哺乳动物乳腺发育不良是导致其泌乳力低下、生产性能低的重要原因,如母猪乳腺发育不良和泌乳力不高会导致仔猪发育不良和死亡率偏高,从而制约我国生猪养殖水平和效率。哺乳动物乳腺的良好发育是其泌乳功能充分发挥的前提。因此,改善动物乳腺发育,提高其泌乳力和生产性能,进而有效提高我国动物养殖水平和效率具有重要的理论和经济价值。
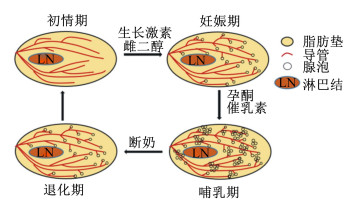
1 小鼠和猪乳腺发育过程及其影响因素哺乳动物乳腺发育主要在出生后,包括初情期、妊娠期、泌乳期和退化期4个阶段(图 1)[1]。雌性动物刚出生时,乳腺只具有简单的原始管状结构。乳腺在初情期之后开始发育,主要表现为乳腺导管的生长和分支;到性成熟期特别是妊娠后,乳腺的腺泡和导管系统都开始发育,在分娩前乳腺组织开始分泌乳汁;在泌乳期整个乳腺基本充满了腺泡和导管,脂肪细胞几乎消失;泌乳末期,乳腺发生退化,即腺泡渐次收缩和消失,而脂肪和结缔组织又开始增生,恢复妊娠前形态[2-3]。总之,性成熟后,伴随每次妊娠、分娩和泌乳,乳腺均经历一次周期性的再生或退化[4]。
|
图 1 哺乳动物出生后乳腺发育过程 Fig. 1 Mammary gland development of mammals after birth[1] |
母猪乳腺位于腹股沟和胸廓之间,在腹中线两侧平行排列,数量一般为6~8对,个别9对以上[5]。母猪乳腺快速发育包括初情期(3月龄到初情期)、妊娠期(妊娠后1/3期)和泌乳期3个关键阶段[6-7]。其中,初情期乳腺发育主要表现为乳腺导管的生长和分支,而妊娠期和泌乳期乳腺发育主要表现为乳腺腺泡数量增加和体积变大。母猪出生时,其乳腺发育不完善,在3月龄后开始快速发育,表现为乳腺组织中DNA的迅速增加(可达4~6倍)[8]。妊娠期的后1/3阶段,乳腺发育主要表现为乳腺实质的生长和乳腺DNA的增长,乳腺的组成也由脂肪和结缔组织转变成具有分泌功能的腺泡小叶组织[9]。进入哺乳期后,母猪乳腺发育仍在继续,乳腺重量及乳腺组织中DNA、蛋白质含量都不断增加[10]。当仔猪断奶后,乳腺的实质组织在7 d内迅速退化,乳腺实质组织的湿重和DNA含量均降低2/3左右[11]。
哺乳动物乳腺发育不仅受遗传(品种)、激素和环境等因素的影响[1, 12-13],营养对乳腺的发育也具有重要的调控作用[14-16]。研究表明,对初情期母猪进行限饲,饲粮蛋白质含量和消化能降低到原来的70%时,乳腺实质重量降低,泌乳量减少[17-18]。而在妊娠和泌乳母猪饲粮中添加10%亚麻籽对母代母猪的乳腺发育无显著作用,但可增加其后代母猪初情期的乳腺实质重量[19]。
2 脂肪酸的分类及其生理调控作用脂肪酸是一类疏水碳氢化合物,属于脂质的一种。根据碳链长度可以分为2~5个碳的短链脂肪酸(SCFA)、6~12个碳的中链脂肪酸(MCFA)和13个碳以上的长链脂肪酸(LCFA)。脂肪酸不饱和键的数量和位置也会影响其理化性质,按照不饱和键数量可分为饱和脂肪酸(SFA)、单不饱和脂肪酸(MUFA)和多不饱和脂肪酸(PUFA)。传统营养观念认为,脂肪酸的主要生理作用是通过有氧氧化来提供能量。越来越多的研究表明,一些脂肪酸还可通过膜受体[G蛋白偶联受体(GPR)、白细胞分化抗原36(CD36)和Toll受体(TLR)等]和核受体[过氧化物酶增殖物激活受体(PPAR)、固醇调节元件结合蛋白(SREBP)和蛋白类视黄醇X受体(RXR)等]来发挥其信号分子作用,调控动物采食、脂肪生成、代谢和炎症/免疫反应等多种生理过程[20-23]。最新研究表明,脂肪酸在动物乳腺发育过程中也发挥着重要的调控作用[24-26]。
3 脂肪酸对小鼠和猪乳腺发育的调控作用及机制 3.1 脂肪酸对小鼠和猪乳腺发育的调控作用饲粮中脂肪酸含量可影响动物乳腺发育。饲喂富含SFA的高脂饲粮,观察到初情期小鼠的乳腺重量虽然增加,但是导管数量和密度显著低于普通饲粮组[27-28]。除此之外,妊娠期大鼠饲喂高脂饲粮,增加其后代的乳腺肿瘤发病率[29-30]。在母猪妊娠末期,由于限饲导致的背膘厚过低会限制乳腺发育,导致乳腺发育不良,乳腺实质重量减少[31]。在妊娠期,母猪营养需求增加,饲粮中补充2%大豆油可改善初乳组成,提高初乳的蛋白质含量和血浆催乳素含量[32]。在泌乳期,不同来源的脂肪酸对母猪的生产性能也有影响,乳中脂肪酸含量及组成和饲粮关系密切[33]。
饲粮中脂肪酸类型可影响动物乳腺发育。饲粮中添加富含n-3 PUFA的鲑鱼油,饲喂小鼠4周后,小鼠乳腺组织终末乳芽(TEB)数量和导管的覆盖百分比显著提高;给小鼠饲喂富含n-6 PUFA的红花籽油的饲粮,其乳腺组织与鲑鱼油饲粮组有类似效果[34]。而终身饮食n-3 PUFA,则会减少TEB数量,同时在乳腺癌模型中有延迟肿瘤发作的作用[35]。饲粮中添加1% cis-10, trans-12共轭亚油酸(CLA)可促进青春期小鼠乳腺导管伸长[36]。将初情期小鼠乳腺进行离体培养,增加n-3 PUFA/n-6 PUFA对导管的伸长没有影响,但观察到了类似妊娠早期的出芽现象,提示n-6 PUFA/n-3 PUFA与乳腺分化可能存在一定联系[37]。摄食n-3 PUFA/n-6 PUFA低的饲粮,可促进乳腺干细胞的自我更新进而增加干细胞库的大小[38]。将泌乳母猪饲粮中的豆油替换为鲑鱼油,其产乳量有增加趋势[39]。在妊娠到泌乳期母猪饲粮中添加1%鱼油会提高乳中n-3 PUFA/n-6 PUFA,减轻断奶时仔猪对急性应激的反应[40]。在妊娠后期和整个泌乳期饲粮中补充β-羟基-β-甲基丁酸可增加乳中脂肪含量和干物质含量,增加初乳产量(初乳成分无影响),但抑制泌乳高峰期乳汁产量,进而影响仔猪的生长[41]。
饲粮中添加单一脂肪酸可影响动物乳腺发育。饲粮中添加中链饱和脂肪酸月桂酸(LA)可促进初情期小鼠的乳腺发育,小鼠乳腺的导管分支和TEB数量极显著高于对照组[25]。饲粮中添加长链单不饱和脂肪酸油酸(OA)可使初情期小鼠乳腺导管分支和TEB数量极显著增加,血清胰岛素样生长因子-1(IGF-1)和雌激素含量也相应增加[24]。但饲粮中添加长链饱和脂肪酸硬脂酸(SA)会抑制初情期小鼠的乳腺导管发育,TEB数量和导管分支显著减少[26]。在细胞水平上,在培养基中添加LA、OA显著促进小鼠乳腺上皮细胞(HC11)的增殖,而添加SA则显著抑制HC11的增殖。本课题组研究也表明,低浓度(10~100 μmol/L)LA极显著促进猪乳腺上皮细胞(PMECs)增殖,而高浓度(200 μmol/L)则极显著抑制PMECs增殖;OA以剂量依赖方式显著促进PMECs增殖,SA以剂量依赖方式显著抑制PMECs增殖(未发表)。在妊娠到泌乳期母猪饲粮中添加1.3% CLA(cis-9,trans-11和trans-10,cis-12异构体混合物),初乳产量有降低趋势,而初乳中脂肪含量显著增加,且仔猪出生1周内死亡率增加[42]。在泌乳母猪饲粮中添加trans-10,cis-12 CLA也降低乳中蛋白质含量[43]。因此,不建议在妊娠和泌乳期母猪饲粮中添加CLA异构体混合物或trans-10, cis-12 CLA。而棕榈油对于乳脂生成的作用显著,用不同浓度的棕榈酸酯处理PMECs,细胞内脂质以剂量依赖方式增加[44]。总之,饲粮中添加不同脂肪酸对动物乳腺发育的影响不尽相同,脂肪酸的含量、种类、构型以及脂肪酸之间的比例均可影响乳腺的发育状况。
3.2 脂肪酸调控小鼠和猪乳腺发育的作用机制脂肪酸调控乳腺发育的机制可能包括2条主要途径:一是通过脂肪酸受体及胞内信号通路发挥其直接调控功能;二是通过调控相关激素的分泌来间接影响乳腺发育。
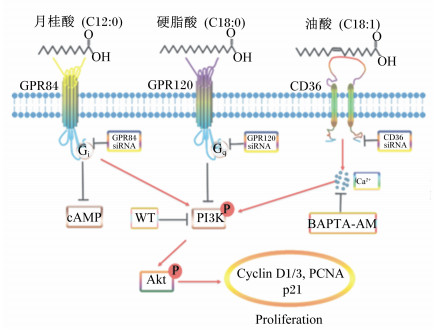
脂肪酸可通过脂肪酸受体直接调控乳腺发育。LA可促进HC11细胞的增殖,同时,LA通过GPR84依赖性激活磷脂酰肌醇-3-激酶/蛋白激酶B(PI3K/Akt)信号通路,而干扰GPR84或阻断PI3K均可逆转LA对小鼠PMECs增殖的促进作用;LA通过GPR84促进PMECs的增殖,干扰GPR84后,促进增殖作用消失。饲粮中添加1% LA可增加小鼠乳腺导管分支和TEB数量,促进GPR84的蛋白表达及PI3K/Akt信号通路的激活[25]。OA可促进HC11细胞的增殖,激活脂肪酸受体CD36,升高胞内Ca2+浓度,进而激活钙调激酶Ⅱ(CaMKⅡ)和PI3K/Akt信号通路;而干扰CD36、螯合胞内Ca2+、阻断CaMKⅡ或PI3K均可逆转OA对HC11细胞增殖的促进作用。饲粮中添加2% OA可显著增加小鼠乳腺导管分支和TEB数量,并激活PI3K/Akt信号通路[24]。SA可抑制HC11细胞的增殖,激活GPR120并以GPR120依赖方式抑制PI3K/Akt信号通路;干扰GPR120可逆转SA对PI3K/Akt信号通路和HC11细胞增殖的抑制作用。饲粮中添加2% SA可显著减少小鼠乳腺导管分支和TEB数量,促进GPR120的蛋白表达,抑制PI3K/Akt信号通路的激活[26]。总之,LA可通过激活细胞膜受体GPR84和PI3K/Akt信号通路促进HC11细胞增殖和初情期小鼠乳腺发育;OA可通过激活CD36-Ca2+-PI3K/Akt信号通路促进HC11细胞增殖和初情期小鼠乳腺发育;SA可通过激活GPR120,进而抑制PI3K/Akt信号通路来抑制HC11细胞增殖和初情期小鼠乳腺发育(图 2)。棕榈酸酯通过CD36激活过氧化物酶体增殖物激活受体γ(PPAR-γ),并上调与乳脂生物合成相关的靶基因甘油-3-磷酸酰基转移酶(GPAM)、1-酰基甘油-3-磷酸酰基转移酶6(AGPAT6)和甘油二酯酰基转移酶1(DGAT1),促进母猪的乳脂生成[44]。
|
GPR84:G蛋白偶联受体84 G protein-coupled receptor 84;GPR120:G蛋白偶联受体120 G protein-coupled receptor 120;CD36:白细胞分化抗原36 leukocyte differentiation antigen 36;siRNA:小干扰RNA small interfering RNA;BAPTA-AM:1, 2-双(2-氨基苯氧基)-乙烷-N, N, N′, N′-四乙酸1, 2-bis (2-aminophenoxy) ethane-N, N, N′, N′-tetraacetic acid;cAMP:环磷酸腺苷cyclic adenosine monophosphate;PI3K:磷脂酰肌醇三激酶phosphatidylinositol 3-kinase;Akt:蛋白激酶B serine/threonine kinase;WT:渥曼青霉素wortmannin;Cyclin D1/3:细胞周期蛋白D1/3;PCNA:增殖细胞核抗原proliferating cell nuclear antigen;p21:细胞周期依赖性蛋白激酶抑制因子1A cyclin-dependent kinase inhibitor 1A;Proliferation:增殖。 图 2 不同脂肪酸调控初情期小鼠乳腺发育的可能机制 Fig. 2 Possible mechanism of regulation of different fatty acids on mammary gland development of puberty mice[45] |
脂肪酸可通过调控激素分泌来间接影响乳腺发育。初情期小鼠饲喂高脂饲粮,其血清雌激素和IGF-1含量显著下降[27],而外源注射雌激素可治疗高脂饲粮导致的乳腺发育受损[46]。研究发现,饲粮中添加LA和OA可显著提高初情期小鼠血液雌激素和IGF-1含量,促进乳腺发育,而添加SA则降低血液雌激素和IGF-1含量,抑制乳腺发育[24-26]。而在怀孕大鼠上,与富含n-6 PUFA的玉米油高脂饲粮相比,饲喂富含n-3 PUFA的鲑鱼油高脂饲粮可显著提高血清雌激素含量,但乳腺TEB数量和密度却显著降低[47]。其原因可能是雌激素含量在生理水平下升高可促进乳腺发育,但过高会抑制乳腺发育。
4 小结本文综述了哺乳动物小鼠和猪乳腺发育过程及影响因素、脂肪酸对乳腺发育的影响及调控作用,并探讨了脂肪酸调控乳腺发育的可能机制,为脂肪酸在调控乳腺发育、提高动物泌乳性能方面的应用提供了理论依据。然而,目前有关脂肪酸影响妊娠期和哺乳期动物乳腺发育的机制研究相对较少;脂肪酸代谢产物对乳腺发育的调控作用也尚不明确。所以,不同生理期脂肪酸的调控作用、脂肪酸代谢物调控乳腺的作用均需要进一步深入研究。
| [1] |
HA W T, JEONG H Y, LEE S Y, et al. Effects of the insulin-like growth factor pathway on the regulation of mammary gland development[J]. Development & Reproduction, 2016, 20(3): 179-185. |
| [2] |
MACIAS H, HINCK L. Mammary gland development[J]. Wiley Interdisciplinary Reviews:Developmental Biology, 2012, 1(4): 533-557. DOI:10.1002/wdev.35 |
| [3] |
AKERS R M. A 100-year review:mammary development and lactation[J]. Journal of Dairy Science, 2017, 100(12): 10332-10352. DOI:10.3168/jds.2017-12983 |
| [4] |
WATSON C J, KREUZALER P A. Remodeling mechanisms of the mammary gland during involution[J]. International Journal of Developmental Biology, 2011, 55(7/8/9): 757-762. |
| [5] |
FARMER C, RENSEN M T. Factors affecting mammary development in gilts[J]. Livestock Production Science, 2001, 70(1/2): 141-148. |
| [6] |
FARMER C, HURLEY W L.Mammary development[M]//Farmer C.The gestating and lactating sow.The Netherlands: Wageningen Academic Publishers, 2015: 73-94.
|
| [7] |
HURLEY W L. Review:mammary gland development in swine:embryo to early lactation[J]. Animal, 2019, 13(Suppl.1): S11-S19. |
| [8] |
S∅RENSEN M T, SEJRSEN K, PURUP S. Mammary gland development in gilts[J]. Livestock Production Science, 2002, 75(2): 143-148. DOI:10.1016/S0301-6226(01)00310-4 |
| [9] |
JI F, HURLEY W L, KIM S W. Characterization of mammary gland development in pregnant gilts[J]. Journal of Animal Science, 2006, 84(3): 579-587. DOI:10.2527/2006.843579x |
| [10] |
KIM S W, HURLEY W L, HAN I K, et al. Changes in tissue composition associated with mammary gland growth during lactation in sows[J]. Journal of Animal Science, 1999, 77(9): 2510-2516. DOI:10.2527/1999.7792510x |
| [11] |
FORD J A, Jr., KIM S W, RODRIGUEZ-ZAS S L, et al.Quantification of mammary gland tissue size and composition changes after weaning in sows[J].Journal of Animal Science, 2003, 81(10): 2583-2589.
|
| [12] |
FARMER C, 何忠武. 激素状态、营养水平与饲养管理方案对猪乳腺发育的影响[J]. 中国猪业, 2014, 9(1): 50-53. DOI:10.3969/j.issn.1673-4645.2014.01.017 |
| [13] |
FARMER C. Review:mammary development in swine:effects of hormonal status, nutrition and management[J]. Canadian Journal of Animal Science, 2013, 93(1): 1-7. DOI:10.4141/cjas2012-066 |
| [14] |
MCNALLY S, STEIN T.Overview of mammary gland development: a comparison of mouse and human[M]//MARTIN F, STEIN T, HOWLIN J.Mammary gland development.New York, Humana Press, 2017.
|
| [15] |
HOLANDA D M, MARCOLLA C S, GUIMARÃES S E F, et al. Dietary L-arginine supplementation increased mammary gland vascularity of lactating sows[J]. Animal, 2019, 13(4): 790-798. DOI:10.1017/S1751731118002069 |
| [16] |
REZAEI R, WU Z L, HOU Y Q, et al. Amino acids and mammary gland development:nutritional implications for milk production and neonatal growth[J]. Journal of Animal Science and Biotechnology, 2016, 7: 20. DOI:10.1186/s40104-016-0078-8 |
| [17] |
FARMER C, PALIN M F, MARTEL-KENNES Y. Impact of diet deprivation and subsequent over-allowance during prepuberty.Part 1.Effects on growth performance, metabolite status, and mammary gland development in gilts[J]. Journal of Animal Science, 2012, 90(3): 863-871. DOI:10.2527/jas.2011-4131 |
| [18] |
FARMER C, PALIN M F, MARTEL-KENNES Y. Impact of diet deprivation and subsequent over-allowance during prepuberty.Part 2.Effects on mammary gland development and lactation performance of sows[J]. Journal of Animal Science, 2012, 90(3): 872-880. DOI:10.2527/jas.2011-4480 |
| [19] |
FARMER C, PALIN M F. Feeding flaxseed to sows during late-gestation and lactation affects mammary development but not mammary expression of selected genes in their offspring[J]. Canadian Journal of Animal Science, 2008, 88(4): 585-590. DOI:10.4141/CJAS08018 |
| [20] |
TAN J K, MCKENZIE C, MARIÑO E, et al. Metabolite-sensing G protein-coupled receptors-facilitators of diet-related immune regulation[J]. Annual Review of Immunology, 2017, 35: 371-402. DOI:10.1146/annurev-immunol-051116-052235 |
| [21] |
SONG T X, ZHOU Y F, PENG J, et al. GPR120 promotes adipogenesis through intracellular calcium and extracellular signal-regulated kinase 1/2 signal pathway[J]. Molecular and Cellular Endocrinology, 2016, 434: 1-13. DOI:10.1016/j.mce.2016.06.009 |
| [22] |
MIYAMOTO J, HASEGAWA S, KASUBUCHI M, et al. Nutritional signaling via free fatty acid receptors[J]. International Journal of Molecular Science, 2016, 17(4): 450. DOI:10.3390/ijms17040450 |
| [23] |
WANG S B, XIANG N N, YANG L S, et al. Linoleic acid and stearic acid elicit opposite effects on AgRP expression and secretion via TLR4-dependent signaling pathways in immortalized hypothalamic N38 cells[J]. Biochemical and Biophysical Research Communications, 2016, 471(4): 566-571. DOI:10.1016/j.bbrc.2016.02.031 |
| [24] |
MENG Y Y, ZHANG J, YUAN C, et al. Oleic acid stimulates HC11 mammary epithelial cells proliferation and mammary gland development in peripubertal mice through activation of CD36-Ca2+ and PI3K/Akt signaling pathway[J]. Oncotarget, 2018, 9(16): 12982-12994. DOI:10.18632/oncotarget.24204 |
| [25] |
MENG Y Y, ZHANG J, ZHANG F L, et al. Lauric acid stimulates mammary gland development of pubertal mice through activation of GPR84 and PI3K/Akt signaling pathway[J]. Journal of Agricultural and Food Chemistry, 2017, 65(1): 95-103. DOI:10.1021/acs.jafc.6b04878 |
| [26] |
MENG Y Y, YUAN C, ZHANG J, et al. Stearic acid suppresses mammary gland development by inhibiting PI3K/Akt signaling pathway through GPR120 in pubertal mice[J]. Biochemical and Biophysical Research Communications, 2017, 491(1): 192-197. DOI:10.1016/j.bbrc.2017.07.075 |
| [27] |
孟莹莹, 张静, 张枫琳, 等. 高脂日粮对初情期小鼠乳腺发育的影响及分子机制[J]. 华南农业大学学报, 2017, 38(3): 9-14. |
| [28] |
KAMIKAWA A, ICHⅡ O, YAMAJI D, et al. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice[J]. Developmental Dynamics, 2009, 238(5): 1092-1099. DOI:10.1002/dvdy.21947 |
| [29] |
LEUNG Y K, GOVINDARAJAH V, CHEONG A, et al. Gestational high-fat diet and bisphenol A exposure heightens mammary cancer risk[J]. Endocrine-Related Cancer, 2017, 24(7): 365-378. |
| [30] |
SIMMEN R.Maternal high fat diet promotion of mammary tumor risk in adult progeny is associated with early expansion of mammary cancer stem-like cells and increased maternal oxidative environment[J].The FASEB Journal, 2013, 27(1): 235.2-.2.
|
| [31] |
FARMER C, COMI M, DUARTE C R A, et al. Differences in body condition of gilts that are maintained from mating to the end of gestation affect mammary development[J]. Journal of Animal Science, 2016, 94(8): 3206-3214. DOI:10.2527/jas.2016-0531 |
| [32] |
PENG X, YAN C, HU L, et al. Effects of fat supplementation during gestation on reproductive performance, milk composition of sows and intestinal development of their offspring[J]. Animals, 2019, 9(4): E125. DOI:10.3390/ani9040125 |
| [33] |
LAURIDSEN C, DANIELSEN V. Lactational dietary fat levels and sources influence milk composition and performance of sows and their progeny[J]. Livestock Production Science, 2004, 91(1/2): 95-105. |
| [34] |
ANDERSON B M, MACLENNAN M B, HILLYER L M, et al. Lifelong exposure to n-3 PUFA affects pubertal mammary gland development[J]. Applied Physiology Nutrition and Metabolism, 2014, 39(6): 699-706. DOI:10.1139/apnm-2013-0365 |
| [35] |
LIU J J, ABDELMAGID S A, PINELLI C J, et al. Marine fish oil is more potent than plant-based n-3 polyunsaturated fatty acids in the prevention of mammary tumors[J]. The Journal of Nutritional Biochemistry, 2018, 55(6): 41-52. |
| [36] |
BERRYHILL G E, GLOVICZKI J M, TROTT J F, et al. Diet-induced metabolic change induces estrogen-independent allometric mammary growth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(40): 16294-16299. DOI:10.1073/pnas.1210527109 |
| [37] |
LIU Y E, PU W P, WANG J D, et al. Activation of Stat5 and induction of a pregnancy-like mammary gland differentiation by eicosapentaenoic and docosapentaenoic omega-3 fatty acids[J]. The FEBS Journal, 2007, 274(13): 3351-3362. DOI:10.1111/j.1742-4658.2007.05869.x |
| [38] |
HILL E M, ESPER R M, SEN A, et al. Dietary polyunsaturated fatty acids modulate adipose secretome and is associated with changes in mammary epithelial stem cell self-renewal[J]. The Journal of Nutritional Biochemistry, 2019, 71: 45-53. DOI:10.1016/j.jnutbio.2019.05.007 |
| [39] |
LAVERY A, LAWLOR P G, MILLER H M, et al. The effect of dietary oil type and energy intake in lactating sows on the fatty acid profile of colostrum and milk, and piglet growth to weaning[J]. Animals, 2019, 9(12): 1092. DOI:10.3390/ani9121092 |
| [40] |
MCAFEE J M, KATTESH H G, LINDEMANN M D, et al. Effect of omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation to lactating sows on growth and indicators of stress in the postweaned pig[J]. Journal of Animal Science, 2019, 97(11): 4453-4463. DOI:10.1093/jas/skz300 |
| [41] |
FLUMMER C, THEIL P K. Effect of β-hydroxy β-methyl butyrate supplementation of sows in late gestation and lactation on sow production of colostrum and milk and piglet performance[J]. Journal of Animal Science, 2012, 90(Suppl.4): 372-374. |
| [42] |
KROGH U, FLUMMER C, JENSEN S K, et al. Colostrum and milk production of sows is affected by dietary conjugated linoleic acid[J]. Journal of Animal Science, 2012, 90(Suppl.4): 366-368. |
| [43] |
SANDRI E C, HARVATINE K J, OLIVEIRA D E. Trans-10, cis-12 conjugated linoleic acid reduces milk fat content and lipogenic gene expression in the mammary gland of sows without altering litter performance[J]. British Journal of Nutrition, 2019, 123(6): 610-618. |
| [44] |
LV Y T, ZHANG S H, GUAN W T, et al. Metabolic transition of milk triacylglycerol synthesis in response to varying levels of palmitate in porcine mammary epithelial cells[J]. Genes & Nutrition, 2018, 13: 18. |
| [45] |
孟莹莹.脂肪酸对初情期小鼠乳腺发育的影响及其分子机制研究[D].硕士学位论文.广州: 华南农业大学, 2017.
|
| [46] |
OLSON L K, TAN Y, ZHAO Y, et al. Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness[J]. International Journal of Obesity, 2010, 34(9): 1415-1426. DOI:10.1038/ijo.2010.51 |
| [47] |
HILAKIVI-CLARKE L, CHO E, CABANES A, et al. Dietary modulation of pregnancy estrogen levels and breast cancer risk among female rat offspring[J]. Clinical Cancer Research, 2002, 8(11): 3601-3610. |




