氨基酸是多肽和蛋白质合成的基本单位,也是调节动物机体各种代谢过程的分子信号传递者。作为机体营养物质的消化吸收部位,胃肠道在机体氨基酸代谢中发挥了至关重要的作用。氨基酸进入哺乳动物胃肠道后,一方面被内分泌细胞表面分布的氨基酸感应受体所识别,通过胞内信号分子的活化,介导胃肠激素分泌,从而调控包括采食、胃肠蠕动等一系列胃肠生理活动;另一方面,位于肠上皮细胞的氨基酸转运载体,除了介导氨基酸的吸收,还通过对氨基酸浓度的感应参与哺乳动物雷帕霉素靶蛋白复合物1(mammalian target of rapamycin complex 1,mTORC1)和一般性调控阻遏蛋白激酶2(general control nonderepressible 2,GCN2)信号通路对氨基酸平衡的调控,在维持细胞内外氨基酸稳态中发挥了重要作用。本文将从胃肠道内分泌系统、氨基酸感应受体及其介导的胃肠激素分泌、氨基酸转运载体及其参与氨基酸平衡调控这几个方面进行综述。
1 胃肠道的氨基酸感应系统 1.1 胃肠道内分泌细胞胃肠道内分泌系统由弥散分布在整个胃肠道黏膜上的内分泌细胞组成,包括L细胞、K细胞、I细胞、D细胞等十几种类型。尽管其数目只占肠上皮细胞总数的1%左右,但却构成了机体最大的内分泌器官[1-2]。内分泌细胞受到肠腔营养物质刺激后,可分泌胃泌素(gastrin)、酪酪肽(peptide YY,PYY)和胆囊收缩素(cholecystokinin,CCK)等数十种胃肠激素。胃肠道作为食物与机体联系的中介,其内分泌细胞分泌的激素是反映机体营养状态和传递代谢信号的关键。内分泌细胞在肠道内分布的部位不同,其产生的激素也相应地调控特定肠道部位的生理功能,包括消化吸收、食欲、胃肠蠕动、pH等。此外,根据与腔内营养物质直接接触与否,内分泌细胞分为“开放式”和“封闭式”2种类型。“开放式”内分泌细胞利用顶部的微绒毛感应肠道内容物或者化学刺激,释放胃肠激素来调控胃肠生理活动及动物的采食;“封闭式”内分泌细胞主要通过感应胃肠机械刺激和其他激素而影响自身分泌[3]。
1.2 胃肠道的氨基酸感应受体饲粮蛋白质降解产生的氨基酸,可被内分泌细胞表面分布的各类氨基酸感应受体识别,其中包括钙敏感受体(calcium sensing receptor,CaSR)、1型味觉受体1/3(type 1 taste receptor 1/3,T1R1/T1R3)、G蛋白偶联受体C家族6组A亚型受体(G protein-coupled receptor class C group 6 member A,GPRC6A)、代谢型谷氨酸受体(metabotropic glutamate receptors,mGLURs)和G蛋白偶联受体142(G protein-coupled receptor 142,GPR142)(表 1)。它们均属于跨膜蛋白,利用胞外的捕蝇夹域(venus flytrap domain,VFD)识别、结合配体[4]。
|
|
表 1 哺乳动物胃肠道分布的氨基酸感应受体 Table 1 Distribution of amino acid sensing receptors in gastrointestinal tract of mammalian animals |
CaSR在胃G细胞、D细胞[13]以及十二指肠Ⅰ细胞中高表达[14],既能感应胞外Ca2+浓度,也能感应L-氨基酸。研究表明,人和啮齿动物胃G细胞上的CaSR可感应阳离子多肽及氨基酸,介导胃泌素的释放[15];胃壁细胞的CaSR通过感应氨基酸等营养素调控H+-K+-ATP酶的活性[16]。在猪上的研究发现,饲粮蛋白质水平影响胃和小肠CaSR的基因表达,并伴随胃蛋白酶活性、血清胃肠激素浓度的显著变化[17]。此外,CaSR能感应芳香族L-苯丙氨酸和L-色氨酸,并促进猪胃肠激素的分泌,包括胃泌素、生长抑素、CCK等[7-9];L-精氨酸也可激活CaSR,通过下游信号分子腺苷酸环化酶(adenylate cyclase,AC)及磷脂酶C(phospholipase C,PLC)的活化,促进十二指肠CCK、葡萄糖依赖性促胰岛素多肽(glucose-dependent insulintropic polypeptide,GIP)的分泌[10]。
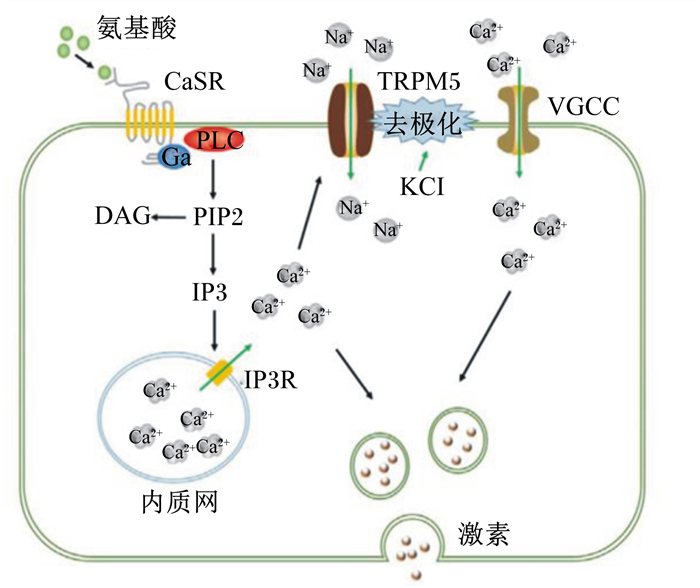
磷脂酰肌醇途径在CaSR介导胃肠激素分泌过程中发挥着重要作用。CaSR被激活后刺激胞内信号分子4,5-二磷酸磷脂酰肌醇(4, 5-phosphatidylinositol bisphosphate,PIP2)的表达,PIP2在PLC的分解作用下产生1,4,5-三磷酸肌醇(1,4,5-inositol triphosphate,IP3),并与内质网上的IP3受体特异性结合,导致Ca2+从内质网钙库释放[18-19]。而瞬时受体电位离子通道蛋白5(transient receptor potential subfamily M member 5,TRPM5)作为细胞膜上重要的通道蛋白之一,胞内Ca2+浓度的升高会激活TRPM5而发生细胞去极化,从而打开电压门控钙离子通道(voltage-gated calcium channel,VGCC),促使胞外Ca2+内流[20]。因此,CaSR通过磷脂酰肌醇途径引起的胞内Ca2+浓度升高可能活化TRPM5,进一步提高了胞内Ca2+浓度,而胞内高浓度的Ca2+与细胞去极化过程相互协同,促进激素的分泌(图 1)。
|
CaSR:钙敏感受体calcium sensing receptor;Gα:G蛋白G protein;PLC:磷脂酶C phospholipase C;PIP2:4,5-二磷酸磷脂酰肌醇4, 5-phosphatidylinositol bisphosphate;IP3:1,4,5-三磷酸肌醇1,4,5-inositol triphosphate;DAG:二酰基甘油diacylglycerol;IP3R:1,4,5-三磷酸肌醇受体1,4,5-inositol triphosphate receptor;TRPM5:瞬时受体电位离子通道蛋白5 transient receptor potential subfamily M member 5;VGCC:电压门控钙离子通道voltage-gated calcium channel。 图 1 CaSR介导氨基酸诱导胃肠激素分泌的可能分子机制 Fig. 1 Possible molecular mechanism of CaSR mediated amino acid induced gastrointestinal hormone secretion |
T1R1/T1R3可识别20多种L-氨基酸,尤其对L-谷氨酸敏感[21]。研究表明,T1R1、T1R3单体与CCK在小鼠Ⅰ细胞共表达;体外培养STC-1细胞和小肠组织发现,L-苯丙氨酸、亮氨酸、异亮氨酸和谷氨酸均能刺激CCK分泌,且这种促分泌效应在加入T1R1/T1R3抑制剂或激活剂后相应地减弱或增强,说明T1R1/T1R3可能参与L-氨基酸诱导的CCK分泌[6,22]。CCK是重要的内源性饱感因子,灌喂谷氨酸钠对大鼠食欲的抑制作用可能是通过上调十二指肠T1R1/T1R3基因表达,诱导CCK分泌,进而下调下丘脑促食因子神经肽Y(neuropeptide Y, NPY)的表达来实现的[23]。相应地,高蛋白质水平饲粮可上调猪胃肠道与该受体偶联的G蛋白的表达,参与机体的代谢和饱感的产生[24]。与其他L-氨基酸感应受体相比,T1R1在STC-1细胞的表达最少,且与CaSR、胰高血糖素样肽-1(glucagon-like peptide-1,GLP-1)和PYY在STC-1细胞共表达[25],说明它可能参与介导GLP-1和PYY的分泌。除内分泌细胞,T1R1/T1R3在胃平滑肌细胞也存在表达,被谷氨酸钠激活后通过降低胞内Ca2+浓度抑制肌肉收缩[26]。
1.3.3 GPRC6AGPRC6A与CaSR结构最相似,氨基酸序列一致性达32%[27],能感应大部分的L-氨基酸,尤其是碱性氨基酸[6],但关于GPRC6A介导胃肠激素分泌的作用存在争议。免疫组化结果显示,GPRC6A与胃泌素和生长激素抑制素在胃窦黏膜中共定位[13]。但一些体外研究显示,氨基酸促进生长激素抑制素的分泌由CaSR介导而非GPRC6A[28]。此外,GPRC6A与PYY、GLP-1在STC-1细胞中共定位,说明它可能介导这2种激素的分泌[25]。GPRC6A介导L-鸟氨酸刺激GLUTag细胞分泌GLP-1[29];然而,对GPRC6A敲除小鼠的研究表明,GPRC6A并非L-精氨酸和L-鸟氨酸诱导GLP-1分泌所必需的[30]。而关于人的研究显示,该受体在细胞膜上的表达很低,而在细胞内的表达似乎更多,因此可能并未参与氨基酸感应[3]。
1.3.4 mGLURsmGLURs共包含8种亚型(mGLUR1~mGLUR8),根据受体序列的相似性又可分为3类:Ⅰ类为mGLUR1和mGLUR5;Ⅱ类为mGLUR2和mGLUR3;Ⅲ类为mGLUR4、mGLUR6、mGLUR7和mGLUR8[31]。其中,mGLUR4和mGLUR1主要在近端小肠表达[6,32],但mGLUR4在胃肠道的作用似乎强于mGLUR1[33],因此,关于mGLUR4在胃肠道中作用的研究较多。研究表明,mGLUR4基因在胃肠道的表达模式因物种而异。在小鼠,mGLUR4在胃肠道的表达量由小肠到大肠逐渐升高;在人和猪,mGLUR4在胃肠道的表达情况较相似,均在十二指肠和结肠的表达量最高[5-6]。有研究报道,谷氨酸钠可促进猪胃和空肠mGLUR4基因表达[32]。
1.3.5 GPR142关于GPR142的研究相对较少。研究发现,GPR142在肠内分泌细胞中选择性表达,可被L-色氨酸激活,而引起GIP和GLP-1的分泌,进一步影响葡萄糖代谢[12]。在小鼠上,GPR142基因在分泌胃饥饿素的D细胞[34]和肠道K细胞中表达量最高[12]。
2 胃肠道氨基酸转运系统 2.1 氨基酸转运系统的分类及特点肠道氨基酸转运是调控机体氨基酸稳态的关键。在转运载体的协助下,氨基酸跨膜进出细胞来发挥重要的生物学功能。根据所转运的氨基酸的性质,转运载体通常分为A、ASC、B0、L、N等系统,例如,A系统主要负责转运小型极性中性氨基酸,L系统主要负责转运疏水性中性氨基酸[35]。此外,转运载体也被纳入溶质载体(solute carriers,SLC)家族,根据基因序列的相似性,分别归为SLC1、SLC3、SLC6、SLC7、SLC15、SLC16等,例如,SLC1包含谷氨酸和中性氨基酸转运载体,SLC16包含芳香族氨基酸转运载体[36]。氨基酸的转运与离子的运动有关,包括Na+、H+、K+和Cl-。不同氨基酸转运载体的作用机制不同,Bröer等[36]总结了哺乳动物肠道上皮细胞顶膜和基底膜氨基酸转运载体的作用,详见表 2。
|
|
表 2 哺乳动物肠道上皮细胞氨基酸转运系统 Table 2 Amino acid transport system in mammalian intestinal epithelial cells |
哺乳动物具有维持机体氨基酸平衡的机制,以保证细胞内氨基酸浓度的正常。mTORC1和GCN2是动物机体调节氨基酸平衡的2条主要信号路径。其中,mTORC1路径主要响应胞外氨基酸浓度的变化,而GCN2路径主要在胞内氨基酸不足时被激活。氨基酸转运载体与mTORC1和GCN2信号通路的相互作用在维持细胞内外氨基酸稳态中发挥了重要作用。
2.2.1 mTORC1信号通路蛋白激酶类哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)以mTORCl和mTORC2的形式在细胞内发挥生物学功能。这2种复合物均包含催化亚基mTOR、稳定mTOR的EC13致死蛋白8(mammalian lethal with SEC 13 protein 8,mLST8)和mTOR负调节因子Deptor(DEP domain-containing mTOR-interacting protein)。此外,mTOR调控相关蛋白(regulatory-associated protein of mTOR,Raptor)和抑制亚基PRAS40(proline-rich Akt substrate 40 ku)是mTORCl的特有组分,其中Raptor负责mTORCl的定位,PRAS40可抑制mTORCl的活性[37]。
众多因子参与调控mTORCl对胞外氨基酸浓度的感应,其中Ras相关鸟苷三磷酸酶(Ras-related guanosine triphosphatases,Rag GTPases)发挥着重要作用。氨基酸充足时,Rag蛋白的调控子复合物(Ragulator)和液泡状腺苷三磷酸酶(vacuolar adenosine triphosphatase,V-ATPase)介导Rag GTPases的活化,并将Rag GTPases锚定在溶酶体上。随后,活化的Rag GTPases将mTORCl招募至溶酶体上激活[38]。因此,溶酶体膜是整合氨基酸感应信号并激活mTORC1的细胞器。激活后的mTORCl可以磷酸化转录激活因子p70 S6激酶1(p70 S6 kinase 1,S6Kl)和转录起始因子真核翻译起始因子4E结合蛋白1(eukaryotic initiation factor 4E binding protein 1,4EBPl)来促进蛋白质的合成[39]。而当氨基酸不足时,mTORC1的活性则会迅速消除,以减少氨基酸的消耗。尽管mTORC1对氨基酸浓度的变化高度敏感,但它对氨基酸的敏感程度并不相同,其中L-亮氨酸、L-精氨酸对其活化尤为重要。当L-亮氨酸浓度发生变化时,胞内的氨基酸感受器亮氨酰tRNA合成酶(leucyl-tRNA synthetase,LRS)以L-亮氨酸依赖性方式激活mTORC1。其过程包括:LRS感知L-亮氨酸浓度并与之结合后,通过与RagD相互作用而促进RagD与核苷酸的结合状态由鸟苷三磷酸(guanosine triphosphate,GTP)转变为鸟苷二磷酸(guanosine diphosphate,GDP),之后LRS与Rag解离,从而激活mTORC1[40]。L-精氨酸可以被溶酶体膜上的跨膜蛋白SLC38A9所感应,之后通过SLC38A9与Rag GTPases和Ragulator的结合而激活mTORC1[41]。
氨基酸转运载体在mTORC1信号通路上游发挥着关键作用。研究表明,细胞对L-谷氨酰胺的摄取是必需氨基酸和生长因子调控mTORC1的限速步骤[42]。其过程包括:L-谷氨酰胺被摄取后,可作为异二聚双向转运载体SLC3A2/SLC7A5的交换底物,引起细胞对L-亮氨酸的摄取,从而调控L-亮氨酸对mTORC1的激活。同样地,SLC1A5作为高亲和力的L-谷氨酰胺转运载体,其抑制会导致mTORC1信号通路的抑制。
2.2.2 GCN2信号通路蛋白质合成中,没有氨基酸可以弥补其他氨基酸的缺失,因此细胞必须能够有效地感应任何一种氨基酸的缺失来防止肽链合成的失败。在核糖体内,氨基酸特异性氨酰tRNA合成酶(amino acid specific aminoacyl tRNA synthetases,aaRSs)负责氨基酸与同源tRNA的结合,因此氨基酸缺乏会导致空载tRNA的积累。而GCN2对所有空载tRNA都有很高的亲和力,GCN2含有的与组氨酸-tRNA合成酶(histidyl-tRNA synthetase,HisRS)同源的调控区域负责与空载tRNA的结合[43]。当胞内氨基酸浓度较低时,GCN2通过与空载tRNA结合引起自身构象变化而被激活。蛋白质翻译的起始包括真核翻译起始因子2(eukaryotic initiation factor 2,eIF2)三聚体与GTP和甲酰甲氨酰tRNA(methionyl-tRNAMet,Met-tRNAMet)形成三元复合物,接着与核糖体40S亚基结合形成43S前起始复合物[44]。翻译启动后,与GDP结合的eIF2被释放,然后通过鸟嘌呤交换因子(guanine exchange factor,GEF)eIF2B恢复到与GTP结合的状态。GCN2激活后,磷酸化的elF2α成为eIF2B的竞争性抑制剂,导致elF2无法形成翻译起始的三元复合物,从而降低胞内氨基酸消耗[45]。而一些特定的mRNA却能够绕过这一翻译障碍,如激活转录因子4(activating transcription factor 4,ATF4)。ATF4是一种控制适应性功能的基因转录调控子,在许多基因的调控区域有结合位点,可以上调包括氨基酸转运体和氨基酸生物合成相关酶等多种基因的转录来调节氨基酸的浓度[46]。
研究发现,在营养充足的培养基中几乎无法检测到细胞表达ATF4蛋白,而缺乏色氨酸或谷氨酰胺时,ATF4蛋白的表达显著升高,并伴随SLC1A5表达的增加;敲除细胞ATF4基因后,培养基中色基酸的缺乏则不会引起SLC1A5表达的改变[47]。这说明色氨酸的缺乏会引起ATF4依赖性转运载体SLC1A5表达的上调,并反过来促进细胞对色氨酸和谷氨酰胺的吸收。此外,该信号通路还可以通过激活相关酶的表达提高氨基酸的转化,进而提高转运载体对氨基酸的摄取,其中包括ATF4依赖性天冬酰胺合成酶(asparagine synthetase,ASNS)。该酶可以将天冬氨酸转变为天冬酰胺,提高了转运载体ASCT2的底物浓度,从而将其他氨基酸转运进入细胞而供其生长代谢所需[48]。
3 小结胃肠氨基酸感应与转运调控机体对氨基酸的吸收和代谢,然而相应的感应机制和调控手段还需进一步研究。首先,发现新的氨基酸感应受体与其配体,深入探究感应体-信号转导-激素分泌的途径,这不仅有助于揭示肠道消化吸收功能的调节过程,也将为通过胃肠调控来治疗代谢性疾病提供新的思路。其次,探究如何有效地调控氨基酸转运受体介导的GCN2和mTORC1信号通路,提高动物机体对氨基酸的利用,从而改善动物对营养物质的吸收。
| [1] |
ADRIAENSSENS A E, REIMANN F, GRIBBLE F M. Distribution and stimulus secretion coupling of enteroendocrine cells along the intestinal tract[J]. Comprehensive Physiology, 2018, 8(4): 1603-1638. |
| [2] |
MACE O J, TEHAN B, MARSHALL F. Pharmacology and physiology of gastrointestinal enteroendocrine cells[J]. Pharmacology Research & Perspectives, 2015, 3(4): e00155. |
| [3] |
SANTOS-HERNÁNDEZ M, MIRALLES B, AMIGO L, et al. Intestinal signaling of proteins and digestion-derived products relevant to satiety[J]. Journal of Agricultural and Food Chemistry, 2018, 66(39): 10123-10131. DOI:10.1021/acs.jafc.8b02355 |
| [4] |
REIMANN F, TOLHURST G, GRIBBLE F M. G-protein-coupled receptors in intestinal chemosensation[J]. Cell Metabolism, 2012, 15(4): 421-431. DOI:10.1016/j.cmet.2011.12.019 |
| [5] |
SYMONDS E L, PEIRIS M, PAGE A J, et al. Mechanisms of activation of mouse and human enteroendocrine cells by nutrients[J]. Gut, 2015, 64(4): 618-626. DOI:10.1136/gutjnl-2014-306834 |
| [6] |
TIAN M, HENG J H, SONG H Q, et al. Branched chain amino acids stimulate gut satiety hormone cholecystokinin secretion through activation of the umami taste receptor T1R1/T1R3 using an in vitro porcine jejunum model[J]. Food & Function, 2018, 10(6): 3356-3367. |
| [7] |
XIAN Y H, ZHAO X Y, WANG C, et al. Phenylalanine and tryptophan stimulate gastrin and somatostatin secretion and H+-K+-ATPase activity in pigs through calcium-sensing receptor[J]. General and Comparative Endocrinology, 2018, 267: 1-8. DOI:10.1016/j.ygcen.2018.05.022 |
| [8] |
ZHAO X Y, XIAN Y H, WANG C, et al. Calcium-sensing receptor-mediated L-tryptophan-induced secretion of cholecystokinin and glucose-dependent insulinotropic peptide in swine duodenum[J]. Journal of Veterinary Science, 2018, 19(2): 179-187. DOI:10.4142/jvs.2018.19.2.179 |
| [9] |
FENG J Y, KANG C C, WANG C, et al. L-phenylalanine increased gut hormone secretion through calcium-sensing receptor in the porcine duodenum[J]. Animals, 2019, 9(8): 476. DOI:10.3390/ani9080476 |
| [10] |
WANG C, KANG C C, XIAN Y H, et al. Sensing of L-arginine by gut-expressed calcium sensing receptor stimulates gut satiety hormones cholecystokinin and glucose-dependent insulinotropic peptide secretion in pig model[J]. Journal of Food Science, 2018, 83(9): 2394-2401. DOI:10.1111/1750-3841.14297 |
| [11] |
STEENSELS S, DEPOORTERE I. Chemoreceptors in the gut[J]. Annual Review of Physiology, 2018, 80: 117-141. DOI:10.1146/annurev-physiol-021317-121332 |
| [12] |
TODA N, HAO X L, OGAWA Y, et al. Potent and orally bioavailable GPR142 agonists as novel insulin secretagogues for the treatment of type 2 diabetes[J]. ACS Medicinal Chemistry Letters, 2013, 4(8): 790-794. DOI:10.1021/ml400186z |
| [13] |
HAID D, WIDMAYER P, BREER H. Nutrient sensing receptors in gastric endocrine cells[J]. Journal of Molecular Histology, 2011, 42(4): 355-364. DOI:10.1007/s10735-011-9339-1 |
| [14] |
LIOU A P, SEI Y, ZHAO X L, et al. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2011, 300(4): G538-G546. DOI:10.1152/ajpgi.00342.2010 |
| [15] |
HERSEY S J, SACHS G. Gastric acid secretion[J]. Physiological Reviews, 1995, 75(1): 155-189. |
| [16] |
DUFNER M M, KIRCHHOFF P, REMY C, et al. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2005, 289(6): G1084-G1090. DOI:10.1152/ajpgi.00571.2004 |
| [17] |
赵秀英, 孟祥龙, 伍力, 等. 日粮不同蛋白水平对猪小肠CaSR基因表达及胃肠激素分泌的影响[J]. 畜牧与兽医, 2016, 48(12): 30-35. |
| [18] |
KLEPAC K, KILIĆ A, GNAD T, et al. The Gq signalling pathway inhibits brown and beige adipose tissue[J]. Nature Communications, 2016, 7(1): 10895. DOI:10.1038/ncomms10895 |
| [19] |
COURJARET R, DIB M, MACHAC K. Spatially restricted subcellular Ca2+ signaling downstream of store-operated calcium entry encoded by a cortical tunneling mechanism[J]. Scientific Reports, 2018, 8(1): 11214. DOI:10.1038/s41598-018-29562-9 |
| [20] |
ZHOU H R, PESTKA J J. Deoxynivalenol (vomitoxin)-induced cholecystokinin and glucagon-like peptide-1 release in the STC-1 enteroendocrine cell model is mediated by calcium-sensing receptor and transient receptor potential ankyrin-1 channel[J]. Toxicological Sciences, 2015, 145(2): 407-417. DOI:10.1093/toxsci/kfv061 |
| [21] |
NELSON G, CHANDRASHEKAR J, HOON M A, et al. An amino-acid taste receptor[J]. Nature, 2002, 416(6877): 199-202. DOI:10.1038/nature726 |
| [22] |
DALY K, AL-RAMMAHI M, MORAN A, et al. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion[J]. American Journal of Physiology:Gastrointestinal and Liver Physiology, 2013, 304(3): G271-G282. DOI:10.1152/ajpgi.00074.2012 |
| [23] |
康翠翠.L-氨基酸通过感应体调控猪CCK分泌的机制及谷氨酸对大鼠CCK和采食的影响[D].硕士学位论文.南京: 南京农业大学, 2018.
|
| [24] |
DE GIORGIO R, MAZZONI M, VALLORANI C, et al. Regulation of α-transducin and α-gustducin expression by a high protein diet in the pig gastrointestinal tract[J]. PLoS One, 2016, 11(2): e0148954. DOI:10.1371/journal.pone.0148954 |
| [25] |
WANG H X, MURTHY K S, GRIDER J R. Expression patterns of L-amino acid receptors in the murine STC-1 enteroendocrine cell line[J]. Cell and Tissue Research, 2019, 378(3): 471-483. DOI:10.1007/s00441-019-03074-y |
| [26] |
CROWE M S, WANG H X, BLAKENEY B A, et al. Expression and function of umami receptors T1R1/T1R3 in gastric smooth muscle[J]. Neurogastroenterology & Motility, 2020, 32(2): e13737. |
| [27] |
CONIGRAVE A D, HAMPSON D R. Broad-spectrum L-amino acid sensing by class 3 G-protein-coupled receptors[J]. Trends in Endocrinology & Metabolism, 2006, 17(10): 398-407. |
| [28] |
NAKAMURA E, HASUMURA M, UNEYAMA H, et al. Luminal amino acid-sensing cells in gastric mucosa[J]. Digestion, 2011, 83(Suppl.1): 13-18. |
| [29] |
OYA M, KITAGUCHI T, PAIS R, et al. The G Protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells[J]. Journal of Biological Chemistry, 2012, 288(7): 4513-4527. |
| [30] |
CLEMMENSEN C, JORGENSEN C V, SMAJILOVIC S, et al. Robust GLP-1 secretion by basic L-amino acids does not require the GPRC6A receptor[J]. Diabetes Obesity and Metabolism, 2017, 19(4): 599-603. DOI:10.1111/dom.12845 |
| [31] |
FERRIGNO A, BERARDO C, DI PASQUA L G, et al. Localization and role of metabotropic glutamate receptors subtype 5 in the gastrointestinal tract[J]. World Journal of Gastroenterology, 2017, 23(25): 4500-4507. DOI:10.3748/wjg.v23.i25.4500 |
| [32] |
ZHANG J, YIN Y L, SHU X G, et al. Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets[J]. Amino Acids, 2013, 45(5): 1169-1177. DOI:10.1007/s00726-013-1573-2 |
| [33] |
YOSHIDA Y, KAWABATA Y, KAWABATA F, et al. Expressions of multiple umami taste receptors in oral and gastrointestinal tissues, and umami taste synergism in chickens[J]. Biochemical and Biophysical Research Communications, 2015, 466(3): 346-349. DOI:10.1016/j.bbrc.2015.09.025 |
| [34] |
ENGELSTOFT M S, PARK W M, SAKATA I, et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells[J]. Molecular Metabolism, 2013, 2(4): 376-392. DOI:10.1016/j.molmet.2013.08.006 |
| [35] |
BRÖER S. Amino acid transport across mammalian intestinal and renal epithelia[J]. Physiologocal Reviews, 2008, 88(1): 249-286. DOI:10.1152/physrev.00018.2006 |
| [36] |
BRÖER S, FAIRWEATHER S J. Amino acid transport across the mammalian intestine[J]. Comprehensive Physiology, 2018, 9(1): 343-373. |
| [37] |
CONDON K J, SABATINI D M. Nutrient regulation of mTORC1 at a glance[J]. Journal of Cell Science, 2019, 132(21): jcs222570. DOI:10.1242/jcs.222570 |
| [38] |
DUAN Y H, LI F N, TAN K R, et al. Key mediators of intracellular amino acids signaling to mTORC1 activation[J]. Amino Acids, 2015, 47(5): 857-867. DOI:10.1007/s00726-015-1937-x |
| [39] |
GOBERDHAN D C I, WILSON C, HARRIS A L. Amino acid sensing by mTORC1:intracellular transporters mark the spot[J]. Cell Metabolism, 2016, 23(4): 580-589. DOI:10.1016/j.cmet.2016.03.013 |
| [40] |
HAN J M, JEONG S J, PARK M C, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway[J]. Cell, 2012, 149(2): 410-424. DOI:10.1016/j.cell.2012.02.044 |
| [41] |
LEI H T, MA J M, MARTINEZ S S. Crystal structure of arginine-bound lysosomal transporter SLC38A9 in the cytosol-open state[J]. Nature Structural & Molecular Biology, 2018, 25(6): 522-527. |
| [42] |
NICKLIN P, BERGMAN P, ZHANG B L, et al. Bidirectional transport of amino acids regulates mTOR and autophagy[J]. Cell, 2009, 136(3): 521-534. DOI:10.1016/j.cell.2008.11.044 |
| [43] |
EFEYAN A, COMB W C, SABATINI D M. Nutrient-sensing mechanisms and pathways[J]. Nature, 2015, 517(7534): 302-310. DOI:10.1038/nature14190 |
| [44] |
JACKSON R J, HELLEN C U T, PESTOVA T V. The mechanism of eukaryotic translation initiation and principles of its regulation[J]. Nature Reviews Molecular Cell Biology, 2010, 11(2): 113-127. DOI:10.1038/nrm2838 |
| [45] |
HARDING H P, NOVOA I, ZHANG Y H, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells[J]. Molecular Cell, 2000, 6(5): 1099-1108. DOI:10.1016/S1097-2765(00)00108-8 |
| [46] |
BRÖER S. Amino acid homeostasis and signalling in mammalian cells and organisms[J]. Biochemical Journal, 2017, 474(12): 1935-1963. DOI:10.1042/BCJ20160822 |
| [47] |
TIMOSENKO E, GHADBANE H, SILK J D, et al. Nutritional stress induced by tryptophan-degrading enzymes results in ATF4-dependent reprogramming of the amino acid transporter profile in tumor cells[J]. Cancer Research, 2016, 76(21): 6193-6204. DOI:10.1158/0008-5472.CAN-15-3502 |
| [48] |
BALASUBRAMANIAN M N, BUTTERWORTH E A, KILBERG M S. Asparagine synthetase:regulation by cell stress and involvement in tumor biology[J]. American Journal of Physiology:Endocrinology and Metabolism, 2013, 304(8): E789-E799. DOI:10.1152/ajpendo.00015.2013 |




