玉米赤霉烯酮(zearalenone,ZEA)是具有类雌激素作用的镰刀属真菌产生的次级代谢产物。玉米和小麦的ZEA污染比较严重,且ZEA具有很强的储存稳定性,高温不易分解[1]。卵巢作为ZEA的靶器官,受ZEA的调控,又反馈调节激素分泌[2]。ZEA通过扰乱动物的生殖激素水平改变卵巢发育,诱导卵巢氧化应激和凋亡[3]。Abbasian等[4]研究表明,ZEA(0.1 mg/kg)诱导小鼠形成多囊卵巢,扰乱胰岛素水平,干扰糖异生过程。ZEA能够诱导颗粒细胞凋亡,影响卵巢颗粒细胞的健康生长[5]。仔猪长期暴露于低浓度的ZEA(20 μg/kg BW)会抑制卵泡增殖,破坏卵巢结缔组织[6]。临床上,雌二醇(estradiol,E2)常用于诱导动物发情和缓解难产[7]。由于E2不易降解,能够通过土壤、水和食物进入食物链,诱导动物子宫内膜增生和性器官发育不良[8-9]。不过,有关ZEA和E2对断奶小母猪养分利用和卵巢组织结构的比较研究尚未见报道。因此,本试验旨在研究ZEA和E2对断奶小母猪生长性能、养分利用率和卵巢形态的影响,以期揭示两者对仔猪危害的异同。
1 材料与方法 1.1 试验材料ZEA:从以色列Fermentek公司采购,为色谱纯,其纯度保证值98%。苯甲酸雌二醇注射液:杭州动物药品厂生产(2 mg/mL)。
1.2 试验动物分组与处理选用健康的28~32日龄、平均体重(13.57±0.41) kg的“杜×长×大”三元杂交断奶小母猪30头,随机分为3组,每组10头(n=10),保证各组间仔猪初始体重差异不显著(P>0.05)。断奶小母猪基础饲粮组成及营养水平见表 1。对照组饲喂基础饲粮,ZEA组在基础饲粮中添加1.0 mg/kg的ZEA,E2组每隔3 d肌肉注射苯甲酸雌二醇0.75 mL(相当于E2含量为1.5 mg/头),ZEA和E2的用量参考Dai等[10]和Yang等[11]设计。小母猪饲养在安装有塑料漏缝地板、乳头饮水器和料槽的单体笼内,自由采食和饮水。试验开始前1周对猪舍及周围环境进行全面清扫和消毒,试验过程中对猪舍每周1次常规消毒处理。舍内安装红外保温灯,试验第1周舍内温度30 ℃左右,随后温度控制在26~28 ℃。猪舍相对湿度为65%左右。试验预试期为7 d,正试期为35 d。试验结束后屠宰全部仔猪。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
将色谱纯(98%)的晶体粉末状的ZEA用乙酸乙酯溶解,将ZEA-乙酸乙酯溶液喷洒到定量滑石粉上,充分混合后过夜,使乙酸乙酯全部挥发,做成1 000 mg/kg的ZEA预混料,用毒素含量低于检测限(委托青岛出入境检测检疫局测定)的玉米粉将上述ZEA预混料稀释成10 mg/kg的ZEA预混料,最后根据试验饲粮ZEA的设计水平,用10 mg/kg的ZEA预混料替代配方中玉米和载体做成ZEA组饲粮,密封储存。饲粮于试验正式开始前1周一次性配合完成,在试验前和试验结束后将饲粮分别取样,检测饲粮养分和毒素含量。取样方法参照《饲料采样方法》(GB/T 14699.1—2005)进行。
1.4 饲粮常规养分和毒素含量的测定饲粮常规养分分析参考张丽英[12]的方法进行:粗蛋白质(CP)含量测定采用凯氏定氮法,钙含量测定采用高锰酸钾滴定法,磷含量测定采用钼黄比色法,氨基酸含量采用日立835-50型氨基酸分析仪测定。
饲粮中毒素(ZEA、呕吐毒素、黄曲霉毒素和烟曲霉毒素)的测定委托青岛出入境检测检疫局。采用液相色谱法结合荧光检测器测定ZEA和黄曲霉毒素含量,免疫亲和柱层析净化,用外标法进行定量。采用液相色谱并结合紫外检测器对烟曲霉毒素和呕吐毒素进行测定,用外标法定量。黄曲霉毒素、ZEA、呕吐毒素和烟曲霉毒素最低检测限分别为1.0 μg/kg及0.1、0.1和0.25 mg/kg。3组饲粮ZEA含量实际的测定值分别为0、(0.96±0.02) mg/kg和0,其他毒素未检到或者低于检测限。
1.5 检测指标 1.5.1 生长性能每天记录各组仔猪采食量和剩料量,试验前、试验末和每周称量各组仔猪体重,计算平均日采食量(ADFI)、平均日增重(ADG)和料重比(F/G)。
1.5.2 养分利用率正试期的第21~27天,每天记录采食量,收集全部粪、尿,计量后4 ℃保存。消化代谢试验结束,分别混匀7 d的粪、尿,按照粪、尿总量的1/5取样加酸(100 g鲜粪或者100 mL尿样加10%的硫酸10 mL)固定氮,用于测定鲜样基础上的CP含量;另取2/5尿样用于测定尿能,2/5鲜粪样于65 ℃条件下烘干,制备风干样本,测定干物质(DM)、有机物质(OM)、CP和粗脂肪(EE)含量以及总能(GE)。粪便和尿液中DM、OM、CP、EE含量及GE测定参照张丽英[12]的方法进行。

|
试验结束,仔猪禁食12 h后采血10 mL于真空促凝管中,3 000×g下离心15 min,分离血清,-20 ℃保存待测。采用全自动生化分析仪(COBUS MIRA Plus,瑞士罗士公司)测定谷丙转氨酶(ALT)、谷草转氨酶(AST)、碱性磷酸酶(ALP)和乳酸脱氢酶(LDH)活性。
1.5.4 卵巢样品的采集与处理试验结束,将小母猪全部屠宰,观察并记录卵巢的发育状况和称重,计算卵巢的器官指数。快速切取双侧的卵巢置于Bouin’s液中固定,用于制备石蜡组织切片,观察卵巢组织学结构。
1.5.5 苏木精-伊红染色将Bouin’s液中固定的卵巢样品切割修整,在流水中洗到组织呈原色,用乙醇逐级进行脱水、二甲苯进行透明,用石蜡包埋后切片(LEICA RM2235,德国),测定所使用切片的厚度为5~6 μm。将切片经二甲苯脱蜡,再用梯度酒精脱水至蒸馏水。然后苏木素染色6 min;在盐酸酒精中进行分化,蓝化15~20 min后,用伊红染色1 min,经酒精梯度脱水、二甲苯透明、中性树胶封片完成。采用光学显微镜(PLYMPUS BX41,日本)观察卵巢形态学结构,拍照分析。
1.6 数据统计试验数据采用SAS 9.3软件进行单因子方差分析,平均值用Duncan氏法进行多重比较。数据用“平均值±标准差”表示,P < 0.05为显著差异。
2 结果与分析 2.1 ZEA和E2对断奶仔猪生长性能的影响由表 2可知,与对照组相比,ZEA组和E2组断奶仔猪末重、ADG、ADFI和F/G均无显著差异(P>0.05)。
|
|
表 2 ZEA和E2对断奶仔猪生长性能的影响 Table 2 Effects of ZEA and E2 on growth performance of weaned piglets (n=10) |
由表 3可知,与对照组相比,ZEA组断奶仔猪BV和NPU显著提高(P < 0.05),E2组断奶仔猪EE的表观消化率、能量表观代谢率(消化能/GE和代谢能/GE)及蛋白质表观代谢率(BV和NPU)显著提高(P < 0.05)。与ZEA组相比,EB组断奶仔猪EE的表观消化率和蛋白质表观代谢率(BV和NPU)显著提高(P < 0.05)。
|
|
表 3 ZEA和E2对断奶仔猪养分利用率的影响 Table 3 Effects of ZEA and E2 on nutrient utilization of weaned piglets (n=10) |
由表 4可知,与对照组相比,ZEA组断奶仔猪血清ALP和LDH活性显著提高(P < 0.05),EB组断奶仔猪血清AST、ALP和LDH活性显著提高(P < 0.05)。与ZEA组相比,EB组断奶仔猪血清LDH活性显著提高(P < 0.05)。
|
|
表 4 ZEA和E2对断奶仔猪血清酶活性的影响 Table 4 Effects of ZEA and E2 on serum enzyme activity of weaned piglets (n=10) |
由图 1可知,与对照组相比,ZEA组和E2组断奶仔猪的卵巢指数均显著提高(P < 0.05)。与ZEA组相比,E2组断奶仔猪的卵巢指数则显著降低(P < 0.05)。
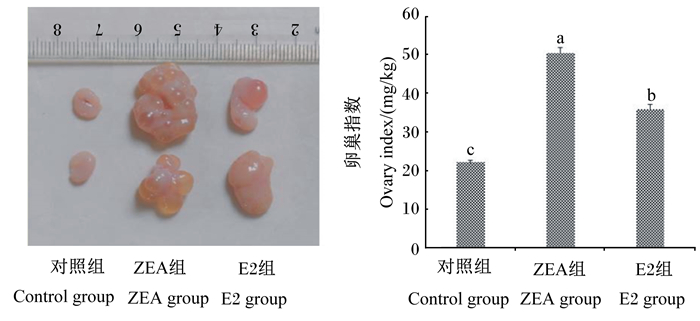
|
数据柱标注不同字母表示差异显著(P < 0.05)。 Date columns with different letter superscripts mean significant difference (P < 0.05). 图 1 ZEA和E2对断奶仔猪卵巢指数的影响 Fig. 1 Effects of ZEA and E2 on ovary index of weaned piglets (n=20) |
由图 2可知,对照组(图 2-A和图 2-D)断奶仔猪卵巢皮质浅层可见规则的原始卵泡(五角星形)成簇排列,偶见闭锁的原始卵泡,皮质深层分布少量的初级生长卵泡(三角形),闭锁的生长卵泡极少(黄箭头处),未见成熟卵泡;ZEA组(图 2-B和图 2-E)断奶仔猪卵巢皮质内可见次级生长卵泡(红箭头处),其体积异常大且卵泡腔充满卵泡液,少见原始卵泡和初级生长卵泡,闭锁的初级生长卵泡较对照组多(黄箭头处);EB组(图 2-C和图 2-F)断奶仔猪卵巢可见大量初级生长卵泡,且大多数发生闭锁(黄箭头处)。
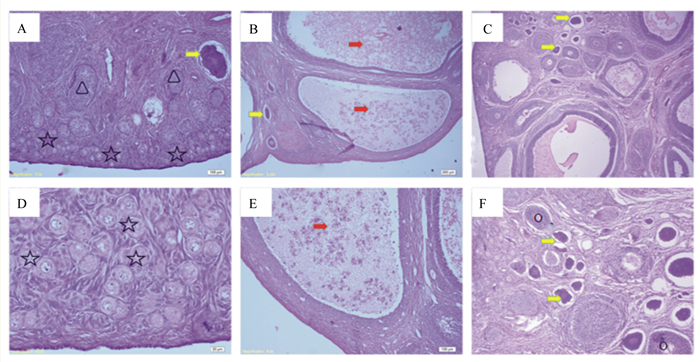
|
A和D为对照组,B和E为ZEA组,C和F为E2组。☆为原始卵泡,△为初级生长卵泡,红箭头处为次级生长卵泡,黄箭头处为闭锁的生长卵泡。A、B和C为样本在40倍下获得的视野;D、E和F为在100倍下获得的视野。 A and D were control group, B and E were ZEA group, and C and F were E2 group. ☆ was a primordial follicle, △ was a primary follicle, the red arrow was a secondary follicle, and the yellow arrow was an atretic growing follicle. A, B and C represented the view of the samples obtained in 40 times, and D, E and F represented the view of the samples obtained in 100 times. 图 2 ZEA和E2对断奶仔猪卵巢形态的影响 Fig. 2 Effects of ZEA and E2 on ovary morphology of weaned piglets |
ZEA的代谢产物玉米赤霉醇(ZEL)能够促进蛋白质的合成,提高饲料利用率,曾经作为牛羊增重剂使用多年[13]。但多年的试验证明,ZEL在食品中的残留影响哺乳动物健康,欧盟1998年禁止ZEL用于畜禽养殖,我国2002年禁止将ZEL用于食品动物。ZEA对猪生长性能的影响报道不一。Marcela等[14]证实3 mg/kg的ZEA对断奶仔猪体重没有显著影响。ZEA(1.0~3.0 mg/kg)不影响断奶仔猪的生长性能,但是线性降低F/G[15]。杨立杰[16]证实饲粮ZEA(0、0.5、1.0和1.5 mg/kg)对断奶仔猪ADFI、ADG和F/G均没有显著影响,但F/G有线性降低的趋势。Jiang等[15, 17]研究表明,ZEA(1.0 mg/kg)显著提高断奶仔猪的ADG和ADFI,推测可能与ZEA的雌激素效应提高仔猪肝脏、肾脏和生殖器官重量有关。然而,给母猪(平均体重64 kg)喂饲霉变玉米饲粮42 d,F/G随着ZEA水平(0、3、6和9 mg/kg)的升高显著降低[18]。雄性大鼠和雌性大鼠饲喂高剂量ZEA(50 mg/kg)饲粮,其增重分别降低了19%和11%[19]。以上结果表明,ZEA对猪生长性能的影响与ZEA水平和动物性别和饲养时间密切相关[20]。本试验条件下,1.0 mg/kg的ZEA和每隔3 d肌肉注射苯甲酸雌二醇0.75 mL(相当于1.5 mg/头E2)对断奶仔猪生长性能都没有显著影响。蒋国礼等[21]研究表明,戊酸雌二醇(相当于0.5 mg/kg E2)通过雌激素受体基因的表达显著降低雌性巴马香猪的体增重及体脂肪沉积率。Hao等[22]研究也表明雌激素通过与肝脏的雌激素受体结合调节体脂肪沉积。不过,ZEA和E2对断奶仔猪体重的分子机制尚需进一步研究。
养分利用率是对动物消化能力的一个综合评价[23]。Jo等[24]表明,10 mg/kg的ZEA处理3 d,没有影响生长猪氨基酸的回肠表观消化率。2 mg/kg的ZEA对仔猪DM和CP表观消化率没有显著影响[25]。给70日龄的约克郡母猪饲喂含有ZEA为2 mg/kg的饲粮,没有影响小母猪生长性能和养分消化率[26]。本试验条件下,ZEA和E2显著提高蛋白质的利用率。E2的经典机制是与雌激素受体结合,形成的复合物进入细胞核,与靶基因反应元件结合后启动DNA转录,调节相应基因的表达和蛋白质合成[27]。本研究中,E2组EE表观消化率显著高于ZEA组,雌激素可以抑制脂肪细胞中脂肪合成相关基因的表达,促进脂肪细胞中的脂肪分解,激活肌肉内脂质氧化,推测体脂肪率的降低可能是EE表观消化率提高的因素之一。以上结果表明,ZEA对生长性能的影响与动物生理阶段和ZEA剂量密切相关。
血清酶活性是肝脏健康水平的重要指标[28]。研究表明,ZEA引起肝脏损伤进而诱导大鼠和家兔血清酶参数改变[29-30]。ZEA(1.0 mg/kg)处理仔猪诱导肝脏中央静脉周围肝细胞的泡状变性和淋巴滤过,其血清生物学结果也证实ZEA造成肝脏损伤的事实[31]。Jiang等[15]研究表明,仔猪饲粮中ZEA(2.0和3.2 mg/kg)使血清ALT活性超出的正常生理范围(31~58 U/L),血清AST和ALP活性也显著升高。血清ALT参与谷氨酸与丙酮酸的转氨作用,AST催化谷氨酸与草酰乙酸的转氨作用,而ALP与骨骼的健康有关,也参与脂肪和蛋白质代谢,LDH可催化丙酸与L-乳酸之间的还原与氧化反应,多用于心肌健康的诊断。本研究中,ZEA组和E2组断奶小母猪血清ALP和LDH活性显著高于对照组,推测ZEA和E2可能诱发心脏疾病,调节钙磷有关的代谢,但需要进一步研究其分子机制。
关于ZEA对卵巢发育影响的研究结果并不一致。Alm等[32]认为,ZEA(30.0 μmol/L)能够通过抑制卵泡刺激素促进孕激素对母猪卵巢发育带来的负面影响。Zhang等[33]也证实,ZEA(30.0 μmol/L)不利于卵泡的发育。ZEA(1.0 mg/kg)诱导断奶仔猪卵巢炎症和血管壁增生与充血,使卵巢血管中大量红细胞和白细胞充满管腔,皮层原始卵泡增多[17]。而杨立杰[16]研究表明,断奶仔猪卵巢器官指数在ZEA低于1.0 mg/kg时线性升高,当ZEA水平为1.5 mg/kg时,卵巢器官指数反而降低。1.1 mg/kg的ZEA能刺激卵泡的发育,而2.0和3.2 mg/kg时发现大量闭锁卵泡[11],这表明ZEA对卵巢发育影响具有剂量依赖效应。雌激素主要通过与雌激素受体结合来调控卵巢的发育[34],E2与雌激素受体结合后会形成激素受体复合物,进而促进孕激素的合成[35],孕激素水平过高诱导卵泡闭锁。本研究中,1.0 mg/kg ZEA促进卵泡的发育进而提高卵巢器官指数,而E2(每隔3 d肌肉注射1.5 mg/头)先是刺激卵泡发育,然后导致大量卵泡闭锁。ZEA在能表达雌激素受体的组织/细胞中(卵巢)表现雌激素样作用,低剂量诱导细胞增殖,高剂量诱导细胞凋亡[36],这与本研究结果一致。
4 结论ZEA和E2均能提高断奶小母猪对蛋白质的表观代谢率,提高血清ALP和LDH活性,刺激卵巢发育和卵泡成熟,诱导卵泡闭锁。
| [1] |
ROGOWSKA A, POMASTOWSKI P, SAGANDYKOVA G, et al. Zearalenone and its metabolites:effect on human health, metabolism and neutralisation methods[J]. Toxicon, 2019, 162: 46-56. |
| [2] |
YANG R, WANG Y M, ZHANG L, et al. Prepubertal exposure to an oestrogenic mycotoxin zearalenone induces central precocious puberty in immature female rats through the mechanism of premature activation of hypothalamic kisspeptin-GPR54 signaling[J]. Molecular and Cellular Endocrinology, 2016, 437: 62-74. |
| [3] |
ZHANG Y Y, JIA Z Q, YIN S T, et al. Toxic effects of maternal zearalenone exposure on uterine capacity and fetal development in gestation rats[J]. Reproductive Sciences, 2014, 21(6): 743-753. |
| [4] |
ABBASIAN N, MOMTAZ S, BAEERI M, et al. Molecular and biochemical evidence on the role of zearalenone in rat polycystic ovary[J]. Toxicon, 2018, 154: 7-14. |
| [5] |
ZHANG G L, SUN X F, FENG Y Z, et al. Zearalenone exposure impairs ovarian primordial follicle formation via down-regulation of Lhx8 expression in vitro[J]. Toxicology and Applied Pharmacology, 2017, 317: 33-40. |
| [6] |
GAJECKA M, RYBARCZYK L, JAKIMIUK E, et al. The effect of experimental long-term exposure to low-dose zearalenone on uterine histology in sexually immature gilts[J]. Experimental and Toxicologic Pathology, 2012, 64(6): 537-542. |
| [7] |
MOGHEISEH A, GHIRI M J M, BANDARIAN E. The clinical follow-up of estradiol benzoate priming during induction of estrus with cabergoline in dogs[J]. Topics in Companion Animal Medicine, 2017, 32(1): 16-19. |
| [8] |
DEGEN G H, BOLT H M. Endocrine disruptors:update on xenoestrogens[J]. International Archives of Occupational and Environmental Health, 2000, 73(7): 433-441. |
| [9] |
REFAIE M M M, EL-HUSSIENY M. The role of interleukin-1b and its antagonist (diacerein) in estradiol benzoate-induced endometrial hyperplasia and atypia in female rats[J]. Fundamental & Clinical Pharmacology, 2017, 31(4): 438-446. |
| [10] |
DAI M L, JIANG S Z, YUAN X J, et al. Effects of zearalenone-diet on expression of ghrelin and PCNA genes in ovaries of post-weaning piglets[J]. Animal Reproduction Science, 2016, 168: 126-137. |
| [11] |
YANG L J, ZHOU M, HUANG L B, et al. Zearalenone-promoted follicle growth through modulation of wnt-1/β-catenin signaling pathway and expression of estrogen receptor genes in ovaries of postweaning piglets[J]. Journal of Agricultural and Food Chemistry, 2018, 66(30): 7899-7906. |
| [12] |
张丽英. 饲料分析及饲料质量检测技术[M]. 4版. 北京: 中国农业大学出版社, 2016.
|
| [13] |
林书康, 游存华. 牛羊增重剂——玉米赤霉醇[J]. 黄牛杂志, 1992, 18(4): 62-66. |
| [14] |
MARCELA Š, LIKER B, ŠPERANDA T, et al. Haematological and biochemical parameters of weaned piglets fed on fodder mixture contaminated by zearalenone with addition of clinoptilolite[J]. Acta Veterinaria, 2006, 56(2/3): 121-136. |
| [15] |
JIANG S Z, YANG Z B, YANG W R, et al. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts[J]. Journal of Animal Science, 2011, 89(10): 3008-3015. |
| [16] |
杨立杰.玉米赤霉烯酮对断奶仔猪免疫毒性及生殖毒性的影响[D].硕士学位论文.泰安: 山东农业大学, 2018. http://cdmd.cnki.com.cn/Article/CDMD-10434-1018169769.htm
|
| [17] |
JIANG S Z, YANG Z B, YANG W R, et al. Effects of feeding purified zearalenone contaminated diets with or without clay enterosorbent on growth, nutrient availability, and genital organs in post-weaning female pigs[J]. Asian-Australasian Journal of Animal Sciences, 2010, 23(1): 74-81. |
| [18] |
YOUNG L G, VESONDER R F, FUNNEL H S, et al. Moldy corn in diets of swine[J]. Journal of Animal Science, 1981, 52(6): 1312-1318. |
| [19] |
NATIONAL TOXICOLOGY PROGRAM. Carcinogenesis bioassay of zearalenone (CAS No.17924-92-4) in F344/N rats and B6C3F1 mice (feed study)[J]. National Toxicology Program Technical Report Series, 1982, 235: 1-155. |
| [20] |
RYKACZEWSKA A, GAJECKA M, DABROWSKI M, et al. Growth performance, selected blood biochemical parameters and body weights of pre-pubertal gilts fed diets supplemented with different doses of zearalenone (ZEN)[J]. Toxicon, 2018, 152: 84-94. |
| [21] |
蒋国礼, 范觉鑫, 张彬, 等. 戊酸雌二醇对雌性巴马香猪脂质代谢的影响及其作用机制[J]. 动物营养学报, 2015, 27(6): 1883-1890. |
| [22] |
HAO L K, WANG Y J, DUAN Y S, et al. Effects of treadmill exercise training on liver fat accumulation and estrogen receptor alpha expression in intact and ovariectomized rats with or without estrogen replacement treatment[J]. European Journal of Applied Physiology, 2010, 109(5): 879-886. |
| [23] |
孙健. 不同添加水平的紫花苜蓿草粉对育肥猪生长性能、养分表观消化率的影响[J]. 中国饲料, 2019(13): 75-77. |
| [24] |
JO H, KONG C, SONG M, et al. Effects of dietary deoxynivalenol and zearalenone on apparent ileal digestibility of amino acids in growing pigs[J]. Animal Feed Science and Technology, 2016, 219: 77-82. |
| [25] |
HAUSCHILD L, LOVATTO P A, LEHNEN C R, et al. Digestibility and metabolism of piglet diets containing zearalenone with addition of organoaluminosilicate[J]. Pesquisa Agropecuária Brasileira, 2007, 42(2): 219-224. |
| [26] |
RAINEY M R, TUBBS R C, BENNETT L W, et al. Prepubertal exposure to dietary zearalenone alters hypothalamo-hypophysial function but does not impair postpubertal reproductive function of gilts[J]. Journal of Animal Science, 1990, 68(7): 2015-2022. |
| [27] |
SHELDAHL L C, SHAPIRO R A, BRYANY D N, et al. Estrogen induces rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane[J]. Neuroscience, 2008, 153(3): 751-761. |
| [28] |
HOMOLKA J.Clinic biochemistry[M]. Prague, Czech: Publ. House SZN, 1969: 434.
|
| [29] |
ČONKOVÁ E, LACIAKOVÁ A, PÁSTOROVÁ B, et al. The effect of zearalenone on some enzymatic parameters in rabbits[J]. Toxicology Letters, 2001, 121(3): 145-149. |
| [30] |
ABBÈS S, OUANES Z, BEN SALAH-ABBÈS J, et al. The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by zearalenone in mice[J]. Toxicon, 2006, 47(5): 567-574. |
| [31] |
JIANG S Z, YANG Z B, YANG W R, et al. Physiopathological effects of zearalenone in post-weaning female piglets with or without montmorillonite clay adsorbent[J]. Livestock Science, 2010, 131(1): 130-136. |
| [32] |
ALM H, GREISING T, BRVSSOW K P, et al. The influence of the mycotoxins deoxynivalenol and zearalenol on in vitro maturation of pig oocytes and in vitro culture of pig zygotes[J]. Toxicology in Vitro, 2002, 16(6): 643-648. |
| [33] |
ZHANG R Q, SUN X F, WU R Y, et al. Zearalenone exposure elevated the expression of tumorigenesis genes in mouse ovarian granulosa cells[J]. Toxicology and Applied Pharmacology, 2018, 356: 191-203. |
| [34] |
赵晓民, 徐小明. 雌激素受体及其作用机制[J]. 西北农林科技大学学报(自然科学版), 2004(12): 154-158. |
| [35] |
朱喜文. 孕激素受体状态对雌激素受体阳性乳腺癌患者预后的影响[J]. 临床合理用药杂志, 2019, 12(15): 166-167. |
| [36] |
ZHENG, BINGJIE WANG, XI LI, et al. Zearalenone promotes cell proliferation or causes cell death?[J]. Toxins, 2018, 10(5): 184. |




