2. 中国科学院亚热带农业生态研究所, 亚热带农业生态过程重点实验室, 湖南省畜禽健康养殖工程技术中心, 长沙 410025;
3. 中国科学院大学, 北京 100049
2. Hunan Provincial Engineering Research Center for Healthy Breeding of Livestock and Poultry, Key Laboratory of Agroecological Processes in Subtropical Region, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410025, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China
在现代养猪生产中,由于断奶、疾病、饲养环境条件等原因,仔猪易受细菌、病毒及非病原体[如脂多糖(LPS)]的侵袭,导致采食量下降、饲料转化率降低和死亡率增加等,从而刺激仔猪产生免疫应激,使肠黏膜屏障功能遭到破坏,肠道通透性增加,肠道炎症损伤加剧,最终导致多脏器功能衰竭。现阶段用于缓解免疫应激的方法主要有改善猪舍环境卫生、减少猪与病原体的接触几率[1]、饲粮中添加抗生素[2]和免疫抑制药物[3-4]等。其中抗生素在饲粮中的大量使用不仅会造成细菌耐药性增强,而且会在动物体内残留,直接影响畜产品质量,严重威胁人类健康。免疫抑制药物虽能缓解免疫应激反应,但也会抑制机体的正常免疫功能。研究表明,饲粮中添加红芪多糖、白头翁素、小檗碱等植物提取物能够缓解免疫应激,其可通过抑制炎性细胞因子的产生,强化免疫系统,提高机体抵抗力[5-7]。血根碱(SAG)是一种苯菲啶异喹啉生物碱[8],其在抗炎[9-10]、抑菌[11-13]、提高机体免疫力[14-15]、改善肠道健康和功能维持[16-17]等方面能发挥着重要作用,且具有易降解、低残留、无环境公害等特点。研究表明,饲粮中添加0.50 mg/kg血根碱可提高断奶仔猪的平均日采食量(ADFI)及血清总蛋白(TP)、免疫球蛋白M(IgM)和免疫球蛋白G(IgG)含量,降低料重比(F/G)和腹泻率[14]。Liu等[18]研究发现,饲粮中添加40 mg/kg血根碱能提高断奶仔猪的ADFI和平均日增重(ADG)。陈家顺等[19]研究发现,饲粮中添加血根碱可通过改善肠道黏膜发育情况,增进肠黏膜免疫功能,进而促进早期断奶仔猪更加健康快速地生长。Bavarsadi等[20]、姬晓琪等[21]和Khadem等[22]研究显示,饲粮中添加血根碱可增强鸡的细胞和体液免疫,降低炎症因子水平,改善肠道结构和健康状况。由此可见,血根碱是一种潜在的可用于缓解畜禽免疫应激的天然植物添加剂。鉴于此,本试验以腹腔注射LPS构建仔猪免疫应激模型,并在饲粮中添加血根碱,探讨其对LPS免疫应激断奶仔猪生长性能、免疫功能及肠道健康的影响,以期为缓解免疫应激提供一种新的选择方向和理论基础,并为血根碱在养猪生产中的应用提供一定的参考。
1 材料与方法 1.1 试验材料血根碱制剂(血根碱含量为1.5%)由湖南美可达生物资源有限公司提供,LPS型号为大肠杆菌(E. coli) serotype 055 : B5,由美国Sigma Chemical公司提供,将其以无菌生理盐水配成浓度为100 μg/mL的稀释液,超滤除菌。
1.2 试验动物与试验设计试验选取(21±1)日龄、平均体重为(6.20±0.09) kg的健康“杜×长×大”断奶仔猪24头,按体重相近原则随机分为4组,分别为对照(CON)组(基础饲粮)、LPS组(基础饲粮+腹腔注射100 μg/kg BW LPS)、SAG组(基础饲粮+50 mg/kg血根碱)和SAG+LPS组(基础饲粮+50 mg/kg血根碱+腹腔注射100 μg/kg BW LPS),每组6个重复,每个重复1头猪。试验期21 d。试验采用2×2双因子设计,主因子为血根碱(饲粮中添加、不添加)和LPS(腹腔注射、不注射)。分别于试验第14、21天给仔猪腹腔注射LPS或等量的生理盐水,于最后一次注射LPS 12 h后麻醉致死取样。
1.3 试验饲粮基础饲粮的配制参照NRC(2012)仔猪营养需要标准,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
本试验于中国科学院亚热带农业生态研究所动物饲养实验室进行,仔猪采用单笼饲养,全封闭管理,栏舍为不锈钢材质,乳头式自由饮水器,并不定期对猪舍进行消毒、卫生清洁。仔猪每天喂食4次,粉料饲喂,时间分别为07:30、11:30、18:30和21:30,自由饮水和采食,以食槽略有剩料为原则。
1.5 样品采集于试验第21天,仔猪腹腔注射LPS 3 h后前腔静脉采血10 mL,静置15 min,4 ℃下3 000 r/min离心15 min分离制备血清,-80 ℃保存待用。腹腔注射LPS 12 h后将仔猪麻醉处死,取十二指肠、空肠、回肠肠断各约2 cm,于10%中性福尔马林溶液中固定,以备小肠形态学检测。
1.6 测定指标及方法 1.6.1 生长性能于试验第1、14和21天对仔猪逐个进行空腹称重,并准确记录;每天准确称量每头仔猪的给料量和剩料量,并记录每天的采食量。分别计算每组仔猪LPS攻毒前、后的ADFI、ADG和F/G。计算公式为:

|
采用全自动生化仪检测血清IgG、IgM含量,测定试验在中国科学院亚热带农业生态研究所生态楼实验室进行;采用酶联免疫吸附试验(ELISA)试剂盒检测血清肿瘤坏死因子-α(TNF-α)、白细胞介素(IL)-8、IL-6、IL-1β和IL-10含量,试剂盒由武汉华美生物工程有限公司提供。
1.6.3 肠道通透性采用试剂盒测定血清二胺氧化酶(DAO)活性和D-乳酸(D-LA)含量,试剂盒由南京建成生物工程研究所提供,操作方法严格按照说明书进行。
1.6.4 肠道形态结构将10%固定液中的十二指肠、空肠、回肠肠道组织取出,经常规水洗、浸蜡、包埋、切片、苏木精-伊红(HE)染色后,在光学显微镜下观察,选取6个走向延伸良好的视野,用Magic Images Advanced 3.2软件测量绒毛高度(VH)和隐窝深度(CD),并计算绒毛高度/隐窝深度(V/C)。
1.7 数据分析所有试验数据均用Excel 2016件初步处理,采用SAS 9.2软件进行2×2双因素统计分析,该统计模型包括血根碱(饲粮中添加、不添加)、LPS(腹腔注射、不注射)及其交互作用。部分数据采用Duncan氏法进行单因素方差分析(one-way ANOVA),P < 0.05表示差异显著。所有数据均以“平均值±标准误”表示。
2 结果与分析 2.1 血根碱对LPS免疫应激断奶仔猪生长性能的影响由表 2可知,LPS攻毒前(1~14 d),饲粮中添加血根碱显著提高断奶仔猪的ADFI和ADG(P < 0.05),显著降低F/G(P < 0.05),对体重无显著影响(P>0.05)。攻毒期间(15~21 d),LPS攻毒显著降低断奶仔猪的ADG和ADFI(P < 0.05),显著提高F/G(P < 0.05),对体重无显著影响(P>0.05);饲粮中添加血根碱显著提高ADFI和ADG(P < 0.05),显著降低F/G(P < 0.05)。饲粮中添加血根碱与LPS攻毒的交互作用对断奶仔猪的体重、ADFI、ADG和F/G均无显著影响(P>0.05)。由此推断,LPS攻毒可以诱发断奶仔猪严重的免疫应激反应,使其生长性能下降;饲粮中添加血根碱不仅可以提高生长性能,还能缓解LPS免疫应激引起的生长性能下降。
|
|
表 2 血根碱对LPS免疫应激断奶仔猪生长性能的影响 Table 2 Effects of SAG on growth performance of immune-stressed weaned piglets challenged with LPS (n=6) |
从表 3可知,攻毒结束后,饲粮中添加血根碱和LPS攻毒均可显著提高断奶仔猪的血清IgG和IgM含量(P < 0.05);与LPS组相比,SAG+LPS组的血清IgG和IgM含量显著增加(P < 0.05)。饲粮中添加血根碱与LPS攻毒的交互作用对断奶仔猪的血清IgG和IgM含量无显著影响(P>0.05)。由此推断,LPS免疫应激激活了断奶仔猪的免疫系统来进行防御和自我保护,饲粮中添加血根碱使这种防护作用进一步加强。
|
|
表 3 血根碱对LPS免疫应激断奶仔猪血清免疫球蛋白含量的影响 Table 3 Effects of SAG on the contents of immunoglobulin in serum of immune-stressed weaned piglets challenged with LPS |
由表 4可知,攻毒结束后,LPS攻毒显著提高断奶仔猪的血清IL-1β、IL-6和TNF-α含量(P < 0.05),显著降低血清IL-10含量(P < 0.05);饲粮中添加血根碱显著降低血清IL-1β、IL-6和TNF-α含量(P < 0.05),显著增加血清IL-10含量(P < 0.05);与LPS组比,SAG+LPS组的血清IL-1β、IL-6和TNF-α含量显著降低(P < 0.05),血清IL-10含量显著增加(P < 0.05)。各组断奶仔猪的血清IL-8含量无显著差异(P>0.05)。饲粮中添加血根碱与LPS攻毒的交互作用对断奶仔猪的血清TNF-α含量有显著影响(P < 0.05)。由此推断,LPS免疫应激可以诱发断奶仔猪的炎症反应,而饲粮中添加血根碱可以缓解炎症反应。
|
|
表 4 血根碱对LPS免疫应激断奶仔猪血清炎症因子含量的影响 Table 4 Effects of SAG on the contents of inflammatory factor in serum of immune-stressed weaned piglets challenged with LPS |
由表 5可知,攻毒结束后,LPS攻毒显著提高断奶仔猪的血清D-LA含量和DAO活性(P < 0.05);饲粮中添加SAG显著降低血清D-LA含量和DAO活性(P < 0.05);与LPS组比,SAG+LPS组的血清D-LA含量和DAO活性显著降低(P < 0.05)。饲粮中添加血根碱与LPS攻毒的交互作用对断奶仔猪的血清D-LA含量和DAO活性有显著影响(P < 0.05)。由此推断,LPS免疫应激可引起断奶仔猪的肠道损伤,使肠道通透性增加,饲粮中添加血根碱可缓解这种肠道损伤和通透性改变。
|
|
表 5 血根碱对LPS免疫应激断奶仔猪肠道通透性的影响 Table 5 Effects of SAG on intestinal permeability of immune-stressed weaned piglets challenged with LPS |
由表 6和图 1可知,攻毒结束后,LPS攻毒显著降低断奶仔猪的十二指肠、回肠VH及V/C,显著提高十二指肠CD(P < 0.05),对空肠VH、CD、V/C和回肠CD无显著影响(P>0.05);饲粮中添加血根碱显著提高十二指肠、空肠、回肠VH及V/C,显著降低十二指肠CD(P < 0.05),对空肠、回肠CD无显著影响(P>0.05);与LPS组相比,SAG+LPS组的十二指肠、空肠和回肠VH和V/C显著增加(P < 0.05),十二指肠CD显著降低(P < 0.05),空肠、回肠CD无显著变化(P>0.05)。饲粮中添加血根碱与LPS攻毒的交互作用对断奶仔猪的十二指肠、空肠和回肠的VH、CD及V/C无显著影响(P>0.05)。由此推断,LPS免疫应激对断奶仔猪的小肠黏膜造成损伤,使其比表面积减小,而饲粮中添加血根碱会在一定程度上缓解这种损伤,有益于断奶仔猪小肠健康及发育。
|
|
表 6 血根碱对LPS免疫应激断奶仔猪肠道形态结构的影响 Table 6 Effects of SAG on intestinal morphology of immune-stressed weaned piglets challenged with LPS |
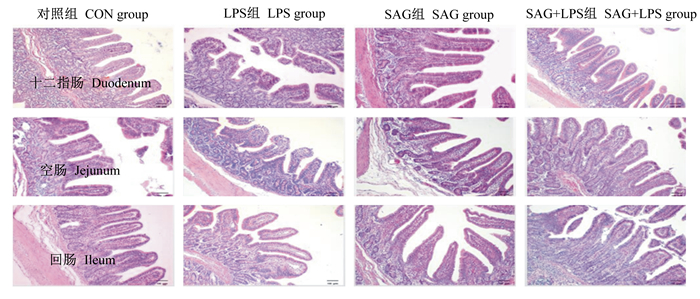
|
图 1 断奶仔猪肠道形态结构 Fig. 1 Intestinal morphology of weaned piglets (100×) |
免疫应激是动物在受到外界抗原侵袭时,机体为抵御其危害,免疫系统被高度激活,进而引发动物代谢和行为的改变,导致生长性能下降、饲料利用率降低、生长迟滞的一种现象[23]。目前腹腔注射一定剂量的LPS是模拟免疫应激最典型的方式之一。LPS是革兰氏阴性菌的重要组分多糖,注射入体内,动物会产生发热、呕吐、腹泻和厌食等多种症状。本试验结果显示,注射LPS后,断奶仔猪出现强烈的高烧、呕吐、腹泻、嗜睡、24 h内几乎不采食、精神状态极差等症状,说明本次试验用LPS营造免疫应激模型是成功的。然而,腹腔注射LPS模拟免疫应激虽为科研工作者普遍采用,但现实猪场中存在的是较温和的持续慢性免疫应激,而LPS刺激产生的是急性免疫应激,反应较剧烈,发挥效价有一定的时间限度,且与LPS的注射剂量、动物的年龄等密切相关,故而在仔猪上科研工作者多采用低剂量多次注射的方法来模拟免疫应激[24-25]。又考虑到短期连续多次注射LPS会使仔猪产生耐受性,还可能出现死亡[26],而且注射过程产生的应激可能会严重影响甚至掩盖LPS导致的应激效果,因此本试验采用第14天和第21天分别注射1次LPS的方法来模拟猪场免疫应激,Zhu等[27]和Yi等[28]报道的LPS注射试验设计与本试验类似。已有报道证实,LPS应激可造成仔猪生长性能显著降低[29-30]。本试验结果显示,注射LPS显著降低断奶仔猪的ADG和ADFI,同时显著提高F/G,与上述报道基本一致。血根碱是一种天然的植物提取物,其作为一种饲料添加剂在动物营养和饲料中的应用,主要是作为抗生素的替代物来发挥促生长作用。Kantas等[31]研究表明,饲粮中添加血根碱可显著提高断奶仔猪的体重、ADG、ADFI和饲料转化率。Chen等[32]研究证实,饲粮中添加血根碱可以显著提高仔猪的ADG和ADFI,对仔猪的F/G有降低的趋势。李美荃等[33]和蔡鹏等[34]研究也证明,饲粮中添加不同水平血根碱可显著提高仔猪的ADG和ADFI,并显著降低F/G。本试验研究发现,LPS攻毒前(1~14 d),饲粮中添加50 mg/kg的血根碱制剂显著提高断奶仔猪的ADG和ADFI,显著降低F/G,这与上述结果基本一致。LPS攻毒期间(15~21 d),饲粮中添加血根碱显著提高断奶仔猪的ADFI和ADG,显著降低F/G,这表示血根碱有益于仔猪抵御LPS诱发的应激反应,调控其导致的生长抑制作用。原因可能是血根碱能够抑制一些炎症因子的过多分泌[10, 35],促进对色氨酸、苯丙氨酸等氨基酸的利用,改善机体健康状况,进而促进生长发育[36]。
3.2 血根碱对LPS免疫应激断奶仔猪免疫功能的影响免疫球蛋白IgM和IgG由B细胞分泌产生,是机体体液免疫的重要组成部分,也是机体免疫功能的重要衡量指标。本试验结果显示,注射LPS使断奶仔猪的血清IgM和IgG含量显著升高,而饲粮中添加血根碱使血清IgM和IgG含量更高,原因可能是仔猪受LPS刺激诱发了免疫应激反应,被激活的免疫系统启动免疫应答,由浆细胞迅速分泌大量的IgM和IgG进行防御,但免球蛋白的合成需要大量的能量,而血根碱既能促进小肠的发育[19-22],使其对营养物质的消化吸收能力更强,同时还能保护细胞线粒体免受LPS诱导氧化应激造成的损伤[37],进而为机体提供更多的能量,最终使浆细胞分泌更多的IgM和IgG抗体。
LPS诱导免疫应激,体内T细胞、巨噬细胞、单核细胞等受到刺激释放细胞因子[9, 38]。IL-1β能引起急性和慢性炎症的发生[39];IL-6通过强化其他炎性细胞因子的作用来参与炎症反应的发展[38];IL-8主要由单核-巨噬细胞产生,能诱导超氧化物的形成,造成细胞和组织损伤[40];TNF-α是一种能发挥多种不同生理作用的促炎细胞因子,能够诱导肠上皮细胞凋亡[41-42];IL-10是一种抑炎因子,能够通过阻止核转录因子-κB(NF-κB)的活化来抑制其他炎性因子的产生,是免疫反应重要的调节因子[43]。本试验结果显示,攻毒结束后,LPS刺激使断奶仔猪的血清IL-1β、IL-6和TNF-α含量显著升高,血清IL-10含量显著降低;饲粮中添加血根碱使血清IL-1β、IL-6和TNF-α含量显著降低,血清IL-10含量显著升高。这表明饲粮中添加血根碱能抑制仔猪免疫应激诱导的炎症反应,促进机体健康,原因可能是血根碱能通过抑制核因子κB抑制因子(IκB)的磷酸化,阻碍NF-κB激活,减少炎性细胞因子的表达,最终起到抑制炎症反应的作用[9]。
3.3 血根碱对LPS免疫应激断奶仔猪肠道健康的影响小肠是动物机体内营养物质消化和吸收的主要场所,因此小肠黏膜形态结构和通透性可以反映出动物的健康状况和对营养物质消化吸收能力的强弱。小肠绒毛越高,隐窝越浅,二者比值越大,说明吸收面积越大,功能越好。Sukhotnik等[44]和王蕾等[45]研究证实,LPS刺激可对小肠黏膜造成损伤,致使绒毛萎缩,隐窝加深。本试验结果显示,攻毒结束后,LPS刺激使断奶仔猪的十二指肠、回肠VH及V/C显著降低,十二指肠CD显著增加,与上述报道相一致。本试验还发现,LPS攻毒后,饲粮中添加血根碱使断奶仔猪的十二指肠、空肠、回肠的VH及V/C显著增加,十二指肠CD显著降低。陈家顺等[19]研究表明,饲粮中添加50 mg/kg的血根碱能显著提高仔猪小肠VH和V/C,有降低空肠CD的趋势,与本试验结果相一致。这说明血根碱可以缓解仔猪免疫应激诱导的小肠形态结构损伤,可能原因如下:1)血根碱能增强食欲,提高采食量,保持了更多的能量供给;2)血根碱能保护细胞线粒体免受LPS诱导氧化应激造成的损伤,改善其能量状态,减少小肠上皮细胞凋亡[37, 46]。
D-LA在哺乳动物体内由肠道微生物代谢产生,但哺乳动物缺乏迅速代谢D-LA的酶,当肠黏膜受损、通透性增加时,D-LA会进入血液,导致血清D-LA含量增加,因此,血清D-LA含量高低可以反映肠道的健康状态[47]。DAO是哺乳动物肠上皮细胞内酶,参与蛋白质、核酸质等的合成[48]。只有肠黏膜损伤时,DAO才会进入血液,因此血清DAO活性也可反映肠道的健康状态。已有报道证实,LPS刺激会导致仔猪肠黏膜紧密连接蛋白表达量下降,通透性增加[44]。本试验结果显示,攻毒结束后,LPS刺激显著提高断奶仔猪的血清D-LA含量和DAO活性,这与以上结果相符。此外,本试验结果还显示,LPS刺激下,饲粮中添加血根碱使断奶仔猪的血清D-LA含量和DAO活性显著降低,这表明LPS刺激造成了小肠黏膜损伤,而血根碱能缓解这种损伤。原因可能是血根碱能促进小肠黏膜紧密连接蛋白的表达,抑制炎性细胞因子的表达,从而降低对肠道黏膜屏障的损伤[49-50]。
4 小结饲粮中添加血根碱可以缓解断奶仔猪免疫应激导致的生长性能下降,提高血清IgG和IgM含量,抑制血清IL-1β、IL-6、TNF-α含量的增加和IL-10含量的降低,进而增强其免疫抵抗能力,缓解肠道通透性增加,并在一定程度上改善小肠黏膜形态结构,从而使断奶仔猪的肠道健康得到改善。因此,血根碱可有效缓解免疫应激,用于减少或替代抗生素在断奶仔猪饲粮中使用。
| [1] |
刘玉兰, 鲁晶, 石君霞, 等. 猪免疫应激研究进展[J]. 畜牧与兽医, 2008, 40(2): 99-102. |
| [2] |
HOPKINS S J, ROTHWELL N J. Cytokines and the nervous system Ⅰ:expression and recognition[J]. Trends in Neurosciences, 1995, 18(2): 83-88. DOI:10.1016/0166-2236(95)80029-2 |
| [3] |
GELMAN L, FRUCHART J C, AUWERX J. An update on the mechanisms of action of the peroxisome proliferator-activated receptors (PPARs) and their roles in inflammation and cancer[J]. Cellular and Molecular Life Sciences CMLS, 1999, 55(6/7): 932-943. |
| [4] |
ZHANG X, YOUNG H A. PPAR and immune system-what do we know?[J]. International Immunopharmacology, 2002, 2(8): 1029-1044. DOI:10.1016/S1567-5769(02)00057-7 |
| [5] |
YU S, ZHANG W, YU J, et al. Inhibiting effect of Radix hedysari polysaccharide (HPS) on endotoxin-induced uveitis in rats[J]. International Immunopharmacology, 2014, 21(2): 361-368. DOI:10.1016/j.intimp.2014.05.016 |
| [6] |
XIAO K, CAO S T, JIAO L F, et al. Anemonin improves intestinal barrier restoration and influences TGF-β1 and EGFR signaling pathways in LPS-challenged piglets[J]. Innate Immunity, 2016, 22(5): 344-352. DOI:10.1177/1753425916648223 |
| [7] |
李红梅, 王一阳, 王华东, 等. 小檗碱和育亨宾对LPS诱导的小鼠肠道损伤和肠上皮细胞增殖抑制的影响[J]. 中国病理生理杂志, 2010, 26(5): 941-946. DOI:10.3969/j.issn.1000-4718.2010.05.022 |
| [8] |
LU J J, BAO J L, CHEN X P, et al. Alkaloids isolated from natural herbs as the anticancer agents[J]. Evidence-Based Complementary and Alternative Medicine, 2012, 2012: 485042. |
| [9] |
CHATURVEDI M M, KUMAR A, DARNAY B G, et al. Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-κB activation, IκBα phosphorylation, and degradation[J]. Journal of Biological Chemistry, 1997, 272(48): 30129-30134. DOI:10.1074/jbc.272.48.30129 |
| [10] |
LI W F, LI H N, YAO H, et al. Pharmacokinetic and anti-inflammatory effects of sanguinarine solid lipid nanoparticles[J]. Inflammation, 2014, 37(2): 632-638. DOI:10.1007/s10753-013-9779-8 |
| [11] |
NANDI R, MAITI M, CHAUDHURI K, et al. Sensitivity of vibrios to sanguinarine[J]. Experientia, 1983, 39(5): 524-525. DOI:10.1007/BF01965187 |
| [12] |
赵东亮, 郁建平, 周晓秋, 等. 博落回生物碱的抑菌作用研究[J]. 食品科学, 2005, 26(1): 45-47. DOI:10.3321/j.issn:1002-6630.2005.01.004 |
| [13] |
郁建平, 赵东亮, 孟祥斌, 等. 博落回生物碱对八种真菌的抑菌作用研究[J]. 贵州大学学报(自然科学版), 2006, 23(1): 77-80. DOI:10.3969/j.issn.1000-5269.2006.01.017 |
| [14] |
何夏阳.血根碱对断奶仔猪生长性能、养分消化率和血液生化指标的影响[D].硕士学位论文.长沙: 湖南农业大学, 2010. http: //cdmd.cnki.com.cn/article/cdmd-10537-1011067414.htm
|
| [15] |
BOJJIREDDY N, SINHA R K, PANDA, et al. Sanguinarine suppresses IgE induced inflammatory responses through inhibition of type Ⅱ PtdIns 4-kinase(s)[J]. Archives of Biochemistry and Biophysics, 2013, 537(2): 192-197. DOI:10.1016/j.abb.2013.07.017 |
| [16] |
刘靖.博落回生物碱对黄羽肉鸡生长的影响[D].硕士学位论文.长沙: 湖南农业大学, 2010. http: //cdmd.cnki.com.cn/article/cdmd-10537-2010226643.htm
|
| [17] |
VRUBLOVA E, VOSTALOVA J, EHRMANN J, et al. The phytogenic feed additive sangrovit modulates dextran sulfate sodium-induced colitis in rats[J]. Veterinarni Medicina, 2010, 55(12): 610-618. DOI:10.17221/2945-VETMED |
| [18] |
LIU G, AGUILAR Y M, ZHANG L, et al. Dietary supplementation with sanguinarine enhances serum metabolites and antibodies in growing pigs[J]. Journal of Animal Science, 2016, 94(Suppl.3): 75-78. |
| [19] |
陈家顺, 康宝聚, 曾建国, 等. 血根碱对断奶仔猪生长性能、肠道形态结构及小肠黏膜免疫功能的影响[J]. 动物营养学报, 2018, 30(5): 1845-1853. DOI:10.3969/j.issn.1006-267x.2018.05.027 |
| [20] |
BAVARSADI M, MAHDAVI A H, ANSARI-MAHYARI S, et al. Effects of different levels of sanguinarine on antioxidant indices, immunological responses, ileal microbial counts and jejunal morphology of laying hens fed diets with different levels of crude protein[J]. Journal of Animal Physiology and Animal Nutrition, 2017, 101(5): 936-948. DOI:10.1111/jpn.12528 |
| [21] |
姬晓琪, 钟杰, 张明军, 等. 血根碱对蛋鸡输卵管炎的治疗作用研究[J]. 中兽医医药杂志, 2017, 36(5): 59-62. |
| [22] |
KHADEM A, SOLER L, EVERAERT N, et al. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties[J]. British Journal of Nutrition, 2014, 112(7): 1110-1118. DOI:10.1017/S0007114514001871 |
| [23] |
JOHNSON W R. Inhibition of growth by pro-inflammatory cytokines:an integrated view[J]. Journal of Animal Science, 1997, 75(5): 1244-1255. DOI:10.2527/1997.7551244x |
| [24] |
BALAJI R, WRIGHT K J, HILL C M, et al. Acute phase responses of pigs challenged orally with Salmonella typhimurium[J]. Journal of Animal Science, 2000, 78(7): 1885-1891. DOI:10.2527/2000.7871885x |
| [25] |
HOU Y Q, WANG L, ZHANG W, et al. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide[J]. Amino Acids, 2012, 43(3): 1233-1242. DOI:10.1007/s00726-011-1191-9 |
| [26] |
黎文彬.酵母硒对脂多糖(LPS)诱导的免疫应激早期断奶仔猪的影响[D].硕士学位论文.雅安: 四川农业大学, 2009. http: //cdmd.cnki.com.cn/Article/CDMD-10626-2009258556.htm
|
| [27] |
ZHU C, WU Y P, JIANG Z Y, et al. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide[J]. International Immunopharmacology, 2015, 28(1): 288-294. DOI:10.1016/j.intimp.2015.04.054 |
| [28] |
YI D, HOU Y Q, WANG L, et al. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets[J]. British Journal of Nutrition, 2014, 111(1): 46-54. DOI:10.1017/S0007114513002171 |
| [29] |
张海文, 徐欣欣, 洪枫, 等. 抗菌肽CJH对LPS刺激的仔猪生长性能及肉品质的影响[J]. 黑龙江畜牧兽医, 2017(22): 154-156. |
| [30] |
KEGLEY E B, SPEARS J W, AUMAN S K. Dietary phosphorus and an inflammatory challenge affect performance and immune function of weanling pigs[J]. Journal of Animal Science, 2001, 79(2): 413-419. DOI:10.2527/2001.792413x |
| [31] |
KANTAS D, PAPATSIROS V G, TASSIS P D, et al. The effect of a natural feed additive (Macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs[J]. Animal Science Journal, 2015, 86(1): 92-98. DOI:10.1111/asj.12240 |
| [32] |
CHEN J S, KANG B J, ZHAO Y R, et al. Effects of natural dietary supplementation with Macleaya cordata extract containing sanguinarine on growth performance and gut health of early-weaned piglets[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(6): 1666-1674. DOI:10.1111/jpn.12976 |
| [33] |
李美荃, 张春勇, 满意, 等. 博落回提取物在仔猪生产中的应用效果研究[J]. 家畜生态学报, 2013, 34(9): 50-55. DOI:10.3969/j.issn.1673-1182.2013.09.010 |
| [34] |
蔡鹏, 孙志良, 曾建国, 等. 不同剂量博落回提取物对断奶仔猪生长性能的影响[J]. 中国畜牧兽医, 2010, 37(5): 41-43. |
| [35] |
NIU X F, FAN T, LI W F, et al. The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages[J]. European Journal of Pharmacology, 2012, 689(1/2/3): 262-269. |
| [36] |
DRSATA J, ULRICHOVÁ J, WALTEROVÁ D. Sanguinarine and chelerythrine as inhibitors of aromatic amino acid decarboxylase[J]. Journal of Enzyme Inhibition, 1996, 10(4): 231-237. DOI:10.3109/14756369609036530 |
| [37] |
王玲, 干学东. 血根碱对脂多糖致H9c2细胞氧化损伤的改善作用[J]. 中国现代应用药学, 2018, 35(10): 1451-1456. |
| [38] |
WEBEL D M, FINCK B N, BAKER D H, et al. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide[J]. Journal of Animal Science, 1997, 75(6): 1514-1520. DOI:10.2527/1997.7561514x |
| [39] |
DINARELLO C A. The Role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1[J]. The New England Journal of Medicine, 2000, 343(10): 732-734. DOI:10.1056/NEJM200009073431011 |
| [40] |
LYONS M J, YOSHIMURA T, MCMURRAY D N. Interleukin (IL)-8(CXCL8) induces cytokine expression and superoxide formation by guinea pig neutrophils infected with Mycobacterium tuberculosis[J]. Tuberculosis, 2004, 84(5): 283-292. DOI:10.1016/j.tube.2003.09.003 |
| [41] |
VAN DULLEMEN H M, VAN DEVENTER S J H, HOMMES D W, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2)[J]. Gastroenterology, 1995, 109(1): 129-135. DOI:10.1016/0016-5085(95)90277-5 |
| [42] |
严瑾, 欧阳钦, 刘卫平, 等. 肿瘤坏死因子-α在溃疡性结肠炎中的表达及其作用探讨[J]. 胃肠病学, 2005, 10(5): 269-272. DOI:10.3969/j.issn.1008-7125.2005.05.004 |
| [43] |
WANG P, WU P, SIEGEL M I, et al. Interleukin (IL)-10 inhibits nuclear factor κB (NF-κB) activation in human monocytes.IL-10 and IL-4 suppress cytokine synthesis by different mechanisms[J]. Journal of Biological Chemistry, 1995, 270(16): 9558-9563. DOI:10.1074/jbc.270.16.9558 |
| [44] |
SUKHOTNIK I, MOGILNER J, KRAUSZ M M, et al. Oral arginine reduces gut mucosal injury caused by lipopolysaccharide endotoxemia in rat[J]. Journal of Surgical Research, 2004, 122(2): 256-262. DOI:10.1016/j.jss.2004.07.004 |
| [45] |
王蕾, 刘坚, 侯永清, 等. α-酮戊二酸对LPS慢性应激仔猪小肠黏膜形态与功能的影响[J]. 畜牧兽医学报, 2010, 41(1): 46-52. |
| [46] |
HONG Y Q, KANG Y, LEI W, et al. Effects of α-ketoglutarate on energy status in the intestinal mucosa of weaned piglets chronically challenged with lipopolysaccharide[J]. British Journal of Nutrition, 2011, 106(3): 357-363. DOI:10.1017/S0007114511000249 |
| [47] |
乔治, 黎沾良, 李基业, 等. 应用血浆D-乳酸水平评价腹部外科手术后肠道屏障功能[J]. 解放军医学杂志, 2005, 30(3): 255-257. DOI:10.3321/j.issn:0577-7402.2005.03.024 |
| [48] |
LUK G D, BAYLESS T M, BAYLIN S B. Plasma postheparin diamine oxidase.Sensitive provocative test for quantitating length of acute intestinal mucosal injury in the rat[J]. The Journal of Clinical Investigation, 1983, 71(5): 1308-1315. DOI:10.1172/JCI110881 |
| [49] |
LIU G, GUAN G P, FANG J, et al. Macleaya cordata extract decreased diarrhea score and enhanced intestinal barrier function in growing piglets[J]. BioMed Research International, 2016, 2016: 1069585. |
| [50] |
王丽杰, 孙梅. 炎症反应与小儿胃肠功能障碍[J]. 国外医学儿科学分册, 2005, 32(4): 223-225. |




