2. 重庆市畜牧技术推广总站, 重庆 401121
2. Chongqing Animal Husbandry Technology Extension Station, Chongqing 401121, China
2018年我国牛肉产量约为729万t,居世界第3位,约占世界牛肉总产量的10%,牛肉进口量约为106万t[1], 进口牛肉中包括相当一部分高档牛肉。随着我国居民消费结构的改变和生活水平的提高,我国对牛肉特别是高档牛肉的需求量将快速增加,但目前我国高档牛肉产量较少,相关的科学研究特别是肉牛的能量供给量和供给阶段方面的研究还较欠缺。
肉牛生长期是肌内脂肪细胞发育的关键阶段,此阶段肉牛肌内脂肪细胞的增殖和分化受营养和激素的调控作用。通过营养供给可调控生长期肉牛肌内脂肪细胞的增殖和分化,即增加肉牛肌内脂肪组织中间充质干细胞转化为肌内前体脂肪细胞的几率和肌内脂肪细胞中甘油三酯的积聚[2]。研究表明,改变饲粮中的淀粉水平可影响肉牛肌内前体脂肪细胞的增殖和分化,国内外也通常给生长育肥期肉牛饲喂高淀粉水平饲粮来生产高档牛肉[3-6],同时促进了皮下脂肪沉积,降低了饲料转化效率和经济效益。
葡萄糖是供给肉牛机体能量需要和脂肪合成的重要能量来源。饲粮中的大量淀粉在瘤胃中降解为丙酸被吸收后进入肝脏经糖异生途径合成葡萄糖;饲粮中的过瘤胃淀粉进入小肠后在胰腺淀粉酶作用下也直接分解为葡萄糖被吸收利用[7-8]。肉牛肌肉葡萄糖的摄取量对肌内脂肪的沉积十分重要。研究表明,在肉牛生长期饲喂生糖指数较高的谷物和增加小肠对淀粉的吸收率可以促进肌内脂肪沉积[9],但是具体机制还不太清晰。因此,本文综述了生长期肉牛肌内脂肪形成的细胞学基础,以及饲粮淀粉水平对生长期肉牛瘤胃发酵的影响和肌内脂肪酶活性和转录因子等方面影响的研究,以期为调控肉牛能量分配和高档牛肉的生产提供理论依据。
1 肌内脂肪组织的细胞学基础牛胎儿在妊娠第80天就出现肾周白色脂肪组织,在妊娠第180天开始出现皮下脂肪组织和肌间脂肪组织,但是肌内脂肪细胞在出生后才被发现(图 1)[10]。除肌内脂肪细胞外的脂肪细胞的增生主要发生在胎儿期和产后早期,此阶段脂肪细胞数量主要受母体营养供给调控[11]。在出生后这些脂肪组织的脂肪细胞数量基本保持稳定,但肌内脂肪细胞数量到250日龄前仍具有可塑性[12]。有研究表明,通过营养调控肉牛肌内脂肪细胞数量的有效期依次为胎儿期、哺乳期、断奶至250日龄,250日龄之后肌内脂肪细胞数量保持在一个相对稳定的水平[13]。
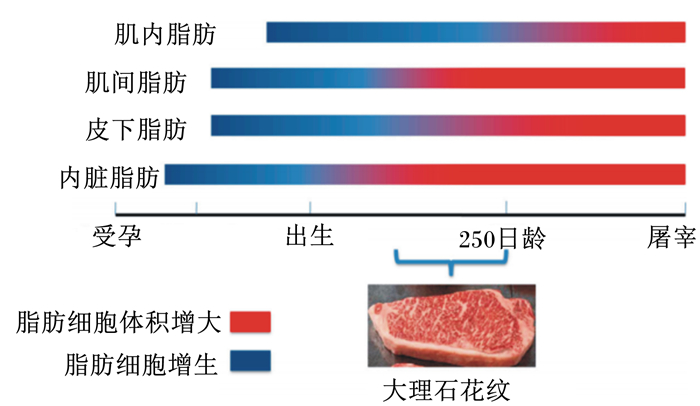
|
图 1 不同的脂肪库中脂肪沉积的形成顺序 Fig. 1 Order of formation of fat deposition in different fat store[2] |
肌内脂肪细胞和骨骼肌细胞来源于共同祖细胞即间充质干细胞[14-15]。间充质干细胞大部分转化为肌原细胞,少部分转化为肌内前体脂肪细胞。在肉牛出生后,营养供给的提高会促进肌内前体脂肪细胞增殖,增加肌内前体脂肪细胞的数量;促进肌内前体脂肪细胞分化为成熟的肌内脂肪细胞,不断积聚甘油三酯并形成脂滴[16]。生长期肉牛骨骼肌中间充质干细胞经诱导可以向骨骼肌细胞和肌内脂肪细胞分化,如果营养调控增加了间充质干细胞向肌内前体脂肪细胞的分化,将减少间充质干细胞向骨骼肌细胞分化,从而增加肌内前体脂肪细胞的数量,这可能是通过营养调控生长期肉牛肌内脂肪沉积的一种重要途径[17]。
骨骼肌中虽然存在大量的间充质干细胞,但是这些细胞会随着动物的生长逐渐减少[18],这表明肉牛的早期发育阶段是调控间充质干细胞向肌内前体脂肪细胞分化的重要阶段。因此从细胞学基础来看,营养供给调控生长发育期肉牛肌内脂肪沉积的主要途径可能是促进间充质干细胞向肌内前体脂肪细胞分化、肌内前体脂肪细胞的增殖和分化以及肌内脂肪细胞中甘油三酯的积聚,从而达到增加肌内脂肪细胞的数量和大小的目的。
2 饲粮淀粉水平对生长期肉牛瘤胃发酵的影响Gimeno等[19]的研究表明,饲喂以碾压的玉米为主(淀粉水平为43.9%)的饲粮的3月龄荷斯坦犊牛瘤胃pH维持在6.0左右,同时还表明饲喂以碾压的玉米(淀粉水平为46.7%)为主的饲粮的5月龄荷斯坦犊牛的瘤胃pH在饲喂后4和8 h分别为6.14和6.01[20],以及饲喂以碾压的玉米(淀粉水平为44.8%)为主的饲粮的6月龄荷斯坦犊牛瘤胃pH在饲喂后4和8 h分别为6.13和5.83[21]。以上研究表明,对于3~6月龄的犊牛饲喂淀粉水平达到了47%的饲粮,并未导致瘤胃pH低于5.6。瘤胃pH低于5.6持续3 h/d通常被认为是亚急性瘤胃酸中毒[22]。但Schoonmaker等[23]研究表明,饲喂高淀粉饲粮(玉米含量为70.79%)的119日龄小公牛瘤胃产生大量的挥发性脂肪酸,其中乙酸、丙酸、丁酸的浓度随饲喂时间增加而逐渐增加,且导致瘤胃pH下降至5.36,且饲喂后6和9 h均低于5.6。这可能与淀粉的加工方式和淀粉水平较高有关。
Fatehi等[24]研究表明,饲喂以玉米为主的淀粉饲粮(玉米含量为55.14%)的8.5月龄的荷斯坦公牛血清挥发性脂肪酸浓度显著增加,其瘤胃液pH维持在6.3左右。由于荷斯坦奶公牛饲粮的淀粉水平较低,瘤胃pH维持在正常水平。De Nardi等[25]研究表明,给育成牛饲喂淀粉水平为33.4%的饲粮时,其瘤胃液pH在5.0~5.5之间持续了90 min/d,低于5.0有12 min/d;而当淀粉水平为42.8%时,瘤胃液pH在5.0~5.5之间持续了80 min/d,瘤胃液pH低于5.0增加至92 min/d。由此可见,当牛在6月龄以上且饲粮淀粉水平高至40%左右就可能发生瘤胃代谢紊乱。
综上可以看出,肉牛在3~6月龄时,饲粮中淀粉水平可最高至47%,瘤胃pH维持在6以上。而当肉牛在6月龄以上时,淀粉水平高至40%左右会导致瘤胃pH降低,诱发亚急性酸中毒的风险增加,表明淀粉水平对瘤胃发酵的影响与牛的月龄有重要的关系。饲粮中的淀粉水平的提高对生长期肉牛瘤胃发酵会存在一定负面影响,但是可通过控制饲粮中的淀粉水平以及选择合适的加工方式,可在对瘤胃发酵未产生负调控作用的基础上影响肌内脂肪的沉积。
3 生长期肉牛葡萄糖代谢特点 3.1 葡萄糖是肌内脂肪细胞脂肪酸从头合成的重要底物饲粮中碳水化合物在反刍动物瘤胃中发酵为挥发性脂肪酸为主,淀粉在小肠中降解为葡萄糖为辅,因此,反刍动物从胃肠道中直接获得的葡萄糖很有限,主要是通过葡萄糖的异生途径合成葡萄糖。一般而言,饲粮中淀粉含量越高,反刍动物从小肠中吸收的葡萄糖的量也会越多。反刍动物的血液葡萄糖浓度(3.0~3.8 mmol/L)低于其他哺乳动物(4.2~4.8 mmol/L),其能量需要的4%~11%来自于葡萄糖,而70%左右来自于乙酸和酮体。这可能是相对于单胃动物而言,反刍动物中枢神经系统能够利用的葡萄糖更少,肝脏缺乏葡萄糖激酶和脂肪组织中的脂肪酸从头合成的底物主要来源于乙酸的原因[26]。尽管血液中葡萄糖的浓度低,但葡萄糖代谢对反刍动物肌内脂肪组织的沉积非常重要。
研究表明,在脂肪酸从头合成的底物利用方面,肉牛肌内脂肪组织和皮下脂肪组织存在底物利用差异性[27]。通过细胞培养研究表明,肌内脂肪细胞优先利用葡萄糖作为脂肪酸从头合成的底物,且葡萄糖比乙酸提供更多的乙酰基用于肌内脂肪细胞脂肪酸从头合成;乙酸和乳酸为肌内脂肪组织脂肪酸从头合成提供的乙酰基不到10%~25%,而葡萄糖提供的乙酰基达到70%~80%,而皮下脂肪组织中则相反[28]。Rhoades等[29]的研究也得出了相似的结论,在肌内脂肪组织中葡萄糖为脂肪酸从头合成提供的乙酰基是乙酸的2倍多,而在皮下脂肪组织中二者提供的乙酰基数量接近。
虽然细胞培养试验表明肌内脂肪组织利用葡萄糖作为脂肪酸的从头合成的底物比例比皮下脂肪组织高,但有活体试验表明乙酸为皮下脂肪组织和肌内脂肪组织脂肪酸从头合成提供的乙酰基均远高于葡萄糖[30]。Nayananjalie等[31]研究表明,在饲喂高淀粉水平饲粮时,肉牛肌内脂肪组织利用乙酸的速率是葡萄糖的10倍。Smith等[32]通过灌注乙酸和葡萄糖研究肌内脂肪组织和皮下脂肪组织脂肪酸从头合成的底物利用的影响,结果表明乙酸和葡萄糖为肌内脂肪组织提供的乙酰基分别为58.8%和41.2%,乙酸和葡萄糖为皮下脂肪组织提供的乙酰基分别为85.1%和14.9%,由此可见,虽然乙酸为肌内脂肪组织和皮下脂肪组织提供的乙酰基均高于葡萄糖提供的乙酰基,但葡萄糖为肌内脂肪组织提供的乙酰基比皮下脂肪组织多。这是饲喂高淀粉水平饲粮时同时促进肌内脂肪和皮下脂肪沉积的根本原因之一。
3.2 饲粮淀粉水平对生长期肉牛肌内脂肪细胞葡萄糖摄取能力的影响葡萄糖转运体(glucose transporters,GLUTs)家族有4种葡萄糖转运体,其中葡萄糖转运蛋白1(glucose transporter 1,GLUT1)广泛表达,负责基础葡萄糖的摄取[33],而葡萄糖转运蛋白4(glucose transporter 4,GLUT4)是一种高亲和力的葡萄糖转运蛋白,在脂肪组织和肌肉中高度表达[34]。胰岛素结合胰岛素受体后通过磷酯酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)/蛋白激酶B(protein kinase B,Akt)信号通路激活GLUT4的囊泡运输,使GLUT4迅速分布于细胞表面,从而激活脂肪组织的葡萄糖摄取功能[35]。反刍动物脂肪组织对葡萄糖的摄取能力远低于单胃动物,因为胰岛素受体底物(insulin receptor substrate,IRS)和GLUT4的低表达。反刍动物肌内脂肪组织胰岛素敏感性高于皮下脂肪组织[36],因为肌内脂肪组织中GLUT4的表达水平高于皮下脂肪组织,且参与葡萄糖转化为长链脂肪酸(即磷酸果糖激酶和ATP-柠檬酸裂解酶)的代谢酶活性较高[37]。肌内脂肪组织内的GLUT4和GLUT1的表达量均比其他脂肪组织高,且GLUT1的表达量是GLUT4的表达量的数倍,而除肌内脂肪组织外的其他脂肪组织的GLUT4的表达量较高,但GLUT1的表达量较低[30, 38]。这说明肌内脂肪组织比皮下脂肪组织更易吸收葡萄糖的原因可能与GLUT1的高表达有关。
GLUT4是胰岛素信号通路的重要靶基因。高淀粉水平饲粮和低淀粉水平饲粮饲喂生长期肉牛的肌内脂肪组织中GLUT4的表达量没有显著差异[39]。有研究发现,饲喂高淀粉水平饲粮和低淀粉水平饲粮肉牛的肌内脂肪组织胰岛素受体(insulin receptor,INSR)、PI3K、AKT和GLUT4的表达量均没有显著差异,胰岛素信号相关的葡萄糖摄取信号通路并没有因为营养摄入而改变,这说明通过调控胰岛素介导的PI3K/Akt信号通路不是高淀粉水平饲粮比低淀粉水平饲粮易促进肌内脂肪组织摄取葡萄糖的主要原因;高淀粉水平饲粮饲喂的肉牛肌内脂肪组织中己糖激酶1(hexokinase 1,HK1)和GLUT1的表达量显著高于饲喂低淀粉组,HK-1是己糖激酶家族的成员之一,主要作用是将葡萄糖转化为葡萄糖-6-磷酸,HK1和GLUT1会促进肌内脂肪组织对葡萄糖的摄取[40]。以上研究均表明饲粮淀粉水平的提高可促进生长期肉牛肌内脂肪葡萄糖的摄取主要与GLUT1有关。
4 饲粮淀粉水平对生长期肉牛肌内脂肪细胞增殖和分化的影响 4.1 饲粮淀粉水平对生长期肉牛肌内脂肪细胞数量和直径的影响大理石花纹的生成不仅与肌内脂肪细胞的肥大(脂肪细胞的直径增加)有关,而且还与脂肪细胞的增生(脂肪细胞的数量增加)有关[41]。在肉牛生长阶段饲喂高淀粉水平饲粮可使前体肌内脂肪细胞数量增加,且促进肌内前体脂肪细胞向成熟肌内脂肪细胞分化,不断积聚甘油三酯,肌内脂肪细胞直径增大。Ebara等[40]研究表明,饲喂高淀粉水平饲粮的10月龄阉牛的肌内脂肪细胞直径比饲喂低淀粉水平饲粮阉牛的大1.82倍,并且过氧化物酶增殖物激活受体γ2(peroxisome proliferator-activated receptor gamma 2,PPARγ2)、CCAAT增强子结合蛋白(CCAAT enhancer binding protein,C/EBPs)、硬脂酰辅酶A去饱和酶(stearoyl-CoA desaturase,SCD)、葡萄糖-6-磷酸脱氢酶(glucose-6-phosphate dehydrogenase,G6PD)、脂肪酸合成酶(fatty acid synthase,FAS)和瘦素的表达量均高于饲喂粗饲料的阉牛。瘦素是由肥大脂肪细胞分泌,在脂肪细胞中高表达是牛肉呈现大理石花纹的标记[42]。
与低淀粉水平饲粮相比,饲喂高淀粉水平饲粮的3月龄安格斯和西门塔尔杂交牛肌内脂肪细胞的平均直径显著增大,肌内脂肪沉积增加,G6PD活性显著增加了约37.6%,同时高淀粉水平饲粮使肉牛皮下脂肪组织中FAS、ATP-柠檬酸裂解酶、6-磷酸葡萄糖酸脱氢酶(6-phosphogluconate dehydrogenase,PGD)、G6PD、苹果酸脱氢酶的活性显著增加[43],表明在此阶段饲喂高淀粉饲粮可增加肌内脂肪细胞的增殖和分化,但同时也会增加皮下脂肪细胞的脂肪代谢相关基因的表达。饲粮淀粉的储存加工方式也会影响淀粉在肉牛机体内的利用率,从而影响肌内脂肪的沉积。Gorocica-Buenfil等[16]研究表明,饲喂干玉米的安格斯肉牛肌内脂肪组织中存在大量肌内脂肪细胞,且肌内脂肪细胞平均直径为70和80 μm的比例高于与饲喂以高水分玉米为主的饲粮,表明不同储存方式的淀粉饲粮可影响肌内脂肪细胞数量和直径。
4.2 饲粮淀粉水平对生长期肉牛肌内脂肪细胞转录因子的影响饲粮淀粉水平会影响生长期肉牛间充质干细胞向前体肌内脂肪细胞的分化。Moisá等[44]研究表明,饲喂高淀粉水平饲粮的生长期肉牛的肌内脂肪组织中前体脂肪细胞数量与锌指蛋白423(zinc finger protein 423,ZFP423)和核受体亚家族2F组成员2(nuclear receptor subfamily 2, F group, member 2,NR2F2)的表达呈显著正相关,ZFP423是调控间充质干细胞向前脂肪细胞分化的重要因子,是前体脂肪细胞的特有标记[45],NR2F2在白色脂肪细胞发育的早期表达,主要促进脂肪细胞的增殖,ZFP423和NR2F2的表达量升高表明高淀粉水平饲粮使肌内前体脂肪细胞数量增加;在高淀粉水平饲粮饲喂早期断奶的肉牛肌内脂肪细胞中葡萄糖反应性转录因子(glucose-responsive transcription factor,MLXIPL)、过氧化物酶增殖物激活受体γ(peroxisome proliferator-activated receptor gamma,PPARγ)、C/EBPs、ZFP423和脂肪原性转录因子甲状腺激素应答蛋白(thyroid hormone response protein,THRSP)、固醇反应元件结合蛋白-1c(sterol reaction element binding protein-1c,SREBFI)、胰岛素诱导基因1(insulin induced gene1,INSIG1)的表达量均逐渐升高并且达到峰值。MLXIPL主要通过调控丙酮酸激酶、乙酰辅酶A羧化酶(acetyl-CoA carboxylase,ACC)和FAS等将葡萄糖转化为甘油三酯[46],INSIG1是调节脂代谢与脂肪细胞分化的重要蛋白质。THRSP是机体脂肪生成所必需的蛋白质,参与调控线粒体三羧酸循环脂肪合成酶系基因的表达,与大理石花纹呈正相关[47]。以上研究表明,高淀粉水平饲粮使生长期肉牛的间充质干细胞向前体肌内脂肪细胞分化的几率增加,并且使肌内脂肪组织脂合成相关基因的表达升高,从而促进肌内脂肪细胞的增殖和分化。
Graugnard等[48]研究表明,与饲喂低淀粉水平饲粮相比,饲喂等氮高淀粉水平饲粮的155日龄安格斯肉牛的肌内脂肪细胞在饲喂56 d后PPARγ调控的靶基因[ACC、FAS、脂肪酸结合蛋白4(fatty acid binding protein 4,FABP4)、SCD]的表达量增加了约2倍,THRSP的表达量增加了6~60倍,INSIG1的表达量增加了4~8倍,而SREBF1的表达量仅在低淀粉组饲喂112 d后达到最大值,这说明SREBF1不是调控肌内脂肪组织脂肪沉积的主要转录因子,而INSIG1可能是与其他转录因子协同调控肌内脂肪沉积的重要因子。综上所述,高淀粉水平饲粮可提高脂代谢相关转录因子的表达,从而促进肌内脂肪细胞的增殖和分化。
5 小结近年来关于饲粮淀粉水平调控生长期肉牛肌内脂肪细胞增殖和分化的研究已取得巨大进展。饲粮淀粉水平的提高促进了生长期肉牛骨骼肌中间充质干细胞向肌内前体脂肪细胞分化以及肌内前体脂肪细胞增殖和分化;增加了生长期肉牛肌内脂肪组织利用葡萄糖作为脂肪酸从头合成的比例;通过调控肌内脂肪细胞中以脂代谢相关转录因子PPARγ、C/EBPs、THRSP和INSIG1为主的基因网络,提高了肌内脂肪沉积相关基因的表达,促进了肌内脂肪沉积。当肉牛处于3~6月龄时,饲粮中淀粉水平可提高至47%,可对瘤胃发酵未产生负面影响的情况下影响肌内脂肪的沉积;但在6月龄以上时饲粮中淀粉水平不能过高,在40%左右为宜,过高会增加亚急性瘤胃酸中毒发生的风险。除饲粮淀粉水平,淀粉来源和加工方式也会显著影响生长期肉牛瘤胃发酵和肌内脂肪细胞的增殖和分化,未来的研究方向应倾向于三者对生长期肉牛肌内脂肪沉积的互作效应及分子机制进一步研究。
| [1] |
中国畜牧业协会牛业分会. 2018年我国肉牛产业发展回顾与2019年展望[J]. 饲料与畜牧, 2019(5): 31-37. |
| [2] |
DU M, HUANG Y, DAS A K, et al. Meat science and muscle biology symposium:manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle[J]. Journal of Animal Science, 2013, 91(3): 1419-1427. |
| [3] |
REDDY K E, JEONG J Y, BAEK Y C, et al. Early weaning of calves after different dietary regimens affects later rumen development, growth, and carcass traits in Hanwoo cattle[J]. Asian-Australasian Journal of Animal Sciences, 2017, 30(10): 1425-1434. |
| [4] |
JOSEPH S J, ROBBINS K R, PAVAN E, et al. Effect of diet supplementation on the expression of bovine genes associated with fatty acid synthesis and metabolism[J]. Bioinformatics and Biology Insights, 2010, 4: 19-31. |
| [5] |
PARK S J, BEAK S H, JUNG D J S, et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle-A review[J]. Asian-Australasian Journal of Animal Sciences, 2018, 31(7): 1043-1061. |
| [6] |
SCHEFFLER J M, MCCANN M A, GREINER S P, et al. Early metabolic imprinting events increase marbling scores in fed cattle[J]. Journal of Animal Science, 2014, 92(1): 320-324. |
| [7] |
BRAKE D W, SWANSON K C. Ruminant nutrition symposium:effects of postruminal flows of protein and amino acids on small intestinal starch digestion in beef cattle[J]. Journal of Animal Science, 2018, 96(2): 739-750. |
| [8] |
MOHARRERY A, LARSEN M, WEISBJERG M R. Starch digestion in the rumen, small intestine, and hind gut of dairy cows—a Meta-analysis[J]. Animal Feed Science and Technology, 2014, 192: 1-14. |
| [9] |
PETHICK D W, HARPER G S, ODDY V H. Growth, development and nutritional manipulation of marbling in cattle:a review[J]. Australian Journal of Experimental Agriculture, 2004, 44(7): 705-715. |
| [10] |
TAGA H, BONNET M, PICARD B, et al. Adipocyte metabolism and cellularity are related to differences in adipose tissue maturity between Holstein and Charolais or Blond d' Aquitaine fetuses[J]. Journal of Animal Science, 2011, 89(3): 711-721. |
| [11] |
MUHLHAUSLER B S, DUFFIELD J A, MCMILLEN I C, et al. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth[J]. Endocrinology, 2007, 148(2): 878-885. |
| [12] |
BONNET M, CASSAR-MALEK I, CHILLIARD Y, et al. Ontogenesis of muscle and adipose tissues and their interactions in ruminants and other species[J]. Animal, 2010, 4(7): 1093-1109. |
| [13] |
DU M, TONG J, ZHAO J, et al. Fetal programming of skeletal muscle development in ruminant animals[J]. Journal of Animal Science, 2010, 88(Suppl.13): E51-E60. |
| [14] |
JOE A W B, YI L, NATARAJAN A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis[J]. Nature Cell Biology, 2010, 12(2): 153-163. |
| [15] |
UEZUMI A, ITO T, MORIKAWA D, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle[J]. Journal of Cell Science, 2011, 124: 3654-3664. |
| [16] |
GOROCICA-BUENFIL M A, FLUHARTY F L, BOHN T, et al. Effect of low vitamin A diets with high-moisture or dry corn on marbling and adipose tissue fatty acid composition of beef steers[J]. Journal of Animal Science, 2007, 85(12): 3355-3366. |
| [17] |
TANG Q Q, LANE M D, et al. Adipogenesis:from stem cell to adipocyte[J]. Annual Review of Biochemistry, 2012, 81(1): 715-736. |
| [18] |
DU M, YIN J D, ZHU M J. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle[J]. Meat Science, 2010, 86(1): 103-109. |
| [19] |
GIMENO A, ALAMI A A, ABECIA L, et al. Effect of type (barley vs. maize) and processing (grinding vs. dry rolling) of cereal on ruminal fermentation and microbiota of beef calves during the early fattening period[J]. Animal Feed Science and Technology, 2015, 199: 113-126. |
| [20] |
GIMENO A, AL ALAMI A, TORAL P G, et al. Effect of grinding or pelleting high grain maize- or barley-based concentrates on rumen environment and microbiota of beef cattle[J]. Animal Feed Science and Technology, 2015, 203: 67-78. |
| [21] |
GIMENO A, AL ALAMI A, YAÑEZ-RUIZ D R, et al. Effect of cereal processing (grinding to 3·5 mm or dry-rolling) in maize- or barley-based high-concentrate diets on rumen environment of beef cattle during the late fattening period[J]. Journal of Agricultural Science, 2016, 154(2): 334-346. |
| [22] |
GOZHO G N, PLAIZIER J C, KRAUSE D O, et al. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response[J]. Journal of Dairy Science, 2005, 88(4): 1399-1403. |
| [23] |
SCHOONMAKER J P, CECAVA M J, FAULKNER D B, et al. Effect of source of energy and rate of growth on performance, carcass characteristics, ruminal fermentation, and serum glucose and insulin of early-weaned steers[J]. Journal of Animal Science, 2003, 81(4): 843-855. |
| [24] |
FATEHI F, DEHGHAN-BANADAKY M, REZA-YAZDI K, et al. Performance, carcass quality and blood metabolites of Holstein bulls on feedlot feeding of different proportions of barley grain to maize grain[J]. Journal of Animal and Feed Sciences, 2013, 22(1): 35-43. |
| [25] |
DE NARDI R, MARCHESINI G, GIANESELLA M, et al. Blood parameters modification at different ruminal acidosis conditions[J]. Agriculturae Conspectus Scientificus, 2013, 78(3): 259-262. |
| [26] |
HOCQUETTE J F, BORNES F, BALAGE M, et al. Glucose-transporter (GLUT4) protein content in oxidative and glycolytic skeletal muscles from calf and goat[J]. Biochemical Journal, 1995, 305(2): 465-470. |
| [27] |
SMITH S B, KAWACHI H, CHOI C B, et al. Cellular regulation of bovine intramuscular adipose tissue development and composition[J]. Journal of Animal Science, 2009, 87(Suppl.14): E72-E82. |
| [28] |
SMITH S B, CROUSE J D. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue[J]. The Journal of Nutrition, 1984, 114(4): 792-800. |
| [29] |
RHOADES R D, SAWYER J E, CHUNG K Y, et al. Effect of dietary energy source on in vitro substrate utilization and insulin sensitivity of muscle and adipose tissues of Angus and Wagyu steers[J]. Journal of Animal Science, 2007, 85(7): 1719-1726. |
| [30] |
LANCASTER P A, SHARMAN E D, HORN G W, et al. Effect of rate of weight gain of steers during the stocker phase.Ⅳ.Rumen fermentation characteristics and expression of genes involved in substrate utilization for fatty acid synthesis in adipose tissues of growing-finishing beef cattle[J]. Journal of Animal Science, 2015, 93(6): 3055-3065. |
| [31] |
NAYANANJALIE W A D, WILES T R, GERRARD D E, et al. Acetate and glucose incorporation into subcutaneous, intramuscular, and visceral fat of finishing steers[J]. Journal of Animal Science, 2015, 93(5): 2451-2459. |
| [32] |
SMITH S B, BLACKMON T L, SAWYER J E, et al. Glucose and acetate metabolism in bovine intramuscular and subcutaneous adipose tissues from steers infused with glucose, propionate, or acetate[J]. Journal of Animal Science, 2018, 96(3): 921-929. |
| [33] |
SCHEEPERS A, JOOST H, SCHÜRMANN A. The glucose transporter families SGLT and GLUT:molecular basis of normal and aberrant function[J]. Journal of Parenteral and Enteral Nutrition, 2004, 28(5): 364-371. |
| [34] |
FERNYHOUGH M E, OKINE E, HAUSMAN G, et al. PPARγ and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte[J]. Domestic Animal Endocrinology, 2007, 33(4): 367-378. |
| [35] |
JI P, OSORIO J S, DRACKLEY J K, et al. Overfeeding a moderate energy diet prepartum does not impair bovine subcutaneous adipose tissue insulin signal transduction and induces marked changes in peripartal gene network expression[J]. Journal of Dairy Science, 2012, 95(8): 4333-4351. |
| [36] |
GILBERT C D, LUNT D K, MILLER R K, et al. Carcass, sensory, and adipose tissue traits of Brangus steers fed casein-formaldehyde-protected starch and/or canola lipid[J]. Journal of Animal Science, 2003, 81(10): 2457-2468. |
| [37] |
HOCQUETTE J F, GONDRET F, BAÉZA E, et al. Intramuscular fat content in meat-producing animals:development, genetic and nutritional control, and identification of putative markers[J]. Animal, 2010, 4(2): 303-319. |
| [38] |
SMITH S B. Cell biology symposium:practical application of the basic aspects of GLUT4 membrane trafficking and insulin signaling on issues related to animal agriculture[J]. Journal of Animal Science, 2017, 95(5): 2185-2197. |
| [39] |
GRAUGNARD D E, PIANTONI P, BIONAZ M, et al. Adipogenic and energy metabolism gene networks in longissimus lumborum during rapid post-weaning growth in Angus and Angus×Simmental cattle fed high-starch or low-starch diets[J]. BMC Genomics, 2009, 10: 142. |
| [40] |
EBARA F, INADA S, MORIKAWA M, et al. Effect of nutrient intake on intramuscular glucose metabolism during the early growth stage in cross-bred steers (Japanese Black male×Holstein female)[J]. Journal of Animal Physiology and Animal Nutrition, 2013, 97(4): 684-693. |
| [41] |
GOTOH T, JOO S T. Characteristics and health benefit of highly marbled wagyu and hanwoo beef[J]. Korean Journal for Food Science of Animal Resources, 2016, 36(6): 709-718. |
| [42] |
BONNET M, FAULCONNIER Y, LEROUX C, et al. Glucose-6-phosphate dehydrogenase and leptin are related to marbling differences among Limousin and Angus or Japanese Black×Angus steers[J]. Journal of Animal Science, 2007, 85(11): 2882-2894. |
| [43] |
SCHOONMAKER J P, FLUHARTY F L, LOERCH S C. Effect of source and amount of energy and rate of growth in the growing phase on adipocyte cellularity and lipogenic enzyme activity in the intramuscular and subcutaneous fat depots of Holstein steers[J]. Journal of Animal Science, 2004, 82(1): 137-148. |
| [44] |
MOISÁ S J, SHIKE D W, FAULKNER D B, et al. Central role of the PPARγ gene network in coordinating beef cattle intramuscular adipogenesis in response to weaning age and nutrition[J]. Gene Regulation and Systems Biology, 2014, 8: S11782. |
| [45] |
HUANG Y, DAS A K, YANG Q Y, et al. Zfp423 promotes adipogenic differentiation of bovine stromal vascular cells[J]. PLoS One, 2012, 7(10): e47496. |
| [46] |
JEONG Y S, KIM D, LEE Y S, et al. Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression[J]. PLoS One, 2011, 6(7): e22544. |
| [47] |
LA B, OH D, LEE Y, et al. Association of bovine fatty acid composition with novel missense nucleotide polymorphism in the thyroid hormone-responsive (THRSP) gene[J]. Animal Genetics, 2013, 44(1): 118. |
| [48] |
GRAUGNARD D E, BERGER L L, FAULKNER D B, et al. High-starch diets induce precocious adipogenic gene network up-regulation in longissimus lumborum of early-weaned Angus cattle[J]. British Journal of Nutrition, 2010, 103(7): 953-963. |




