在目前的集约化养殖模式下,许多因素会导致仔猪发生氧化应激,如猪舍中的有毒有害气体、高温高湿及细菌与病毒感染等。猪处于氧化应激状态时,产生大量活性氧,机体内产生的自由基得不到及时有效清除,使体内氧化系统与抗氧化系统失衡,导致机体损伤[1-2]。氧化应激是许多疾病发生的病理生理基础,它会造成动物生长性能下降、饲料转化效率降低、畜产品品质下降、免疫力降低、发病率升高等,给养殖业带来经济损失[3-5]。
肝脏是脂质代谢的重要场所,在机体能量稳态中具有不可忽视的作用,它是脂肪酸合成和脂质循环的中枢,而脂肪酸是最常见的能量储存和循环形式。研究表明,脂肪从头合成对于肝脏中脂肪沉积贡献率达到26%,肝脏脂质代谢紊乱会加重肝脏负荷,影响仔猪生长性能[6],因此研究肝脏中脂质合成是必要的。固醇调节元件结合蛋白-1c(SREBP-1c)在肝脏脂质合成基因的转录中发挥重要作用,它主要调节脂肪酸合成相关基因如脂肪酸合成酶(FAS)、硬脂酰辅酶A去饱和酶1(SCD1)以及乙酰辅酶A羧化酶(ACC)等的转录,从而调节脂肪酸的合成。研究表明,氧化应激会导致肝细胞中脂质积聚,机体在处于氧化应激状态时,SREBP-1c表达上调,其下游基因FAS、ACC、SCD1表达量增加,脂质合成增多,同时氧化应激还会增加甘油三酯(TG)在肝脏内的积聚,造成脂质积累[7-9]。因此,为了减少氧化应激带来的负面影响,降低经济损失,探索开发绿色安全的天然抗氧化剂显得尤为重要。
山竹醇(garcinol)是一种来自藤黄属植物的黄色晶体化合物,含有酚羟基和β-二酮,属于聚异戊二烯化二苯甲酮衍生物[10-11]。许多研究报道,山竹醇具有广泛的药理作用,如抗炎、抗氧化、清除自由基等,具有广泛的研究前景[12-15]。Yamaguchi等[16]研究表明,在次黄嘌呤/黄嘌呤氧化酶体系中,山竹醇对超氧阴离子的抑制作用与维生素E几乎相同;在芬顿反应体系中,山竹醇对羟基自由基的抑制作用强于维生素E。然而,当仔猪受到刺激发生氧化应激时,山竹醇的抗氧化保护效应尚不清楚,且山竹醇对于猪氧化应激状态下脂质合成方面影响的研究还鲜见报道。因此,本研究在验证正常饲养条件下山竹醇在仔猪上应用效果的基础上,通过腹腔注射敌草快(diquat)建立仔猪氧化应激模型,进一步研究山竹醇对氧化应激状态下仔猪生长性能、抗氧化功能及肝脏脂质合成的影响,为山竹醇在仔猪饲粮中的合理应用提供理论依据。
1 材料与方法 1.1 试验材料山竹醇购自陕西昊辰有限公司,有效含量为30%;diquat购自Toronto Research Chemicals公司,使用时用灭菌生理盐水配成diquat溶液。
1.2 试验设计与试验动物选取健康状况良好、胎次相近的(9.20±0.38) kg“杜×长×大”三元杂交断奶仔猪30头,根据体重相近原则随机分为5个组,分别为对照组、应激组和3个山竹醇组,每组6个重复,每个重复1头猪。对照组和应激组仔猪饲喂基础饲粮,山竹醇组仔猪饲喂在基础饲粮中分别添加200、400、600 mg/kg山竹醇的试验饲粮,预饲7 d后开始正式试验,试验期28 d。应激组和山竹醇组仔猪于试验第15天按照10 mg/kg BW的剂量腹腔注射diquat溶液[17-18],对照组腹腔注射等量灭菌生理盐水。各组仔猪全程单笼饲养。
1.3 试验饲粮基础饲粮参照NRC(2012)仔猪营养需要进行配制,其组成及营养水平见表 1。在基础饲粮中添加试验设计剂量的山竹醇配成试验饲粮。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
试验在华中农业大学代谢室进行,室温保持在26 ℃左右,相对湿度保持在55%~65%,每天喂料4次(08:00、12:00、16:00、20:00),每次以仔猪吃饱后料槽内略有余料为度,自由饮水。圈舍每天打扫,注意通风换气,定期消毒。
1.5 样品采集与处理 1.5.1 血清所有仔猪禁食12 h后,于试验第15天和第29天早上前腔静脉采血,置于普通采血管中,静置30 min后3 500 r/min离心10 min,分离血清,分装后于-20 ℃保存待测。
1.5.2 肝脏于试验第29天早上称重采血后,仔猪注射戊巴比妥钠麻醉后,放血、剖开腹腔。迅速分离肝脏,用预冷生理盐水冲洗,迅速取样。肝脏分装入离心管后放入液氮速冻,于-80 ℃保存待测。
1.6 测定指标与方法 1.6.1 生长性能试验期间准确记录每个重复试验猪每天的采食量,在试验第1、15、和29天早上试验猪空腹称重,计算第1~14天和第15~28天的平均日增重(ADG)、平均日采食量(ADFI)和料重比(F/G)。
1.6.2 血清、肝脏生化指标采用试剂盒测定血清、肝脏总抗氧化能力(T-AOC),超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)活性以及丙二醛(MDA)含量,所有试剂盒均购自南京建成生物工程研究所,详细操作方法参见试剂盒说明书。血清TG和总胆固醇(TC)含量使用全自动生化分析仪测定。
1.6.3 肝脏组织形态肝组织支持石蜡切片后苏木精-伊红(HE)染色,染色步骤如下:切片入苏木素染3~8 min,自来水洗,1%的盐酸酒精分化数秒,自来水冲洗,0.6%氨水返蓝,流水冲洗;切片入伊红染液中染色1~3 min;将切片依次放入95%酒精Ⅰ (5 min)、95%酒精Ⅱ (5 min)、无水乙醇Ⅱ (5 min)、无水乙醇Ⅰ (5 min)、二甲苯Ⅰ(5 min)、二甲苯Ⅱ (5 min)中脱水透明,将切片从二甲苯中拿出来稍晾干,中性树胶封片,镜检并拍照,观察肝组织病理学变化。
1.6.4 肝脏脂质沉积将OCT包埋的冰冻组织切片取出复温,将油红O母液与蒸馏水按3 : 2比例混合,混合后静置10 min,过滤后为工作液;切片经蒸馏水洗后,入60%异丙醇浸洗;将工作液滴加到组织上染色10 min;将切片入60%异丙醇分色,镜下控制时间,蒸馏水洗;切片入Harris苏木素染3~8 min,自来水洗,1%的盐酸酒精分化数秒,自来水冲洗,0.6%氨水返蓝,流水冲洗;将切片上的水分甩干,用甘油封片。封片后尽快镜检并拍照,观察肝脏脂质沉积情况。
1.6.5 肝脏脂质合成相关基因mRNA表达量按试剂盒说明采用Trizol法提取肝脏总RNA,然后用1%琼脂糖凝胶电泳检测所提RNA的完整性,并使用核酸蛋白测定仪检测RNA的浓度与纯度,吸光度(OD)260/280在1.8~2.0较为理想。利用反转录试剂盒(Prime ScriptTM Reagent Kit,TaKaRa,日本)进行反转录合成cDNA,置于-20 ℃保存备用。
采用Primer 5.0软件设计引物,PCR引物序列见表 2。以cDNA为模板,采用荧光定量PCR技术检测肝脏中脂质合成相关基因SREBP-1c、FAS、ACC、SCD1 mRNA的表达量。PCR反应体系为10 μL:5 μL SYBR Premix Ex Taq(TaKaRa,日本)、0.4 μL上游引物、0.4 μL下游引物、1 μL cDNA、3.2 μL ddH2O。PCR扩增条件为:95 ℃预变性30 s;95 ℃变性5 s,在各基因最佳退火温度下退火20 s,共40个循环;PCR后绘制熔解曲线以判断扩增产物的正确性,温度以每5 s 0.5 ℃的速度从60 ℃上升到95 ℃。以β-肌动蛋白(β-actin)为内参基因,对得到的各样品Ct值进行均一化处理,在各目的基因与β-肌动蛋白的扩增效率基本一致条件下,以对照组基因mRNA的表达量为基准,通过2-ΔΔCt法计算相关基因mRNA的表达量。
|
|
表 2 基因引物序列 Table 2 Primer sequences of genes |
所有数据均采用SPSS 17.0统计软件进行统计分析。试验数据采用单因素方差分析,Duncan氏法进行多重比较,结果以“平均值±标准差”形式表示,P<0.05为差异显著。
2 结果 2.1 山竹醇对仔猪生长性能的影响由表 3可知,注射diquat前(第1~14天),与对照组相比,饲粮添加200、400、600 mg/kg山竹醇对仔猪的ADG、ADFI和F/G均无显著影响(P>0.05)。注射diquat后(第15~28天),与对照组相比,应激组仔猪的ADG和ADFI显著降低(P<0.05),F/G显著升高(P<0.05);200、400、600 mg/kg山竹醇组的ADFI和ADG显著高于应激组(P<0.05),400 mg/kg山竹醇组的F/G显著低于应激组(P<0.05),其他2个山竹醇组的F/G与应激组无显著差异(P>0.05)。
|
|
表 3 饲粮添加山竹醇和注射diquat对仔猪生长性能的影响 Table 3 Effects of garcinol supplementation and diquat injection on growth performance of piglets |
由表 4可知,注射diquat前(第15天),饲粮添加200 mg/kg山竹醇对仔猪血清SOD、GSH-Px活性、T-AOC及MDA含量无显著影响(P>0.05);饲粮添加400、600 mg/kg山竹醇显著提高了仔猪血清SOD活性以及T-AOC(P<0.05),但对血清GSH-Px活性以及MDA含量无显著影响(P>0.05)。注射diquat后(第29天),与对照组相比,应激组血清SOD、GSH-Px活性显著降低(P<0.05),MDA含量显著增加(P<0.05),T-AOC无显著变化(P>0.05);饲粮添加200、400和600 mg/kg山竹醇均显著提高了氧化应激仔猪血清SOD、GSH-Px活性(P<0.05),显著降低了MDA含量(P<0.05),血清SOD、GSH-Px活性随着山竹醇添加量的增加呈现先升高后下降的趋势,以山竹醇添加量为400 mg/kg时效果最好。
|
|
表 4 山竹醇对氧化应激仔猪血清抗氧化指标的影响 Table 4 Effects of garcinol supplementation on serum antioxidant indexes of piglets under oxidative stress |
由表 5可知,注射diquat后(第29天),与对照组相比,应激组肝脏SOD、GSH-Px活性显著降低(P<0.05),MDA含量显著提高(P<0.05);与应激组相比,饲粮添加400、600 mg/kg山竹醇均显著提高了肝脏SOD、GSH-Px活性(P<0.05),并随着山竹醇添加量的增加呈现先升高后下降的趋势,其中山竹醇添加量为400 mg/kg效果最好,且各山竹醇组肝脏MDA含量显著低于应激组(P<0.05)。
|
|
表 5 山竹醇对氧化应激仔猪肝脏抗氧化指标的影响 Table 5 Effects of garcinol supplementation on liver antioxidant indexes of piglets under oxidative stress |
肝脏HE染色结果(图 1)显示,对照组仔猪肝脏结构正常,肝细胞大小一致,排列整齐;应激组仔猪肝脏发生明显病理改变,肝小叶结构紊乱,肝细胞肿胀;200和600 mg/kg山竹醇组肝细胞部分肿胀,与应激组相比肝脏组织结构有所恢复;400 mg/kg山竹醇组与应激组相比肝细胞无明显肿胀,结构清晰。油红O染色结果(图 1)显示,对照组仔猪肝细胞中基本无红染颗粒,结构正常,肝细胞排列整齐;应激组仔猪肝脏组织中有大量红染颗粒,脂质沉积明显增加,结构被破坏,肝细胞排列紊乱;饲粮添加200、400和600 mg/kg山竹醇均减少了肝脏中脂质沉积,其中以400 mg/kg山竹醇的改善效果最为明显。
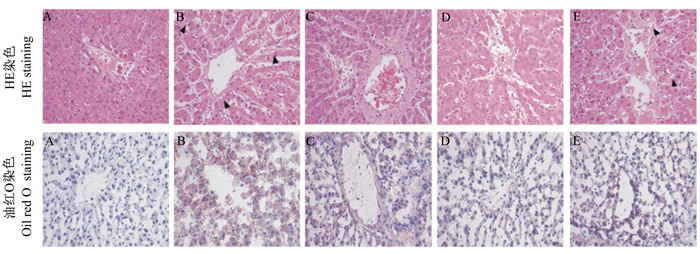
|
A:对照组;B:应激组;C:200 mg/kg山竹醇组;D:400 mg/kg山竹醇组;E:600 mg/kg山竹醇组。A: control group; B: stress group;C: 200 mg/kg garcinol group; D: 400 mg/kg garcinol group; E: 600 mg/kg garcinol group. 图 1 山竹醇对氧化应激仔猪肝脏组织形态和脂质沉积的影响 Fig. 1 Effects of garcinol supplementation on liver tissue morphology and lipid deposition of piglets under oxidative stress (200×) |
由图 2可知,注射diquat后(第29天),应激组血清TG和TC含量显著高于对照组(P<0.05)。饲粮添加200 mg/kg山竹醇可显著降低氧化应激仔猪血清TC含量(P<0.05),对血清TG含量无显著影响(P>0.05);饲粮添加400、600 mg/kg山竹醇可显著降低氧化应激仔猪血清TG含量(P<0.05),且与对照组无显著差异(P>0.05);饲粮添加400、600 mg/kg山竹醇可显著降低氧化应激仔猪血清TC含量(P<0.05),且400 mg/kg山竹醇组与对照组无显著差异(P>0.05)。
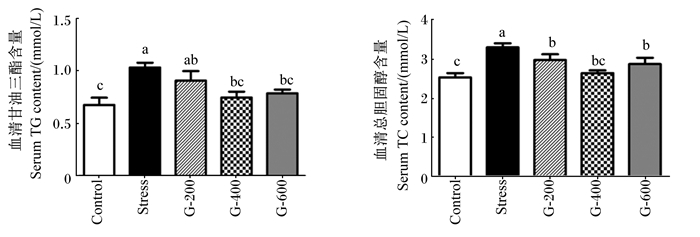
|
Control:对照组;Stress:应激组;G-200:200 mg/kg山竹醇组;G-400:400 mg/kg山竹醇组;G-600:600 mg/kg山竹醇组。数据柱标注不同小写字母表示差异显著(P < 0.05),相同字母表示差异不显著(P>0.05)。下图同。 Control: control group; Stress: stress group; G-200: 200 mg/kg garcinol group; G-400: 400 mg/kg garcinol group; G-600: 600 mg/kg garcinol group. Value columns with different small letters mean significant difference (P < 0.05), and with the same letter superscripts mean no significant difference (P > 0.05). The same as below. 图 2 山竹醇对氧化应激仔猪血清TG和TC含量的影响 Fig. 2 Effects of garcinol supplementation on serum TG and TC contents of piglets under oxidative stress |
由图 3可知,与对照组相比,应激组肝脏中SREBP-1c、FAS、ACC、SCD1 mRNA的表达量显著上调(P<0.05)。饲粮添加200 mg/kg山竹醇可以显著降低氧化应激仔猪肝脏中SREBP-1c、ACC mRNA的表达量(P<0.05),但对肝脏中FAS、SCD1 mRNA的表达量无显著影响(P>0.05);饲粮添加400 mg/kg山竹醇可以显著降低氧化应激仔猪肝脏中SREBP-1c、FAS、ACC、SCD1 mRNA的表达量(P<0.05);饲粮添加600 mg/kg山竹醇可以显著降低氧化应激仔猪肝脏中SREBP-1c、FAS、ACC mRNA的表达量(P<0.05),但对肝脏中SCD1 mRNA的表达量无显著影响(P>0.05);饲粮添加200、400、600 mg/kg山竹醇对肝脏中FAS、ACC、SCD1 mRNA表达量的影响无显著差异(P>0.05)。
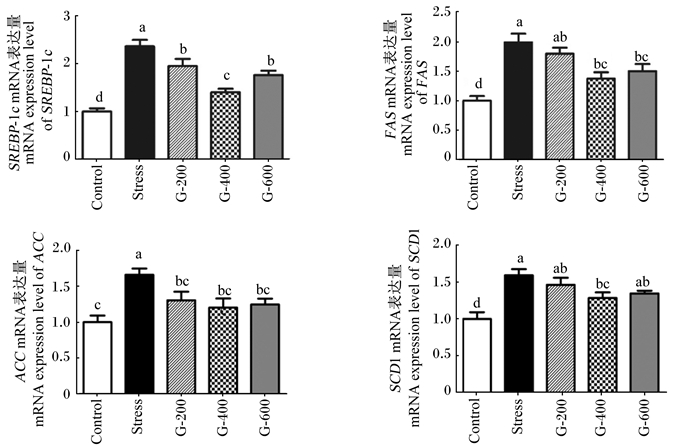
|
图 3 山竹醇对氧化应激仔猪肝脏脂质合成相关基因mRNA表达量的影响 Fig. 3 Effects of garcinol supplementation on lipid synthesis related genes mRNA expression levels of piglets under oxidative stress |
本试验通过对仔猪生长性能、血清及肝脏抗氧化指标进行测定可知,腹腔注射diquat溶液成功诱导仔猪产生氧化应激。Zheng等[19]通过给仔猪一次性腹腔注射diquat,观察仔猪生长性能和血浆抗氧化指标建立了氧化应激模型。刘通等[17]研究发现,综合仔猪生长性能、血液生化指标可知,按照10 mg/kg BW的剂量一次性给仔猪腹腔注射diquat溶液可建立稳定的氧化应激模型。
有研究表明,diquat诱导的氧化应激可以显著降低仔猪的ADFI和ADG,并且显著提高F/G,降低仔猪的生长性能[20-21]。本试验结果发现,在正常饲养条件下饲粮添加山竹醇对仔猪的生长性能无显著影响。Majeed等[22]研究发现,山竹醇添加量达到2 000 mg/kg时对啮齿动物的生长发育无任何负面作用。上述结果表明在仔猪饲粮中应用山竹醇是切实可行的。本试验中应激组仔猪的ADFI和ADG显著低于对照组,且F/G显著高于对照组。在饲粮中添加不同水平的山竹醇后,氧化应激仔猪的ADFI和ADG显著升高,F/G不同程度降低,说明山竹醇可以缓解氧化应激导致的仔猪生长性能下降情况。
仔猪正常生理状态下,体内氧化系统和抗氧化系统处于平衡状态,可以及时清除自由基,保护机体免受损伤;而氧化应激状态下,仔猪体内氧化系统和抗氧化系统的平衡被打破[1]。SOD、GSH-Px是动物体内天然的自由基清除剂,T-AOC则反映了机体总的抗氧化能力的强弱。研究发现,diquat诱导的氧化应激会显著降低仔猪血清中的SOD、GSH-Px、CAT等抗氧化酶的活性,提高脂质过氧化物MDA的含量[18, 23]。肝脏作为机体内非常活跃的器官,在氧化应激状态下容易受到损伤,氧化应激可以显著降低仔猪肝脏中抗氧化酶的活性,提高MDA的含量[24-25]。本试验结果也表明,diquat显著降低了仔猪血清、肝脏中抗氧化酶SOD、GSH-Px的活性,且显著升高了血清、肝脏中脂质过氧化物MDA的含量,降低了仔猪的抗氧化能力,使仔猪处于氧化应激状态,与前人研究结果一致;而在饲粮中添加山竹醇之后,可以显著缓解氧化应激导致的仔猪血清和肝脏中SOD、GSH-Px活性的下降以及MDA含量的上升,且山竹醇添加量为400 mg/kg时效果最佳。仔猪处于正常生理状态时,饲粮添加山竹醇也可提高血清抗氧化功能。研究发现,饲粮添加抗氧化剂不仅可以改善应激猪的抗氧化能力,还可提高正常生理状态下猪的抗氧化功能[5]。因山竹醇具有抗氧化活性,推测它对氧化应激仔猪的保护效应可能是通过增强机体的抗氧化功能实现的。对山竹醇的抗氧化机制进行研究,体外试验和体内试验结果都显示山竹醇能够抑制超氧阴离子和一氧化氮等自由基的形成,或通过单电子转移与氧自由基反应,形成共振稳定中间体,可以防止自由基种类的增加,从而减少氧化损伤,最终发挥其抗氧化作用[26-29]。
本试验中,氧化应激导致仔猪肝脏组织结构损伤,并且使得肝脏中脂质沉积明显增加,而在饲粮中添加山竹醇后,肝脏组织结构有了一定程度的恢复,同时也减少了氧化应激导致的脂质沉积的增加,在3个不同添加量的山竹醇组中,400 mg/kg山竹醇组肝脏组织形态和脂质沉积的改善情况较应激组最为明显。Panda等[30]研究发现,山竹醇可以增加肝脏内源抗氧化剂,减少脂质过氧化反应,具有保护肝脏的作用。由此可见,在饲粮中添加山竹醇可以提高仔猪的抗氧化能力,从而减少氧化应激带来的组织损伤。
肝脏在脂类的合成、分解、转运等过程发挥重要作用,而肝脏作为氧化应激的重要靶器官,在氧化应激条件下会发生脂质代谢紊乱。氧化应激会影响线粒体脂肪酸氧化,损害脂蛋白的组装和分泌,使合成的TG在肝脏内积聚[31-32]。氧化应激还会干扰胰岛素介导的信号转导,使胰岛素信号通路受损,导致细胞的胰岛素抵抗[33-34]。胰岛素抵抗和脂质代谢失衡导致脂肪在肝细胞中的沉积,尤其是脂肪酸和TG[35]。肝脏中含有丰富的与脂质合成相关的酶类,包括FAS、ACC、SCD1等,它们在脂质合成中发挥重要作用,而SREBP-1c是调节脂质合成的重要转录因子之一,因此我们将它们作为反映山竹醇调控脂质合成作用的重要指标。氧化应激会导致仔猪肝脏中SREBP-1c、FAS、ACC、SCD1 mRNA表达量升高,且随着氧化应激的加重,上述基因表达量随之升高;氧化应激还会导致脂质沉积增多,细胞内TG含量上升[7]。有研究显示,山竹醇对脂质合成具有调控作用,它可以通过下调脂肪细胞内过氧化物酶增殖激活受体γ(PPARγ)和FAS的基因和蛋白表达,抑制脂肪细胞内TG合成和脂质沉积[36]。除此以外,山竹醇也可通过降低脂肪酸诱导的肝细胞内FAS的表达来减少脂质沉积[37]。本试验发现,与对照组相比,应激组肝脏中SREBP-1c、FAS、ACC、SCD1 mRNA的表达量显著上调;与应激组相比,各山竹醇组肝脏中SREBP-1c、FAS、ACC、SCD1 mRNA的表达量均有不同程度降低,且随着饲粮中山竹醇添加量的升高呈现先降低后上升的趋势,在山竹醇添加量为400 mg/kg时改善效果最佳。综上所述,山竹醇可能通过提高仔猪的抗氧化功能,保护肝脏结构和功能的完整性,缓解氧化应激造成的生长性能下降,减少氧化应激状态下肝脏脂质合成的增加,但山竹醇对脂质合成的分子调节机制仍需进一步研究。
4 结论① 饲粮添加山竹醇可通过改善仔猪机体的抗氧化功能,缓解diquat诱导的氧化应激,提高氧化应激仔猪的生长性能,其中以山竹醇添加量为400 mg/kg时效果最好。
② 饲粮添加山竹醇可改善氧化应激导致仔猪脂质合成的增加,减少脂质沉积,保护肝脏结构和功能的完整性,且以山竹醇添加量为400 mg/kg时效果最好。
| [1] |
BHAT A H, DAR K B, ANEES S, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight[J]. Biomedicine & Pharmacotherapy, 2015, 74: 101-110. |
| [2] |
APEL K, HIRT H. Reactive oxygen species:metabolism, oxidative stress, and signal transduction[J]. Annual Review of Plant Biology, 2004, 55: 373-399. DOI:10.1146/annurev.arplant.55.031903.141701 |
| [3] |
BHATTACHARYYA A, CHATTOPADHYAY R, MITRA S, et al. Oxidative stress:an essential factor in the pathogenesis of gastrointestinal mucosal diseases[J]. Physiological Reviews, 2014, 94(2): 329-354. DOI:10.1152/physrev.00040.2012 |
| [4] |
YUAN S B, CHEN D W, ZHANG K Y, et al. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs[J]. Asian-Australasian Journal of Animal Sciences, 2007, 20(10): 1600-1605. DOI:10.5713/ajas.2007.1600 |
| [5] |
田刚, 黄琳慧, 宋晓华, 等. 壳寡糖对氧化应激仔猪生长性能、抗氧化能力及空肠养分消化和转运能力的影响[J]. 动物营养学报, 2018, 30(7): 2652-2661. DOI:10.3969/j.issn.1006-267x.2018.07.025 |
| [6] |
FOUFELLE F, FERRÉ P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose:a role for the transcription factor sterol regulatory element binding protein-1c[J]. Biochemical Journal, 2002, 366(2): 377-391. DOI:10.1042/bj20020430 |
| [7] |
SEKIYA M, HIRAISHI A, TOUYAMA M, et al. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells[J]. Biochemical and Biophysical Research Communications, 2008, 375(4): 602-607. DOI:10.1016/j.bbrc.2008.08.068 |
| [8] |
FANG D L, WAN Y, SHEN W, et al. Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells[J]. Molecular and Cellular Biochemistry, 2013, 381(1/2): 127-137. |
| [9] |
BAICEANU A, MESDOM P, LAGOUGE M, et al. Endoplasmic reticulum proteostasis in hepatic steatosis[J]. Nature Reviews Endocrinology, 2016, 12(12): 710-722. DOI:10.1038/nrendo.2016.124 |
| [10] |
JAGTAP P, BHISE K, PRAKYA V. A phytopharmacological review on Garcinia indica[J]. International Journal of Herbal Medicine, 2015, 3(4): 2-7. |
| [11] |
BALASUBRAMANYAM K, ALTAF M, VARIER R A, et al. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression[J]. Journal of Biological Chemistry, 2004, 279(32): 33716-33726. DOI:10.1074/jbc.M402839200 |
| [12] |
AHMAD A, SARKAR S H, BITAR B, et al. Garcinol regulates EMT and Wnt signaling pathways in vitro and in vivo, leading to anticancer activity against breast cancer cells[J]. Molecular Cancer Therapeutics, 2012, 11(10): 2193-2201. DOI:10.1158/1535-7163.MCT-12-0232-T |
| [13] |
PADHYE S, AHMAD A, OSWAL N, et al. Emerging role of garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs[J]. Journal of Hematology & Oncology, 2009, 2: 38. |
| [14] |
CHENG A C, TSAI M L, LIU C M, et al. Garcinol inhibits cell growth in hepatocellular carcinoma Hep3B cells through induction of ROS-dependent apoptosis[J]. Food & Function, 2010, 1(3): 301-307. |
| [15] |
HUNG W L, LIU C M, LAI C S, et al. Inhibitory effect of garcinol against 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumorigenesis in mice[J]. Journal of Functional Foods, 2015, 18: 432-444. DOI:10.1016/j.jff.2015.07.019 |
| [16] |
YAMAGUCHI L F, SAITO M, ARIGA T, et al. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind[J]. Journal of Agricultural and Food Chemistry, 2000, 48(6): 2320-2325. DOI:10.1021/jf990908c |
| [17] |
刘通, 李泽青, 闫峻, 等. 构建Diquat诱导断奶仔猪氧化应激模型的研究[J]. 畜牧兽医杂志, 2016, 35(6): 61-63, 66. DOI:10.3969/j.issn.1004-6704.2016.06.018 |
| [18] |
WANG A N, CAI C J, ZENG X F, et al. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat[J]. Journal of Applied Microbiology, 2013, 114(6): 1582-1591. DOI:10.1111/jam.12188 |
| [19] |
ZHENG P, YU B, LV M, et al. Effects of oxidative stress induced by diquat on arginine metabolism of postweaning pigs[J]. Asian-Australasian Journal of Animal Sciences, 2010, 23(1): 98-105. |
| [20] |
XU Y Q, XING Y Y, WANG Z Q, et al. Pre-protective effects of dietary chitosan supplementation against oxidative stress induced by diquat in weaned piglets[J]. Cell Stress and Chaperones, 2018, 23(4): 703-710. DOI:10.1007/s12192-018-0882-5 |
| [21] |
CAO S T, WU H, WANG C C, et al. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets[J]. Journal of Animal Science, 2018, 96(5): 1795-1805. DOI:10.1093/jas/sky104 |
| [22] |
MAJEED M, BANI S, BHAT B, et al. Safety profile of 40% garcinol from Garcinia indica in experimental rodents[J]. Toxicology Reports, 2018, 5: 750-758. DOI:10.1016/j.toxrep.2018.06.009 |
| [23] |
LV M, YU B, MAO X B, et al. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs[J]. Animal, 2012, 6(6): 928-934. DOI:10.1017/S1751731111002382 |
| [24] |
MAO X B, LV M, YU B, et al. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets[J]. Journal of Animal Science and Biotechnology, 2014, 5(1): 49. DOI:10.1186/2049-1891-5-49 |
| [25] |
袁施彬, 陈代文. 氧化应激对断奶仔猪组织抗氧化酶活性和病理学变化的影响[J]. 中国兽医学报, 2009, 29(1): 74-78. |
| [26] |
TANAKA T, KOHNO H, SHIMADA R, et al. Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats[J]. Carcinogenesis, 2000, 21(6): 1183-1189. DOI:10.1093/carcin/21.6.1183 |
| [27] |
LIAO C H, HO C T, LIN J K. Effects of garcinol on free radical generation and NO production in embryonic rat cortical neurons and astrocytes[J]. Biochemical and Biophysical Research Communications, 2005, 329(4): 1306-1314. DOI:10.1016/j.bbrc.2005.02.110 |
| [28] |
SANG S M, LIAO C H, PAN M H, et al. Chemical studies on antioxidant mechanism of garcinol:analysis of radical reaction products of garcinol with peroxyl radicals and their antitumor activities[J]. Tetrahedron, 2002, 58(51): 10095-10102. DOI:10.1016/S0040-4020(02)01411-4 |
| [29] |
YAMAGUCHI F, ARIGA T, YOSHIMURA Y, et al. Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind[J]. Journal of Agricultural and Food Chemistry, 2000, 48(2): 180-185. DOI:10.1021/jf990845y |
| [30] |
PANDA V S, ASHAR H D. Antioxidant and hepatoprotective effects of Garcinia indica choisy fruits in carbon tetrachloride-induced liver injury in rats[J]. Journal of Food Biochemistry, 2012, 36(2): 240-247. DOI:10.1111/j.1745-4514.2010.00531.x |
| [31] |
PAN M H, CEDERBAUM A I, ZHANG Y L, et al. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production[J]. Journal of Clinical Investigation, 2004, 113(9): 1277-1287. DOI:10.1172/JCI19197 |
| [32] |
BONNARD C, DURAND A, PEYROL S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice[J]. Journal of Clinical Investigation, 2008, 118(2): 789-800. |
| [33] |
EVANS J L, GOLDFINE I D, MADDUX B A, et al. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction?[J]. Diabetes, 2003, 52(1): 1-8. DOI:10.2337/diabetes.52.1.1 |
| [34] |
RAINS J L, JAIN S K. Oxidative stress, insulin signaling, and diabetes[J]. Free Radical Biology and Medicine, 2011, 50(5): 567-575. DOI:10.1016/j.freeradbiomed.2010.12.006 |
| [35] |
DAY C P, JAMES O F W. Steatohepatitis:a tale of two "hits"?[J]. Gastroenterology, 1998, 114(4): 842-845. DOI:10.1016/S0016-5085(98)70599-2 |
| [36] |
HSU C L, LIN Y J, HO C T, et al. Inhibitory effects of garcinol and pterostilbene on cell proliferation and adipogenesis in 3T3-L1 cells[J]. Food & Function, 2012, 3(1): 49-57. |
| [37] |
俞慧宏, 沈薇, 于圣杰. Garcinol对非酒精性脂肪肝细胞内脂质合成的作用及机制[J]. 肝胆外科杂志, 2017, 25(4): 308-312. DOI:10.3969/j.issn.1006-4761.2017.04.024 |




