2. 山东农业大学动物科技学院, 泰安 271018;
3. 西藏自治区农牧科学院畜牧兽医研究所, 拉萨 850000
2. College of Animal Science and Technology, Shandong Agricultural University, Tai'an 271018, China;
3. Institute of Animal Husbandry and Veterinary, Tibet Academy of Agricultural and Animal Husbandry Science, Lhasa 850000, China
拉萨白鸡是以白来航鸡(父本)与河谷藏鸡(母本)杂交培育的良种蛋鸡群体[1],对西藏高海拔地区表现出良好的适应性,具有抗病力强、耐粗放管理、成熟早和生产性能良好的特点,受到西藏广大农牧民的欢迎,已成为西藏农区大规模养殖的当家品种,产生了良好的社会效益和经济效益[2]。由于拉萨白鸡养殖于青藏高原,其种蛋孵化率受到海拔高度、遗传因素、种蛋品质和贮存条件等因素的严重制约[2]。种蛋孵化率低严重影响拉萨白鸡的群体扩大和推广,限制了西藏农牧民增收致富的渠道。研究表明,孵化后期是拉萨白鸡胚胎死亡的高峰期,孵化后期胚胎死亡数占死亡总数的44.2%[3]。孵化后期,鸡胚基础代谢升高,呼吸方式转为肺呼吸,转身、啄壳等活动增强,生理和代谢发生巨大变化,鸡胚处于营养物质和能量紧缺状态,能量供应不足限制鸡胚发育,影响种蛋孵化性能[4]。
胚蛋给养是一种在孵化期为家禽胚胎提供外源营养物质的营养调控手段,胚蛋给养适宜的营养物质可促进家禽胚胎生长,提高家禽种蛋孵化性能,改善其出壳后的生长性能,具有良好的应用价值[5],孵化后期羊膜腔给养对家禽种蛋孵化性能的改善效果尤为突出[6]。孵化后期是鸡胚肠道形态和功能性发育的重要时期,鸡胚小肠绒毛形成于15胚龄,随着胚龄增加,不同发育阶段肠道绒毛呈现火箭形(rocket-shaped)、手指形(finger-shaped)和梨形(pear-shaped)3种形态[7-8],谢文惠等[9]研究表明出壳后肉仔鸡十二指肠、空肠和回肠肠道绒毛均为手指形绒毛,提示手指形绒毛具有更好的消化吸收功能。从17胚龄到出壳当天,肉仔鸡空肠绒毛高度可增长200%~300%[10],小肠重量可增加近2倍[11],肠道消化酶和营养物质转运载体基因表达水平迅速升高[12]。孵化后期,营养物质缺乏限制肠道的发育,甚至造成鸡胚死亡,胚蛋给养可改善鸡胚营养状况,促进胚胎和肠道发育,提高鸡胚存活率,改善孵化性能[13-17]。N-乙酰-L-谷氨酸(N-acetyl-L-glutamate,NAG)是一种氨基酸衍生物,可激活内源性精氨酸合成过程中关键酶氨甲酰磷酸合成酶Ⅰ和吡咯啉合成酶,并提高其活性[18]。研究表明,饲粮添加NAG及其结构类似物可有效促进仔猪肠道瓜氨酸和精氨酸合成[18],提高仔猪肠道营养物质转运载体基因表达水平,增强肠道消化吸收功能,改善其生长性能[19],而NAG对家禽孵化性能和胚胎期肠道发育的影响鲜有报道。
胚蛋给养是否具有促进拉萨白鸡孵化后期肠道发育和提高其种蛋孵化率的作用目前尚不清楚。因此,本试验以拉萨白鸡为试验动物,以NAG作为给养功能物质,研究羊膜腔给养NAG对拉萨白鸡孵化性能的影响,并通过对鸡胚肠道形态的研究探讨胚蛋给养NAG对鸡胚肠道发育的影响,为胚蛋给养促进家禽胚胎生长,改善家禽孵化性能和提高雏鸡质量应用提供理论基础和实践依据。
1 材料与方法 1.1 试验材料与试验动物将NAG(纯度为98%)用0.85%生理盐水配制成1.5% NAG营养液。拉萨白鸡受精蛋由西藏自治区农牧科学院畜牧兽医研究所提供,采用DMS-9600型孵化器(山东省青岛兴仪电子设备有限公司)进行孵化,孵化试验于西藏自治区农牧科学院畜牧兽医研究所蔡公堂乡拉萨白鸡养殖基地进行。
1.2 试验设计选取发育正常的17胚龄拉萨白鸡活胚蛋240枚,随机分为对照组和NAG给养组,每个组设6个重复,每个重复20枚活胚蛋。于17.5胚龄,调整孵化间温度和相对湿度接近孵化条件,对照组种蛋不做处理,直接转入出雏器;用75%乙醇溶液擦拭NAG给养组种蛋气室端,确定羊膜腔位置后,在合适部位钻1个直径为1 mm的小孔,用1 mL注射器(针头长25 mm)吸取0.1 mL 1.5% NAG营养液(NAG补给量为1.5 mg/枚),经羊膜腔注射到种蛋中,注射后用指甲油封口[20]。以重复为单位进行注射,注射时间不超过2 min,注射完成后,将种蛋转移至出雏器,孵化条件不变。17.5胚龄注射程序完成后,将种蛋转移至出雏器中,温湿度(出雏器温度37.8 ℃、相对湿度75%)与孵化器保持一致,19胚龄时按照表 1所示孵化程序调整出雏器温度和相对湿度。本试验以0.85%生理盐水溶液为溶剂配制1.5% NAG营养液,因本实验室以往试验研究以及诸多相关文献均表明胚蛋给养生理盐水对家禽孵化性能、胚胎生长与肠道形态以及幼雏生长性能无显著影响[15-17],故本试验未设置生理盐水注射组。
|
|
表 1 孵化程序 Table 1 Incubation program |
种蛋孵化采用恒温孵化程序,孵化程序见表 1。1~18胚龄每1.5 h翻蛋1次,翻蛋角度为90 °,每4 h记录1次孵化器温度和相对湿度。10和17胚龄照蛋,淘汰未受精和发育异常种蛋。
1.4 测定指标和方法 1.4.1 鸡胚生长指标在19.5胚龄,以重复为单位,挑选1枚重量接近平均重量的活胚蛋,每个组共计挑选6枚活胚蛋,称量蛋重、胚重(含卵黄囊)和卵黄囊重,计算胚重/蛋重、绝对体重(不含卵黄囊)、卵黄囊指数和绝对体指数。绝对体重、卵黄囊指数和绝对体指数计算公式如下:
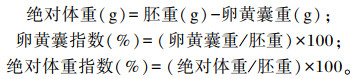
|
测定完鸡胚生长指标后,将鸡胚解剖,分离肠段,取十二指肠、空肠和回肠中间部分各0.5 cm肠段,置于4%多聚甲醛溶液中固定,参照周建民等[21]方法将肠道组织经脱水和石蜡包埋后制为4 μm切片,用苏木精-伊红(HE)染色并封固切片。将切片置于荧光显微镜(BK-FL4)下观测肠道绒毛形态。于显微镜(100×)下选择合适的视野,观测并统计连续50根绒毛的类型,统计各类型绒毛占总绒毛的比例,以百分数形式表示[8]。每个切片随机抽取5个非连续性视野,每个视野选取3根手指形绒毛,测量绒毛顶端到绒毛基部的长度,作为绒毛高度(VH),于绒毛中部位置测量绒毛宽度(VW)。
1.4.3 孵化性能出壳当天,以重复为单位统计未出雏蛋数、健康雏鸡数和弱雏鸡数。健康雏鸡判别标准为活泼、叫声响亮清脆,外表羽毛清洁、干燥、有光泽,脐部愈合良好无血痕,肛门无粪便黏附。健康雏鸡数和弱雏鸡数总和记为总雏鸡数,总雏鸡数和未出雏蛋数总和记为总蛋数。按照下列公式计算孵化率和健雏率。

|
数据统计采用SAS Version 8e统计软件的t检验进行分析,试验数据用平均值±标准误(mean±SE)表示,P < 0.05表示处理效应差异显著,0.05≤P < 0.10表示有差异显著性趋势。
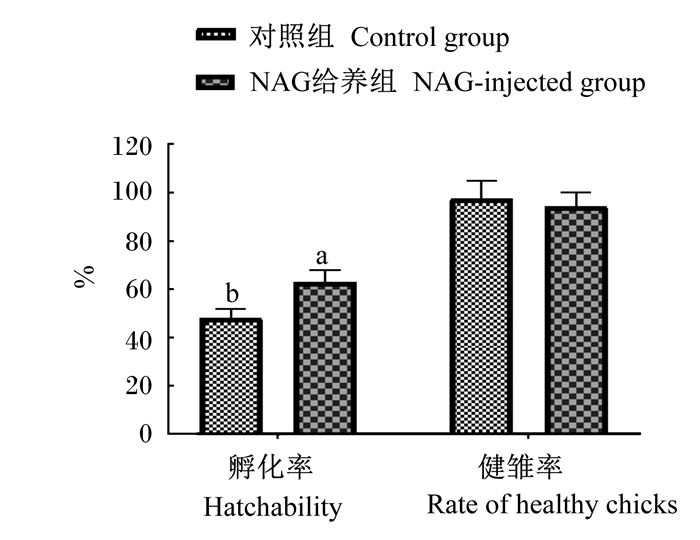
2 结果与分析 2.1 胚蛋给养NAG对种蛋孵化性能的影响本试验中,NAG给养组共有42枚未出雏蛋,其中死胚17枚,弱胚25枚;对照组共有59枚未出雏蛋,其中死胚26枚,弱胚33枚。由图 1可知,对照组的孵化率为48.19%,NAG给养组的孵化率为63.16%,NAG给养组较对照组提高14.97%(P < 0.05);胚蛋给养NAG对健雏率无显著影响(P>0.05),19.5胚龄采样后,NAG给养组和对照组各有114枚种蛋,NAG给养组可比对照组多提供14只健康雏鸡。
|
图柱无字母标注表示差异不显著(P>0.05),标注不同字母表示差异显著(P < 0.05)。 Date columns with no letter mean no significant difference (P > 0.05), while with different letters mean significant difference (P < 0.05). 图 1 胚蛋给养NAG对种蛋孵化性能的影响 Fig. 1 Effects of in ovo feeding of NAG on hatching performance of hatching eggs |
由表 2可知,NAG给养组蛋重、胚重、卵黄囊重、绝对体重、胚重/蛋重、卵黄囊指数和绝对体重指数均与对照组无显著差异(P>0.05)。
|
|
表 2 胚蛋给养NAG对鸡胚生长的影响 Table 2 Effects of in ovo feeding of NAG on growth of chicken embryos |
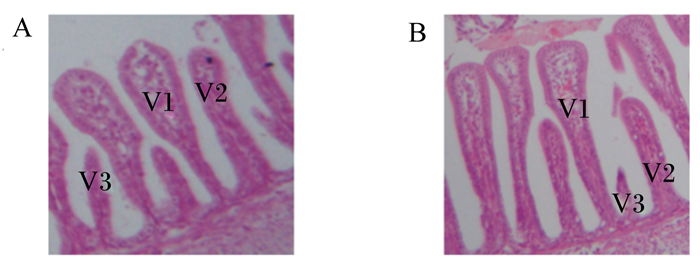
由图 2可知,鸡胚十二指肠绒毛可分为梨形(V1)、手指形(V2)和火箭形(V3),梨形绒毛特征为顶部膨大,火箭形绒毛顶部呈尖形,手指形绒毛形似手指。鸡胚空肠和回肠绒毛也呈现这3种类型,与十二指肠一致。
|
绒毛分为梨形(V1)、手指形(V2)和火箭形(V3)。 Villus were divided into pear-shaped (V1), rocket-shaped (V2) and finger-shaped (V3). 图 2 对照组(A)和NAG给养组(B)鸡胚十二指肠绒毛形态 Fig. 2 Duodenal villi morphology of chicken embryos from control group (A) and NAG-injected group (B) (100×) |
由表 3可知,与对照组相比,胚蛋给养NAG可显著降低鸡胚十二指肠火箭形绒毛比例(P < 0.05),对十二指肠梨形和手指形绒毛比例无显著影响(P>0.05);胚蛋给养NAG对空肠和回肠各类型绒毛比例均无显著影响(P>0.05)。
|
|
表 3 胚蛋给养NAG对鸡胚肠道各类型绒毛比例的影响 Table 3 Effects of in ovo feeding of NAG on proportions of different types of intestinal villus of chicken embryos |
由表 4可知,NAG给养组鸡胚回肠手指形绒毛高度显著高于对照组(P < 0.05);与对照组相比,胚蛋给养NAG有提高鸡胚空肠手指形绒毛宽度的趋势(0.05≤P < 0.10)。
|
|
表 4 胚蛋给养NAG对鸡胚肠道手指形绒毛高度和宽度的影响 Table 4 Effects of in ovo feeding of NAG on height and width of finger-shaped villi in intestine of chicken embryo |
孵化性能不降低是胚蛋给养投入应用的重要前提。在前人的研究中,胚蛋给养对家禽孵化性能和幼雏质量的影响结果并不一致。17.5胚龄羊膜腔给养β-羟基-β-甲基丁酸(HMB)可使新生雏鸡体重提高5%~6%,而对孵化率无显著影响[22-23];6胚龄卵黄囊给养L-肉毒碱降低了火鸡孵化率,17或18胚龄羊膜腔给养L-肉毒碱对肉鸡孵化率无显著影响[10];马友彪[24]分别于7(气室)、14(气室)和18胚龄(羊膜腔)为爱拔益加(AA)肉鸡胚蛋给养HMB溶液,结果表明7胚龄气室给养HMB显著提高了种蛋孵化率,而14胚龄气室给养和18胚龄羊膜腔给养HMB对孵化率无显著影响;Nowaczewski等[25]研究发现,20胚龄胚蛋给养4或8 mg维生素C可显著提高北京鸭种蛋孵化率,而对肉鸡种蛋孵化率无显著影响。可见,胚蛋给养对家禽孵化性能的影响受给养时间、给养部位、营养物质的种类和剂量以及家禽品种等因素的影响。本试验中,鸡胚死亡集中于孵化后期,与佘永新等[3]的研究结果一致,孵化后期胚蛋给养NAG显著提高了拉萨白鸡种蛋孵化率。拉萨白鸡是西藏第1个育成的优良家禽品种,对藏地环境表现出较好的适应性,在保留了藏鸡优良肉质的同时,产蛋性能也得到极大提升[1],具有较好的经济效益和社会效益,但种蛋孵化率低是制约其大规模推广的重要因素。本试验结果表明,胚蛋给养NAG是提高拉萨白鸡种蛋孵化率的有效手段,为拉萨白鸡生产推广提供了新思路,同时也可为胚蛋给养用于改善藏鸡孵化性能提供理论依据。
孵化后期,鸡胚基础代谢加强,肠道和肝脏快速发育,肺呼吸、转身和破壳等活动消耗大量能量,鸡胚处于能量缺乏状态[26],胚蛋给养为家禽胚胎提供外源营养物质,可增加胚胎能量储备。研究发现,17.5胚龄胚蛋给养葡萄糖和一水肌酸混合物可显著提高AA肉仔鸡肝糖原和胸肌肌酸含量,节省卵黄囊营养物质消耗,提高新生雏鸡卵黄囊重量[27];23胚龄胚蛋给养HMB或精氨酸、17.5胚龄胚蛋给养碳水化合物或肉毒碱、14.5胚龄胚蛋给养蔗糖和麦芽糖混合营养液可分别提高火鸡、罗斯肉鸡和太平王鸽胚胎或幼雏肝糖原含量,增加肝糖原储备[28-30]。孵化期间,卵黄囊内脂肪酸氧化是鸡胚获取能量的唯一方式,孵化前2周,鸡胚对卵黄囊内营养物质的利用速度较慢,而最后1周,卵黄囊内营养物质消耗速度较快[26]。出壳前1周卵黄囊内脂质利用情况直接影响鸡胚发育,Yadgary等[31]发现,18~21胚龄卵黄囊内脂肪消耗量低对鸡胚发育和孵化性能均有负面影响,因此,卵黄囊内营养物质利用情况可作为衡量鸡胚发育状况的指标之一。本试验中,胚蛋给养NAG对拉萨白鸡胚胎生长无显著影响。
3.2 胚蛋给养NAG对拉萨白鸡鸡胚肠道形态的影响种蛋内物质是鸡胚的唯一营养来源,出壳后,肉仔鸡通过采食饲粮获得营养物质以满足自身生长发育需要,孵化后期,鸡胚肠道经历一系列形态和功能发育,使肉仔鸡出壳后具有一定的消化和吸收能力[32]。孵化期间,鸡胚肠道黏膜不存在隐窝结构,出壳后数小时隐窝开始逐渐形成,直至2~3 d肉仔鸡肠道才形成典型的隐窝结构[33]。本试验中,未检测到19.5胚龄鸡胚肠道隐窝的存在,与Uni等[7]的研究结果一致。Uni等[7]研究发现鸡胚肠道绒毛形成于15胚龄,17胚龄鸡胚可观测到肠道绒毛呈现不同形态,20胚龄鸡胚肠道可观测到3种形态绒毛[7]。Nazem等[8]研究发现19胚龄时鸡胚肠道绒毛就已呈现梨形、手指形和火箭形3种形态。本试验中,19.5胚龄鸡胚十二指肠、空肠和回肠样品均可观测到3种形态绒毛,与Uni等[7]和Nazem等[8]的研究结果基本一致。在十二指肠中,火箭形绒毛最多,手指形绒毛其次,梨形绒毛最少;在空肠和回肠中,手指形绒毛最多,火箭形绒毛多于梨形绒毛。肠道绒毛结构与消化吸收功能具有一定关系,绒毛高度与肠上皮细胞数量呈正相关,绒毛越高,面积越大,小肠对营养物质的消化吸收能力越强[28]。出壳前后,小肠绒毛高度增长迅速,而绒毛宽度增长缓慢,绒毛表面积的增加主要是由于绒毛高度的增长[34],孵化期小肠绒毛高度的增加有助于提高肠道绒毛表面积,增加肠上皮细胞数量,促进消化酶的分泌,增强小肠消化吸收能力,提高肉仔鸡出壳后对饲粮的利用效率,改善其生长性能[8, 35-36]。孵化后期,经羊膜腔给养的营养物质可以被鸡胚吞食并进入胃肠道,促进鸡胚肠道早期发育[37, 10]。本试验中,胚蛋给养NAG改善孵化后期鸡胚肠道形态可能与NAG促进肠道瓜氨酸和精氨酸的合成,促进肠道发育有关[38],具体作用机制有待于后续研究阐明。
较多胚蛋给养相关研究表明,家禽种蛋孵化率的提高与胚期肠道功能的改善相关性不强[39-42]。本试验中,胚蛋给养NAG提高拉萨白鸡种蛋孵化率的原因可能是补充外源氨基酸增加了种蛋内氨基酸的含量,促进了鸡胚蛋白质合成,同时抑制了蛋白质降解[8, 43]。此外,胚蛋给养NAG对拉萨白鸡出壳后生长发育的影响仍需进一步研究。
4 结论孵化后期(17.5胚龄)经由羊膜腔给养1.5 mg/枚NAG可有效提高拉萨白鸡种蛋的孵化率,显著降低鸡胚十二指肠幼稚型绒毛比例,增加回肠绒毛高度,促进鸡胚肠道发育。因此,胚蛋给养NAG可以作为一种促进早期肠道发育和提高孵化率的方式应用于拉萨白鸡。
| [1] |
刘会杰, 王燕, 冯静, 等. 拉萨白鸡生长性能和肉品质研究[J]. 中国家禽, 2017, 39(14): 11-14. |
| [2] |
冯静, 王燕, 王莉, 等. 影响拉萨白鸡种蛋孵化率的主要因素[J]. 中国家禽, 2015, 37(2): 63-65. |
| [3] |
佘永新, 田发益, 赵晓玲, 等. 低氧环境对良种鸡蛋孵化率的影响[J]. 西南农业学报, 2001, 14(3): 71-74. |
| [4] |
LEKSRISOMPONG N, ROMERO-SANCHEZ H, PLUMSTEAD P W, et al. Broiler incubation.1.Effect of elevated temperature during late incubation on body weight and organs of chicks[J]. Poultry Science, 2007, 86(12): 2685-2691. |
| [5] |
齐广海, 张海军, 马友彪, 等. 肉仔鸡胚期给养研究进展[J]. 饲料工业, 2019, 40(15): 1-8. |
| [6] |
PEEBLES D E. In ovo applications in poultry:a review[J]. Poultry Science, 2018, 97(7): 2322-2338. DOI:10.3382/ps/pey081 |
| [7] |
UNI Z, TAKO E, GAL-GARBER O, et al. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo[J]. Poultry Science, 2003, 82(11): 1747-1754. |
| [8] |
NAZEM M N, SAJJADIAN S M, KHEIRANDISH R, et al. Histomorphometric analysis of the small intestine of broiler chick embryos injected in ovo with methionine[J]. Animal Production Science, 2019, 59(1): 133-139. DOI:10.1071/AN17269 |
| [9] |
谢文惠, 姜宁, 王鑫, 等. 复合益生菌制剂对肉仔鸡养分表观利用率、血清生化指标和肠道黏膜形态的影响[J]. 动物营养学报, 2018, 30(4): 1495-1503. |
| [10] |
高天.胚蛋注射精氨酸对肉仔鸡早期肠道发育和功能的影响及其作用机制研究[D].博士学位论文.南京: 南京农业大学, 2017: 20-30.
|
| [11] |
UNI Z, SMIRNOV A, SKLAN D. Pre-and posthatch development of goblet cells in the broiler small intestine:effect of delayed access to feed[J]. Poultry Science, 2003, 82(2): 320-327. DOI:10.1093/ps/82.2.320 |
| [12] |
CHOTINSKY D, TONCHEVA E, PROFIROV Y. Development of disaccharidase activity in the small intestine of broiler chickens[J]. British Poultry Science, 2001, 42(3): 389-393. |
| [13] |
COLLIN A, BERRI C, TESSERAUD S, et al. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens[J]. Poultry Science, 2007, 86(5): 795-800. DOI:10.1093/ps/86.5.795 |
| [14] |
UNI Z, FERKET P R.Enhancement of development of oviparous species by in ovo feeding: 20020035965[P].2001-07-31.
|
| [15] |
GAO T, ZHAO M M, ZHANG L, et al. Effects of in ovo feeding of L-arginine on the development of lymphoid organs and small intestinal immune barrier function in posthatch broilers[J]. Animal Feed Science and Technology, 2017, 225: 8-19. |
| [16] |
GAO T, ZHAO M M, LI Y J, et al. Effects of in ovo feeding of L-arginine on the development of digestive organs, intestinal function and post-hatch performance of broiler embryos and hatchlings[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(1): e166-e175. |
| [17] |
GAO T, ZHAO M, ZHANG L, et al. Effect of in ovo feeding of L-arginine on the hatchability, growth performance, gastrointestinal hormones, and jejunal digestive and absorptive capacity of posthatch broilers[J]. Journal of Animal Science, 2017, 95(7): 3079-3092. |
| [18] |
WU G Y, KNABE D A, WOO K S. Arginine nutrition in neonatal pigs[J]. The Journal of Nutrition, 2004, 134(10): 2783S-2790S. |
| [19] |
YANG H S, FU D Z, KONG X F, et al. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets[J]. Journal of Animal Science, 2013, 91(6): 2740-2748. DOI:10.2527/jas.2012-5795 |
| [20] |
AL-DARAJI H J, AL-MASHADANI A A, AL-MASHADANI W K, et al. Effect of in ovo injection with L-arginine on productive and physiological traits of Japanese quail[J]. South African Journal of Animal Science, 2012, 42(2): 139-145. |
| [21] |
周建民, 马友彪, 张海军, 等. 白酒糟酵母培养物对肉仔鸡生长性能、血清抗氧化指标和肠道形态结构的影响[J]. 动物营养学报, 2019, 31(5): 2357-2366. |
| [22] |
UNI Z, FERKET P R, TAKO E, et al. In ovo feeding improves energy status of late-term chicken embryos[J]. Poultry Science, 2005, 84(5): 764-770. DOI:10.1093/ps/84.5.764 |
| [23] |
OHTA Y, TSUSHIMA N, KOIDE K, et al. Effect of amino acid injection in broiler breeder eggs on embryonic growth and hatchability of chicks[J]. Poultry Science, 1999, 78(11): 1493-1498. |
| [24] |
马友彪.胚蛋给养β-羟基-β-甲基丁酸对肉仔鸡肌肉发育的影响[D].硕士学位论文.北京: 中国农业科学院, 2016: 12-25.
|
| [25] |
NOWACZEWSKI S, KONTECKA H, KRYSTIANIAk S. Effect of in ovo injection of vitamin C during incubation on hatchability of chickens and ducks[J]. Folia Biologica, 2012, 60(1/2): 93-97. |
| [26] |
UNI Z, YADGARY L, YAIR R. Nutritional limitations during poultry embryonic development[J]. Journal of Applied Poultry Research, 2012, 21(1): 175-184. |
| [27] |
ZHANG L, ZHU X D, WANG X F, et al. Individual and combined effects of in-ovo injection of creatine monohydrate and glucose on somatic characteristics, energy status, and posthatch performance of broiler embryos and hatchlings[J]. Poultry Science, 2016, 95(10): 2352-2359. DOI:10.3382/ps/pew130 |
| [28] |
FOYE O T, UNI Z, MCMURTRY J P, et al. The effects of amniotic nutrient administration, "in-ovo feeding" of arginine and/or β-hydroxy-β-methyl butyrate (HMB) on insulin-like growth factors, energy metabolism and growth in turkey poults[J]. International Journal of Poultry Science, 2006, 5(4): 309-317. DOI:10.3923/ijps.2006.309.317 |
| [29] |
SHAFEY T M, AL-BATSHAN H A, AL-OWAIMER A N, et al. Effects of in ovo administration of L-carnitine on hatchability performance, glycogen status and insulin-like growth factor-1 of broiler chickens[J]. British Poultry Science, 2010, 51(1): 122-131. |
| [30] |
董信阳.鸽早期小肠发育及碳水化合物对其调控的研究[D].博士学位论文.杭州: 浙江大学, 2013: 32-43.
|
| [31] |
YADGARY L, CAHANER A, KEDAR O, et al. Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens[J]. Poultry Science, 2010, 89(11): 2441-2452. |
| [32] |
LI H, GILBERT E R, ZHANG Y, et al. Expression profiling of the solute carrier gene family in chicken intestine from the late embryonic to early post-hatch stages[J]. Animal Genetics, 2008, 39(4): 407-424. |
| [33] |
UNI Z, GEYRA A, BEN-HUR H, et al. Small intestinal development in the young chick:crypt formation and enterocyte proliferation and migration[J]. British Poultry Science, 2000, 41(5): 544-551. |
| [34] |
SMIRNOV A, TAKO E, FERKET P R, et al. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates[J]. Poultry Science, 2006, 85(4): 669-673. |
| [35] |
VAEZI G, TESHFAM M, BAHADORAN S, et al. Effects of different levels of lysine on small intestinal villous morphology in starter diet of broiler chickens[J]. Global Veterinaria, 2011, 7(6): 523-526. |
| [36] |
BARTELL S M, BATAL A B. The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract, and humoral immune response of broilers[J]. Poultry Science, 2007, 86(9): 1940-1947. |
| [37] |
DE OLIVEIRA J, UNI Z, FERKET P. Important metabolic pathways in poultry embryos prior to hatch[J]. World's Poultry Science Journal, 2008, 64(4): 488-499. |
| [38] |
WU G Y, BAZER F W, DAVIS T A, et al. Important roles for the arginine family of amino acids in swine nutrition and production[J]. Livestock Science, 2007, 112(1/2): 8-22. |
| [39] |
SALMANZADEH M, EBRAHIMNEZHAD Y, SHAHRYAR H A, et al. The effects of in ovo feeding of glutamine in broiler breeder eggs on hatchability, development of the gastrointestinal tract, growth performance and carcass characteristics of broiler chickens[J]. Archives Animal Breeding, 2016, 59(2): 235-242. |
| [40] |
SALMANZADEH M, SHAHRYAR H A, LOTFI A. Effect of in ovo feeding of butyric acid on hatchability, performance and small intestinal morphology of turkey poults[J]. Kafkas Vniversitesi Veteriner Fakültesi Dergisi, 2015, 21(1): 19-25. |
| [41] |
KERMANSHAHI H, GOLIAN A, EMAMI N K, et al. Effects of in ovo injection of threonine on hatchability, intestinal morphology, and somatic attributes in Japanese quail (Coturnix japonica)[J]. Journal of Applied Animal Research, 2017, 45(1): 437-441. |
| [42] |
SHAFEY T M, ALODAN M A, ALRUQAIE I M, et al. In ovo feeding of carbohydrates and incubated at a high incubation temperature on hatchability and glycogen status of chicks[J]. South African Journal of Animal Science, 2012, 42(3): 210-220. |
| [43] |
OHTA Y, KIDD M, ISHIBASHI T. Embryo growth and amino acid concentration profiles of broiler breeder eggs, embryos, and chicks after in ovo administration of amino acids[J]. Poultry Science, 2001, 80(10): 1430-1436. |




