型胆汁酸:X=NHCH2CO2H(甘氨酸)或NHCH2CH2SO3H(牛磺酸)。CA:胆酸;CDCA:鹅去氧胆酸;DCA:去氧胆酸;LCA:石胆酸;UDCA:熊去氧胆酸。 Free bile acids: X=OH; conjugated bile acids: X=NHCH2CO2H (glycine) or NHCH2CH2SO3H (taurine). CA: cholic acid; CDCA: chenodeoxycholic acid; DCA: deoxycholic acid; LCA: lithocholic acid; UDCA: ursodesoxycholic acid. 图 1 胆汁酸的结构与组成 Fig. 1 Structure and composition of bile acids[15-16] |
进入肠道中的胆汁酸在回肠末端有约95%会通过回肠末端的胆汁酸转运体溶质载体家族10(SLC10A2,也称ASBT)吸收进入肠道上皮细胞,并通过异二聚体有机溶质转运体α和β(OSTα/β)在基底外侧膜上分泌出来,再通过肝门静脉重吸收回肝脏,有学者将通过此过程回收到肝脏的次级胆汁酸称为三级胆汁酸[17]。剩余的5%未被吸收再次利用的胆汁酸,一部分经过肠道微生物群的解聚,被动地被结肠重新吸收,例如DCA可以被重吸收回肝脏再利用[18];另一部分则是经粪便排出。被吸收的胆汁酸通过肠系膜上静脉和门静脉返回肝脏,通过牛磺胆酸钠共转运多肽(NTCP,也称SLC10A1)和有机阴离子转运多肽(OATP,也称SLCO1A2)等肝细胞窦状隙中的活性转运蛋白重新摄取[19]。而有一些胆汁酸虽然可以进入到门静脉循环中,却无法被肝细胞回收,而是随着尿液排出。在肝脏内,胆固醇会重新代谢合成新的胆汁酸,再与肝脏内已有的胆汁酸及重吸收回肝脏的胆汁酸一起分泌到胆小管中,补偿粪便和尿液损失的那部分胆汁酸。同时,也会存在少量胆汁酸既没有被肝脏重新吸收,也没有随尿液和粪便排出体外,而是在血浆中与血浆蛋白[主要是白蛋白(约80%)和脂蛋白(约20%)][20]结合在一起,被运送到周围其他组织和器官,与相应受体结合发挥功能[2]。
胆汁酸的合成与转运如图 2所示。
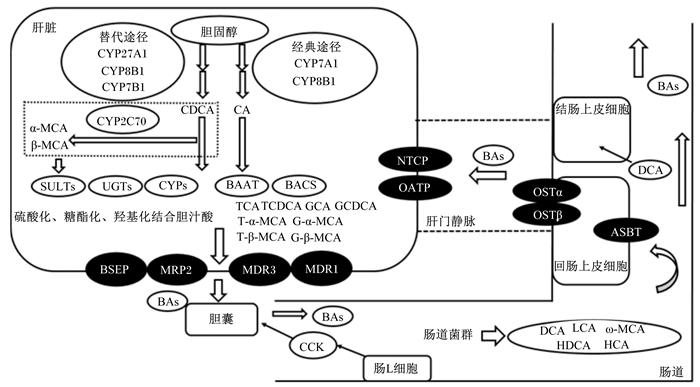
|
CYP27A1:甾醇-27羟化酶sterol-27 hydroxylase;CYP7A1:胆固醇7α-羟化酶cholesterol 7 alpha-hydroxylase;CYP7B1:氧固醇7α羟化酶oxysterol 7 alpha-hydroxylase;CYP8B1:甾醇12α羟化酶sterol 12-alpha-hydroxylase;CYP2C70:细胞色素P450 2C家族酶cytochrome P450, family 2, subfamily C, polypeptide 70;α-MCA:α-鼠胆酸α-muricholic acid;β-MCA:β-鼠胆酸β-muricholic acid;ω-MCA:ω-鼠胆酸ω-muricholic acid;CA:胆酸cholic acid;CDCA:鹅去氧胆酸chenodeoxycholic acid;DCA:去氧胆酸deoxycholic acid;LCA:石胆酸lithocholic acid;SULTs:磺基转移酶家族sulfotransferase family;UGTs:葡萄糖醛酸基转移酶glucuronide transferase;CYPs:细胞色素P450 cytochromes P450;BACS:胆汁酸辅酶A合成酶bile acid coenzyme A synthase;BAAT:胆汁酸辅酶A氨基酸N-乙酰基转移酶bile acid CoA amino acid N-acetyltransferase;BAs:胆汁酸bile acids;TCA:牛磺胆酸taurocholic acid;TCDCA:牛磺鹅去氧胆酸taurochenodeoxycholic acid;GCA:甘氨胆酸glycocholic acid;GCDCA:甘氨鹅去氧胆酸glycochenodeoxycholic acid;T-α-MCA:牛磺-α-鼠胆酸tauro α-muricholic acid;T-β-MCA:牛磺-β-鼠胆酸tauro β-muricholic acid;G-α-MCA:甘氨酸-α-鼠胆酸glycine-α-muricholic acid;G-β-MCA:甘氨酸-β-鼠胆酸glycine-β-Muricholic acid;NTCP:牛磺胆酸钠共转运多肽Na+/taurocholate cotransporting polypeptide;OATP:有机阴离子转运多肽organic anion transporting polypeptides;OSTα:有机溶质转运体α organic solute transporter α;OSTβ:有机溶质转运体β organic solute transporter β;ASBT:顶端钠胆汁酸转运蛋白apical sodium-bile acid transporter;HCA:猪胆酸hyocholic acid;HDCA:猪去氧胆酸hyodeoxycholic acid;CCK:胆囊收缩素cholecystokinin;BSEP:胆盐输出泵bile salt export pump;MRP2:多耐药蛋白2 multidrug resistance-associated protein 2;MDR3:多药耐药蛋白3 multidrug resistanec-associated protein 3;MDR1:多药耐药蛋白1 multidrug resistanec-associated protein 1。 图 2 胆汁酸的合成与转运 Fig. 2 Synthesis and transport of bile acids |
胆汁酸受体有2种类型:核受体和G蛋白偶联受体(GPCR)。核受体包括法尼酯X受体(FXR)、孕烷X受体(PXR)等,GPCR包括G蛋白偶联胆汁酸受体1(TGR5,也称GPBAR1)、鞘氨醇-1-磷酸受体2(S1PR2)等。在肠肝循环中FXR、PXR、TGR5及S1PR2都有相应的调控作用,胆汁酸与这几种受体结合后,通过调节相应胆汁酸信号通路参与胆汁酸代谢、糖脂代谢以及一些能量代谢和炎症过程等多种生理功能的调节[21-22]。其中胆汁酸激活核受体后,主要参与调节机体肠肝循环的胆汁酸代谢过程及糖脂代谢,也参与药物的代谢和解毒反应;而TGR5被激活后,虽可调控糖代谢过程,但主要参与调节机体能量代谢和免疫反应[23];S1PR2近些年被发现,在肝脏中对糖脂代谢调控具有重要作用。
2.1 胆汁酸受体与肠肝循环FXR是控制胆汁酸代谢,参与影响肠肝循环系统的最重要的受体,其主要表达于肝脏、肠道、肾脏和肾上腺等器官[24]。已有研究显示FXR在心脏、脂肪(白色脂肪)[25-26]和血管系统中低表达,但具体功能不十分清楚。FXR通过诱导肝脏中的小异源二聚体伴侣(SHP)降低肝受体同源物1(LRH1,也称NR5A2)的生成,从而调节CYP7A1和CYP8B1的活性,调节胆汁酸的合成。在肠道中胆汁酸激活肠上皮细胞的FXR受体,进而促进成纤维细胞生长因子19(FGF19)[鼠中是成纤维细胞生长因子15(FGF15)]的表达并从回肠末端释放进入肝门静脉及肠系膜静脉运输到肝脏,作用于肝脏的成纤维细胞生长因子受体4(FGFR4)及β-klotho(也称KLB)受体,二者激活后共同抑制肝脏CYP7A1和CYP8B1的活性[27-28]。高水平的胆汁酸对细胞有害,而且会以负反馈的形式抑制FXR激活的胆汁酸合成途径,因此FXR在调节胆汁酸合成中起着关键作用。FXR会通过多种方式影响胆汁酸含量,调节胆汁酸的合成、结合、回收和摄取。在小鼠中,FXR的缺失与许多严重疾病的病理过程有关,说明FXR介导的胆汁酸信号通路对胆汁酸通路和代谢稳态都至关重要[29]。
在胆汁酸产生的初始阶段,FXR除通过诱导SHP抑制胆汁酸合成,还通过诱导负调控因子过氧化物酶体增殖物激活受体α(PPARα)[30]或环磷酸腺苷反应元件结合蛋白(CREB)转录共激活因子2(CRTC2)间接抑制FXR的转录[27]。SHP与LRH1相互作用形成异二聚体时,也导致CYP7A1和SHP自身的抑制[19, 27, 30-32]。FXR通过介导SHP,抑制肝细胞核因子4α(HNF4α),从而抑制CYP8B1的表达[32],CYP8B1的表达控制着CA与CDCA的比率。天然FXR激动剂即是机体自身通过合成等途径产生的胆汁酸,其对胆汁酸受体也存在激活和抑制2种方式,已有研究表明FXR天然的激活剂的顺序为CDCA>DCA>CA>LCA,天然抑制剂为牛磺-α-鼠胆酸(T-α-MCA)、牛磺-β-鼠胆酸(T-β-MCA)和UDCA[19],所以机体内产生的胆汁酸类型和含量会调控FXR的表达。BACS和BAAT这2种参与胆汁酸结合的关键酶均受FXR的正向调控[30],参与胆汁酸与牛磺酸或甘氨酸的结合。
FXR同样会参与胆汁酸的分泌与转运过程。FXR通过调节BSEP和MRP2转运体的表达控制胆汁酸的分泌。FXR还通过调节几种转运蛋白抑制胆汁酸的重吸收途径。胆汁酸对ASBT的负反馈调节是由FXR通过作用SHP,抑制ASBT的启动子LRH-1的激活介导的[33]。有学者研究表明FXR可以激活回肠中胆汁酸结合蛋白(IBABP,也称为胃促素)促进胆汁酸盐从肠细胞的顶端移动到基底外侧膜[34],从而加速胆汁酸重吸收回肝脏。FXR通过诱导有机溶质转运体α(OSTα)和有机溶质转运体β(OSTβ)[35]的表达使胆汁酸释放到门静脉循环中以返回肝脏。胆汁酸抵达肝脏处被NTCP和OATP再次主动摄取,这2种转运体均受FXR的负反馈调节,防止肝脏组织内胆汁酸含量过高,引起胆汁淤积造成的肝脏毒性作用。并且,FXR激活后可以激活修饰胆汁酸基因的表达,其中包括使胆汁酸羟基化的CYP3A4和CYP3A11以及硫酸化的SULT2A1和糖脂化的UGT2B4。此外,FXR已被证明能预防药物引起的肝脏毒性[10]。
肝脏PXR可被胆汁酸激活,主要参与肝脏胆汁酸代谢、药物代谢和解毒功能调节。PXR激活后,可结合到靶基因启动子内的激素反应原件位点,直接调控靶基因的转录[36]。研究证明,肝脏CYP7A1、MRP2、MDR3、有机阴离子转运多肽2(OATP2)和FGFR4以及肠道FGF15(鼠为FGF19)均为PXR的靶基因,通过调节这些靶基因的转录,抑制胆汁生成,促进胆汁排出,从而维持体内胆汁酸代谢平衡[37-38]。TGR5主要在胆囊、脑[2]、骨骼肌、肠道(尤其是在回肠和结肠)[39]及棕色脂肪细胞[40]、白色脂肪细胞中表达,在肝细胞中几乎不表达[41]。所以其参与胆汁酸代谢的作用较小,而在激活TGR5时,次级胆汁酸比初级胆汁酸更有效[42]。LCA是最有效的TGR5天然激活剂[43]。天然胆汁酸激活TGR5的顺序为LCA>DCA>CDCA>CA[19]。S1PR2是另外一种G蛋白偶联受体,其主要在肝细胞中被结合型胆汁酸激活,发挥一定的作用。S1PR2同TGR5类似,几乎不参与肝肠循环中的胆汁酸代谢途径。
2.2 胆汁酸受体与糖脂代谢 2.2.1 对脂代谢的调控FXR通过调节肝脏脂质生成、脂质分泌、血浆脂质清除以及肠道胆固醇吸收来调节脂质代谢。FXR的激活已被证明其调节脂质生成的关键途径。研究发现,在SHP缺失小鼠体内调节元件结合转录因子-1c(SREBP-1c)的表达没有受到抑制,推测FXR可通过介导SHP[44]和FGF15[45]来调控SREBP-1c的表达,进而减少脂肪的生成。在生理条件下,乙酰辅酶A羧化酶(ACC)和脂肪酸合成酶(FAS)等许多酶参与脂肪生成,已有学者证明了这2种酶均受SHP调控[46],因此推测FXR可通过调控SHP间接调控脂肪的生成。此外,微粒体甘油三酯转移蛋白(MTP)和载脂蛋白B(ApoB)基因表达也均受到FXR的调节[47],从而降低极低密度脂蛋白(VLDL)的分泌[44]。有研究表明,FXR通过激活脂蛋白脂酶(LPL)的激活剂载脂蛋白CⅡ(ApoCⅡ)[48]的表达,同时抑制LPL的抑制剂载脂蛋白CⅢ(ApoCⅢ)的活性[49],从而增强LPL的活性,使血液中甘油三酯(TG)的含量降低。FXR还可以介导极低密度脂蛋白受体(VLDL-R)的表达[50],调节脂质代谢。FXR的激活抑制胆固醇转化成胆汁酸,导致肝脏胆固醇含量增加,低密度脂蛋白受体(LDL-R)含量降低,使血浆低密度脂蛋白胆固醇(LDL-C)含量升高。FXR还通过抑制载脂蛋白AⅠ(ApoAⅠ)的表达[51],从而使清道夫受体-B1(SR-B1)[52]和转运抑制蛋白(CETP)[53]的表达升高,继而增加对高密度脂蛋白胆固醇(HDL-C)的清除能力,降低血浆HDL-C含量,从而对胆固醇转运(RCT)和HDL-C代谢起关键作用。最近有学者发现,FXR的激活可抑制碳水化合物反应原件结合蛋白(ChREBP)表达,抑制其下游基因肝脏丙酮酸激酶(LPK)表达,阻止糖类转化为脂肪,最终抑制脂肪酸和TG的合成[54]。研究还发现,肝脏FXR被胆汁酸激活也可诱导PPARα的高表达,继而上调脂肪酸β氧化相关酶,如酰基辅酶A氧化酶1(ACOX1)、乙酰辅酶A合酶(ACS)、LPL和肉毒碱棕榈酰转移酶1(CPT1)等的表达,促进脂肪酸氧化,减少脂质沉积[55]。值得注意的是,通过小鼠试验证明,FGF15可通过肝脏FGFR4通路下调乙酰辅酶A羧化酶2(ACC2)、硬脂酰辅酶A去饱和酶1(SCD1)、SREBP1c和肉毒碱棕榈酰转移酶1α(CPT1α)表达,减少肝脏脂肪、TG和胆固醇含量,推测动物体内FGF15也可能抑制肝脏脂质的合成等过程[56]。
2.2.2 对糖代谢的调控胆汁酸可以通过直接作用于肠道中的FXR和TGR5,调节葡萄糖稳态;也可通过促进肠道中FXR诱导FGF15的合成,间接调节葡萄糖稳态。在小鼠体内,FGF15不仅在肠道中可以合成,在下丘脑中也可合成,并发出相应信号抑制胰高血糖素的生成[57]。在小肠中,FXR调节吸收葡萄糖的动力学作用,这一作用在FXR敲除的小鼠中没有观察到[58]。FXR通过抑制肝脏中的糖酵解和脂肪生成过程,以降低餐后葡萄糖水平[44, 59-60],而FGF15的合成可以加快糖原的生成[61]。胆汁酸可以激活肠内分泌L细胞上的TGR5,由此调节胰高血糖素样肽-1(GLP-1)的分泌,此过程在回肠段最为活跃。TGR5的激活还可以调节胰高血糖素合成相关基因的表达[62-63]。由于TGR5是在基底外侧膜上而不是在顶端L细胞膜上表达,其激动剂的吸收和局部释放可能是激活TGR5的先决条件[64]。相比之下,FXR激活后,可抑制肠道L细胞的糖酵解过程以及ChREBP的活性,并抑制回肠中胰高血糖素合成相关基因的表达和GLP-1的分泌[65]。这2种受体对GLP-1合成及分泌的作用相反,但由于TGR5介导的胞内信号是快速的,而FXR的转录调控是缓慢的,所以通常情况下TGR5在肠道中的激活作用更为重要。CYP8B1的表达抑制或缺失会使小鼠GLP-1分泌增多,从而改善葡萄糖稳态。CYP8B11缺失的小鼠CA与MCA的比值降低,小肠内GLP-1的分泌增加[66]。此外,MCA与肠道中FXR结合后,可以抑制肠道FXR,使GLP-1的分泌增多[65]。
在胰岛中,FXR和TGR5在胰腺β细胞中均有表达,并正向调节胰岛素的合成以及葡萄糖稳态[67]。此外,胰腺α细胞中TGR5的激活可诱导胰高血糖素转化酶原-1的表达,催化胰高血糖素原生成GLP-1,继而转移到β细胞,与细胞膜上的GLP-1受体(GLP-1R)结合,增强β细胞的功能[68]。
胆汁酸也可结合肝脏中FXR,调节葡萄糖稳态。研究已证明,FXR缺失的小鼠磷酸烯醇丙酮酸羧激酶(PEPCK)和葡萄糖-6-磷酸酶(G6P)的表达量均降低[59-60]。FXR缺失的小鼠禁食后,进行空腹再饲喂试验,结果表明再饲喂高糖食物的小鼠反应格外明显,再次证实了FXR诱导糖酵解和脂肪生成过程中相关基因的表达,并显著抑制调控糖原生成基因的表达[59]。最近有报道显示,一个先前与解毒相关的醛酮还原酶1B7(Akr1b7)可降低血糖水平,并抑制糖原合成酶和脂质合成酶的表达[10, 14]。因此,Akr1b7有可能参与激活FXR,但具体机制还不明确。最近研究发现,受FXR调控的FGF15通过抑制CREB、下调过氧化物酶体增殖物激活受体γ共激活因子1-α (PGC-1α),抑制肝脏的糖异生过程[69]。
除了FXR和TGR5在调节糖脂代谢方面作用显著外,最近研究发现S1PR2的拮抗剂JTE-013可以阻断细胞外信号调节激酶(ERK1/2)和蛋白激酶B(AKT)通道[70],ERK1/2和AKT信号通路在调节肝脏糖脂代谢方面起着重要作用[71],所以S1PR2在调节肝脏糖脂代谢方面也起着重要作用[72-73]。SHP同样受FXR调控,可以通过调节ERK1/2和AKT信号通路抑制CYP7A1基因的转录,并抑制胆汁酸的合成[74]。
胆汁酸受体介导肠肝循环及其调控糖脂代谢的主要机制如图 3所示。
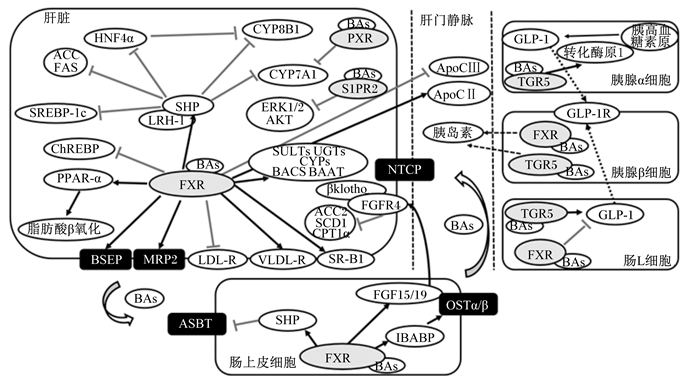
|
BAs:胆汁酸bile acids;FXR:法尼酯X受体farnesoid X receptor;SHP:小异源二聚体伴侣small heterodimer partner;LRH-1:肝受体同源物1 liver receptor homolog-1;CYP7A1:胆固醇7α-羟化酶cholesterol 7 alpha-hydroxylase;CYP8B1:甾醇12α羟化酶sterol 12-alpha-hydroxylase;HNF4α:肝细胞核因子4α hepatocyte nuclear factor 4;ACC:乙酰辅酶A羧化酶acetyl CoA carboxylase;FAS:脂肪酸合成酶fatty acid synthase;SREBP-1c:调节元件结合转录因子-1c sterol regulatory element-binding protein 1;ChREBP:碳水化合物反应原件结合蛋白carbohydrate reaction element binding protein;PPAR-α:过氧化物酶体增殖物激活受体α peroxisome proliferator-activated receptor α;MRP2:多耐药蛋白2 multidrug resistance-associated protein 2;LDL-R:低密度脂蛋白受体low density lipoprotein receptor;VLDL-R:极低密度脂蛋白受体very low density lipoprotein receptor;SR-B1:清道夫受体-B1 scavenger receptor class B, type I;FGFR4:成纤维细胞生长因子受体4 fibroblast growth factor receptor 4;ACC2:乙酰辅酶A羧化酶2 acetyl CoA carboxylase 2;SCD1:硬脂酰辅酶A去饱和酶1 stearoyl-CoA desaturase 1;CPT1α:肉毒碱棕榈酰转移酶1α carnitine palmitoyltransferase 1α;BACS:胆汁酸辅酶A合成酶bile acid coenzyme A synthase;BAAT:胆汁酸辅酶A氨基酸N-乙酰基转移酶bile acid CoA amino acid N-acetyltransferase;SULTs:磺基转移酶家族sulfotransferase family;UGTs:葡萄糖醛酸基转移酶glucuronide transferase;S1PR2:鞘氨醇-1-磷酸受体2 sphingosine-1-phosphate receptor 2;PXR:孕烷X受体pregnane X receptor;NTCP:牛磺胆酸钠共转运多肽Na+-taurocholate cotransporting polypeptide;ASBT:顶端钠胆汁酸转运蛋白apical sodium-bile acid transporter;IBABP:胆汁酸结合蛋白ileal bile acid binding protein;FGF15/19:成纤维细胞生长因子15/19 fibroblast growth factor15/19;OSTα/β:异二聚体有机溶质转运体α/β organic solute transporter α/β;TGR5:G蛋白偶联胆汁酸受体5 G protein-coupled bile acid receptor 5;GLP-1:胰高血糖素样肽-1 glucagon-like peptide 1;GLP-1R:胰高血糖素样肽1受体glucagon-like peptide 1 receptor;ApoCⅡ:载脂蛋白CⅡ apolipoprotein CⅡ;ApoCⅢ:载脂蛋白CⅢ apolipoprotein CⅢ;ERK1/2:细胞外调节蛋白激酶extracellular regulated protein kinases;AKT:蛋白激酶B protein kinase B。 图 3 胆汁酸受体介导肠肝循环及其调控糖脂代谢的主要机制 Fig. 3 Bile acid receptors mediate enterohepatic circulation and the main mechanism of glycolipid metabolism regulated by bile acid receptors |
综上所述,胆汁酸参与肝肠循环的许多重要环节均受胆汁酸受体的调控,包括胆汁酸的合成、分泌、排泄、再吸收等,通过这些途径维持机体的代谢平衡。肠肝循环系统的紊乱将导致机体代谢障碍,而调控胆汁酸受体是调节胆汁酸代谢的最直接、最有效的途径。虽然对胆汁酸代谢通路的研究已进行了数十年,但对于其在各种代谢型疾病上的作用,特别是在非酒精性脂肪型肝炎(NASH)、非酒精性脂肪性肝病(NAFLD)、2型糖尿病(T2DM)、代谢综合征(MS)、肠道相关炎症疾病等上的研究是近些年学者们才关注的热点。作为现今防治此类疾病的新策略,胆汁酸代谢通路及胆汁酸受体的作用是值得深入研究的。
尽管有许多引人注目的发现,但目前关于胆汁酸和胆汁酸代谢如何影响机体各方面功能的机制尚不完全清楚。胆汁酸在各个种属中类型繁多、作用复杂,还需要进一步的探究。胆汁酸受体的表达部位分布很广,除肠道、肝脏以外,胆汁酸受体还在很多器官和组织中表达并发挥着一定作用,其作用机制仍然需要进一步探索。在未来,相关机制阐明后,可通过影响胆汁酸代谢、改变胆汁酸类型等措施,为优化畜禽养殖业以及为动物相关疾病的防治提供新的思路与靶点。
致谢: 感谢沈阳农业大学杨建成教授对文稿所提的宝贵意见。| [1] |
VOLTA U, BONAZZI C, BIANCHI F B, et al. IgA antibodies to dietary antigens in liver cirrhosis[J]. International Journal of Clinical and Laboratory Research, 1987, 17(3): 235-242. |
| [2] |
LEFEBVRE P, CARIOU B, LIEN F, et al. Role of bile acids and bile acid receptors in metabolic regulation[J]. Physiological Reviews, 2009, 89(1): 147-191. DOI:10.1152/physrev.00010.2008 |
| [3] |
RUSSELL D W, SETCHELL K D R.Bile acid biosynthesis[M].Biochemistry, 1992, 31(20): 4737-4749.
|
| [4] |
VLAHCEVIC Z R, STRAVITZ R T, HEUMAN D M, et al. Quantitative estimations of the contribution of different bile acid pathways to total bile acid synthesis in the rat[J]. Gastroenterology, 1997, 113(6): 1949-1957. DOI:10.1016/S0016-5085(97)70015-5 |
| [5] |
DUANE W C, JAVITT N B. 27-hydroxycholesterol:production rates in normal human subjects[J]. Journal of Lipid Research, 1999, 40(7): 1194-1199. |
| [6] |
TAKAHASHI S, FUKAMI T, MASUO Y, et al. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans[J]. Journal of Lipid Research, 2016, 57(12): 2130-2137. DOI:10.1194/jlr.M071183 |
| [7] |
KUIPERS F, BLOKS V W, GROEN A K. Beyond intestinal soap-bile acids in metabolic control[J]. Nature Reviews Endocrinology, 2014, 10(8): 488-498. DOI:10.1038/nrendo.2014.60 |
| [8] |
陈志维, 黎玉翠, 暴梅佳, 等. 柱前衍生化高效液相色谱法测定猪胆粉结合胆酸中甘氨酸和牛磺酸的含量[J]. 药物分析杂志, 2010, 30(2): 288-290. |
| [9] |
邓启华, 姚颖, 刘婷, 等. 猪胆汁中三种主要胆汁酸的提取分离[J]. 中国生化药物志, 2012, 33(4): 405-407. |
| [10] |
LEE F Y, DE AGUIAR VALLIM T Q, CHONG H K, et al. Activation of the farnesoid X receptor provides protection against acetaminophen-induced hepatic toxicity[J]. Molecular Endocrinology, 2010, 24(8): 1626-1636. DOI:10.1210/me.2010-0117 |
| [11] |
DE VREE J M, JACQUEMIN E, STURM E, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis[J]. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(1): 282-287. DOI:10.1073/pnas.95.1.282 |
| [12] |
STRAUTNIEKS S S, BULL L N, KNISELY A S, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis[J]. Nature Genetics, 1998, 20(3): 233-238. DOI:10.1038/3034 |
| [13] |
KÖNIG J, NIES A T, CUI Y H, et al. Conjugate export pumps of the multidrug resistance protein (MRP) family:localization, substrate specificity, and MRP2-mediated drug resistance[J]. Biochimica et Biophysica Acta:Biomembranes, 1999, 1461(2): 377-394. DOI:10.1016/S0005-2736(99)00169-8 |
| [14] |
RIDLON J M, HARRIS S C, BHOWMIK S, et al. Consequences of bile salt biotransformations by intestinal bacteria[J]. Gut Microbes, 2016, 7(1): 22-39. DOI:10.1080/19490976.2015.1127483 |
| [15] |
LI Y Y, JADHAV K, ZHANG Y Q. Bile acid receptors in non-alcoholic fatty liver disease[J]. Biochemical Pharmacology, 2013, 86(11): 1517-1524. DOI:10.1016/j.bcp.2013.08.015 |
| [16] |
王朋, 林森, 吴德, 等. 胆汁酸的营养生理作用及代谢调控研究进展[J]. 动物营养学报, 2019, 32(5): 2002-2011. |
| [17] |
CHIANG J Y L. Regulation of bile acid synthesis:pathways, nuclear receptors, and mechanisms[J]. Journal of Hepatology, 2004, 40(3): 539-551. DOI:10.1016/j.jhep.2003.11.006 |
| [18] |
DOWLING R H. The enterohepatic circulation of bile acids as they relate to lipid disorders[J]. Journal of Clinical Pathology, 1973, 5: 59-67. |
| [19] |
RIDLON J M, KANG D J, HYLEMON P B. Bile salt biotransformations by human intestinal bacteria[J]. Journal of Lipid Research, 2006, 47(2): 241-259. DOI:10.1194/jlr.R500013-JLR200 |
| [20] |
STEINER C, HOLLEBOOM A G, KARUNA R, et al. Lipoprotein distribution and serum concentrations of 7α-hydroxy-4-cholesten-3-one and bile acids:effects of monogenic disturbances in high-density lipoprotein metabolism[J]. Clinical Science, 2012, 122(8): 385-400. DOI:10.1042/CS20110482 |
| [21] |
MONTE M J, MARIN J J, ANTELO A, et al. Bile acids:chemistry, physiology, and pathophysiology[J]. World Journal of Gastroenterology, 2009, 15(7): 804-816. DOI:10.3748/wjg.15.804 |
| [22] |
VITEK L, HALUZIK M. The role of bile acids in metabolic regulation[J]. The Journal of Endocrinology, 2016, 228(3): R85-R96. DOI:10.1530/JOE-15-0469 |
| [23] |
SCHMID A, SCHLEGEL J, THOMALLA M, et al. Evidence of functional bile acid signaling pathways in adipocytes[J]. Molecular and Cellular Endocrinology, 2019, 483: 1-10. DOI:10.1016/j.mce.2018.12.006 |
| [24] |
KADIR S H S A, MIRAGOLI M, ABU-HAYYEH S, et al. Bile acid-induced arrhythmia is mediated by muscarinic M2 receptors in neonatal rat cardiomyocytes[J]. PLoS One, 2010, 5(3): e9689. DOI:10.1371/journal.pone.0009689 |
| [25] |
ABDELKARIM M, CARON S, DUHEM C, et al. The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-γand interfering with the Wnt/β-catenin pathways[J]. Journal of Biological Chemistry, 2010, 285(47): 36759-36767. DOI:10.1074/jbc.M110.166231 |
| [26] |
CARIOU B, VAN HARMELEN K, DURAN-SANDOVAL D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice[J]. Journal of Biological Chemistry, 2006, 281(16): 11039-11049. DOI:10.1074/jbc.M510258200 |
| [27] |
GOODWIN B, JONES S A, PRICE R R, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis[J]. Molecular Cell, 2000, 6(3): 517-526. DOI:10.1016/S1097-2765(00)00051-4 |
| [28] |
INAGAKI T, CHOI M, MOSCHETTA A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis[J]. Cell Metabolism, 2005, 2(4): 217-225. DOI:10.1016/j.cmet.2005.09.001 |
| [29] |
SINAL C J, TOHKIN M, MIYATA M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis[J]. Cell, 2000, 102(6): 731-744. DOI:10.1016/S0092-8674(00)00062-3 |
| [30] |
PIRCHERP C, KITTO J L, PETROWSKI M L, et al. Farnesoid X receptor regulates bile acid-amino acid conjugation[J]. Journal of Biological Chemistry, 2003, 278(30): 27703-27711. DOI:10.1074/jbc.M302128200 |
| [31] |
LU T T, MAKISHIMA M, REPA J J, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors[J]. Molecular Cell, 2000, 6(3): 507-515. DOI:10.1016/S1097-2765(00)00050-2 |
| [32] |
ZHANG M, CHIANG J Y L. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1):roles of hepatocyte nuclear factor 4α in mediating bile acid repression[J]. Journal of Biological Chemistry, 2001, 276(45): 41690-41699. DOI:10.1074/jbc.M105117200 |
| [33] |
CHEN F, MA L, DAWSON P A, et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter[J]. Journal of Biological Chemistry, 2003, 278(22): 19909-19916. DOI:10.1074/jbc.M207903200 |
| [34] |
GROBER J, ZAGHINI I, FUJII H, et al. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene.Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer[J]. Journal of Biological Chemistry, 1999, 274(42): 29749-29754. DOI:10.1074/jbc.274.42.29749 |
| [35] |
LANDRIER J F, ELORANTA J J, VAVRICKA S R, et al. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-α and-β genes[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2006, 290(3): G476-G485. DOI:10.1152/ajpgi.00430.2005 |
| [36] |
YU C D, WANG F, KAN M, et al. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4[J]. Journal of Biological Chemistry, 2000, 275(20): 15482-15489. DOI:10.1074/jbc.275.20.15482 |
| [37] |
WANG L L, WU G X, WU F H, et al. Geniposide attenuates ANIT-induced cholestasis through regulation of transporters and enzymes involved in bile acids homeostasis in rats[J]. Journal of Ethnopharmacology, 2017, 196: 178-185. DOI:10.1016/j.jep.2016.12.022 |
| [38] |
辛瑜, 杨暄, 奚涛, 等. 孕烷X受体:肝脏疾病的潜在药物靶标[J]. 药学进展, 2018, 42(12): 922-928. |
| [39] |
KEITEL V, CUPISTI K, ULLMER C, et al. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders[J]. Hepatology, 2009, 50(3): 861-870. DOI:10.1002/hep.23032 |
| [40] |
WATANABE M, HOUTEN S M, MATAKI C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation[J]. Nature, 2006, 439(7075): 484-489. DOI:10.1038/nature04330 |
| [41] |
KEITEL V, ULLMER C, HÄUSSINGER D. The membrane-bound bile acid receptor TGR5 (GPBAR-1) is localized in the primary cilium of cholangiocytes[J]. Biological Chemistry, 2010, 391(7): 785-789. |
| [42] |
FOORD S M, BONNER T I, NEUBIG R R, et al. International Union of Pharmacology.XLVI.G protein-coupled receptor list[J]. Pharmacological Reviews, 2005, 57(2): 279-288. DOI:10.1124/pr.57.2.5 |
| [43] |
KAWAMATA Y, FUJII R, HOSOYA M, et al. A G protein-coupled receptor responsive to bile acids[J]. Journal of Biological Chemistry, 2003, 278(11): 9435-9440. DOI:10.1074/jbc.M209706200 |
| [44] |
WATANABE M, HOUTEN SM, WANG L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c[J]. Journal of Clinical Investigation, 2004, 113(10): 1408-1418. DOI:10.1172/JCI21025 |
| [45] |
BHATNAGAR S, DAMRON H A, HILLGARTNER F B. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis[J]. Journal of Biological Chemistry, 2009, 284(15): 10023-10033. DOI:10.1074/jbc.M808818200 |
| [46] |
RAO A, KOSTERS A, MELLS J E, et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice[J]. Science Translational Medicine, 2016, 8(357): 357ra122. DOI:10.1126/scitranslmed.aaf4823 |
| [47] |
HIROKANE H, NAKAHARA M, TACHIBANA S, et al. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4[J]. Journal of Biological Chemistry, 2004, 279(44): 45685-45692. DOI:10.1074/jbc.M404255200 |
| [48] |
KAST H R, NGUYEN C M, SINAL C J, et al. Farnesoid X-activated receptor induces apolipoprotein C-Ⅱ transcription:a molecular mechanism linking plasma triglyceride levels to bile acids[J]. Molecular Endocrinology, 2001, 15(10): 1720-1728. DOI:10.1210/mend.15.10.0712 |
| [49] |
CLAUDEL T, INOUE Y, BARBIER O, et al. Farnesoid X receptor agonists suppress hepatic apolipoprotein CⅢ expression[J]. Gastroenterology, 2003, 125(2): 544-555. DOI:10.1016/S0016-5085(03)00896-5 |
| [50] |
SIRVENT A, CLAUDEL T, MARTIN G, et al. The farnesoid X receptor induces very low density lipoprotein receptor gene expression[J]. FEBS Letters, 2004, 566(1/2/3): 173-177. |
| [51] |
CLAUDEL T, STURM E, DUEZ H, et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element[J]. Journal of Clinical Investigation, 2002, 109(7): 961-971. DOI:10.1172/JCI0214505 |
| [52] |
ZHANG Y Q, YIN L Y, ANDERSON J, et al. Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia[J]. Journal of Biological Chemistry, 2010, 285(5): 3035-3043. DOI:10.1074/jbc.M109.083899 |
| [53] |
GAUTIER T, DE HAAN W, GROBER J, et al. Farnesoid X receptor activation increases cholesteryl ester transfer protein expression in humans and transgenic mice[J]. Journal of Lipid Research, 2013, 54(8): 2195-2205. DOI:10.1194/jlr.M038141 |
| [54] |
D'AGATI V D, CHAGNAC A, DE VRIES A P J, et al. Obesity-related glomerulopathy:clinical and pathologic characteristics and pathogenesis[J]. Nature Reviews Nephrology, 2016, 12(8): 453-471. DOI:10.1038/nrneph.2016.75 |
| [55] |
PREIDIS G A, KIM K H, MOORE D D. Nutrient-sensing nuclear receptors PPARα and FXR control liver energy balance[J]. Journal of Clinical Investigation, 2017, 127(4): 1193-1201. DOI:10.1172/JCI88893 |
| [56] |
ALVAREZ-SOLA G, URIARTE I, LATASA M U, et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis:development of an FGF19-based chimeric molecule to promote fatty liver regeneration[J]. Gut, 2017, 66(10): 1818-1828. DOI:10.1136/gutjnl-2016-312975 |
| [57] |
PICARD A, SOYER J, BERNEY X, et al. A genetic screen identifies hypothalamic FGF15 as a regulator of glucagon secretion[J]. Cell Reports, 2016, 17(7): 1795-1806. DOI:10.1016/j.celrep.2016.10.041 |
| [58] |
VAN DIJK T H, GREFHORST A, OOSTERVEER M H, et al. An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr-/- mice[J]. Journal of Biological Chemistry, 2009, 284(16): 10315-10323. DOI:10.1074/jbc.M807317200 |
| [59] |
DURAN-SANDOVAL D, CARIOU B, PERCEVAULT F, et al. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition[J]. Journal of Biological Chemistry, 2005, 280(33): 29971-29979. DOI:10.1074/jbc.M501931200 |
| [60] |
CARON S, HUAMAN SAMANEZ C, DEHONDT H, et al. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes[J]. Molecular and Cellular Biology, 2013, 33(11): 2202-2211. DOI:10.1128/MCB.01004-12 |
| [61] |
KIR S, BEDDOW S A, SAMUEL V T, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis[J]. Science, 2011, 331(6024): 1621-1624. DOI:10.1126/science.1198363 |
| [62] |
KATSUMA S, HIRASAWA A, TSUJIMOTO G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1[J]. Biochemical and Biophysical Research Communications, 2005, 329(1): 386-390. DOI:10.1016/j.bbrc.2005.01.139 |
| [63] |
THOMAS C, GIOIELLO A, NORIEGA L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis[J]. Cell Metabolism, 2009, 10(3): 167-177. DOI:10.1016/j.cmet.2009.08.001 |
| [64] |
BRIGHTON C A, RIEVAJ J, KUHRE R E, et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors[J]. Endocrinology, 2015, 156(11): 3961-3970. DOI:10.1210/en.2015-1321 |
| [65] |
TRABELSI M S, DAOUDI M, PRAWITT J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells[J]. Nature Communications, 2015, 6: 7629. DOI:10.1038/ncomms8629 |
| [66] |
KAUR A, PATANKAR J V, DE HAAN W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1[J]. Diabetes, 2015, 64(4): 1168-1179. |
| [67] |
POPESCU I R, HELLEBOID-CHAPMAN A, LUCAS A, et al. The nuclear receptor FXR is expressed in pancreatic β-cells and protects human islets from lipotoxicity[J]. FEBS Letters, 2010, 584(13): 2845-2851. DOI:10.1016/j.febslet.2010.04.068 |
| [68] |
KUMAR D P, ASGHARPOUR A, MIRSHAHI F, et al. Activation of transmembrane bile acid receptor TGR5 modulates pancreatic islet α cells to promote glucose homeostasis[J]. Journal of Biological Chemistry, 2016, 291(13): 6626-6640. DOI:10.1074/jbc.M115.699504 |
| [69] |
POTTHOFF M J, BONEY-MONTOYA J, CHOI M, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway[J]. Cell Metabolism, 2011, 13(6): 729-738. DOI:10.1016/j.cmet.2011.03.019 |
| [70] |
JONES H, ALPINI G, FRANCIS H. Bile acid signaling and biliary functions[J]. Acta Pharmaceutica Sinica B, 2015, 5(2): 123-128. DOI:10.1016/j.apsb.2015.01.009 |
| [71] |
KWONG E, LI Y Z, HYLEMON P B, et al. Bile acids and sphingosine-1-phosphate receptor 2 in hepatic lipid metabolism[J]. Acta Pharmaceutica Sinica B, 2015, 5(2): 151-157. DOI:10.1016/j.apsb.2014.12.009 |
| [72] |
STUDER E, ZHOU X Q, ZHAO R P, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes[J]. Hepatology, 2012, 55(1): 267-276. DOI:10.1002/hep.24681 |
| [73] |
STRUB G M, MACEYKA M, HAIT N C, et al. Extracellular and intracellular actions of sphingosine-1-phosphate[J]. Oxygen Transport to Tissue ⅩⅩⅩⅢ, 2010, 688: 141-155. |
| [74] |
MIAO J, XIAO Z, KANAMALURU D, et al. Bile acid signaling pathways increase stability of small heterodimer partner (SHP) by inhibiting ubiquitin-proteasomal degradation[J]. Genes & Development, 2009, 23(8): 986-996. |




