2. 中国科学院大学, 北京 100008;
3. 湖南农业大学动物科技学院, 长沙 410128
2. University of Chinese Academy of Sciences, Beijing 100008, China;
3. College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, China
畜禽生产过程中的各种应激因素严重影响了机体的健康。在应激状态下,畜禽体内会产生过量活性氧(reactive oxygen species, ROS),导致抗氧化系统紊乱,造成氧化应激,从而降低畜禽的抗病能力和饲料转化效率,最终导致生产性能降低。ROS包括超氧化物(O2-)、过氧化氢(H2O2)、羟自由基(·OH)和烷氧基自由基(RO·)等物质,是正常生理状态下线粒体氧化磷酸化和还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶作用下的产物,可以参与杀菌、抗炎及多种代谢途径的调节。但当机体处于多种应激原刺激或病原菌感染时,机体产生的ROS水平高于抗氧化系统清除正常ROS产生的能力,会诱发氧化应激,造成严重的脂质过氧化,损伤DNA,破坏细胞的完整性[1]。近年来大量研究表明,细胞自噬在缓解机体氧化应激反应中发挥重要作用。细胞自噬是依赖于溶酶体,通过清除胞内错误折叠的蛋白质、受损的细胞器及入侵的病原菌等来抵御各种应激,减缓细胞死亡;而当自噬缺陷时,细胞将更易受到氧化应激损伤[2-3]。
1 自噬的分类和发生过程 1.1 自噬的分类自噬是广泛存在于真核细胞中的重要生命现象,已被发现在细胞生长、发育、免疫防御以及缓解环境刺激等生理和病理过程中发挥重要的作用。按照待降解物质运送到溶酶体的方式不同,细胞自噬分为大自噬、小自噬和分子伴侣介导的自噬。大自噬是目前研究最多的,细胞内自噬相关蛋白(autophagy-related protein, Atg)通过将胞质中受损的蛋白质或细胞器包裹形成自噬体,与溶酶体结合,形成自噬溶酶体,溶酶体降解包裹的物质,降解后的一些小分子物质可再次被细胞所利用[4]。小自噬是指通过溶酶体膜自身凹陷包裹待降解物质的过程,而分子伴侣介导的自噬是指溶酶体选择性地降解特定氨基酸序列的物质。近年来随着研究深入,除了常见的几种自噬类型以外,还存在对底物降解具有专一性的选择性自噬的形式,包括线粒体自噬(mitophagy)、过氧化物酶体自噬(pexophagy)、聚集体自噬(aggrephagy)、内质网自噬(reticulophagy)和核糖体自噬(ribophagy)等。
1.2 自噬的发生过程在诱导因子的作用下,细胞自噬的发生过程一般包括自噬体的形成、自噬体与溶酶体的融合和底物的降解与回收等[4](图 1)。自噬诱导开始阶段,Atg1、Atg7和Atg13构成的丝氨酸/苏氨酸蛋白激酶——Unc-51样自噬激活激酶(Unc-51 like autophagy activating kinase, ULK)复合物参与调控,哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)信号通路起主要作用。受到外界刺激时,mTOR受到抑制,Atg13去磷酸化,与Atg1和Atg7解离,激活Atg1激酶并诱导自噬[5]。Atg14、空泡蛋白分选相关的蛋白(Vps)15、Vps34形成复合体,Atg21和Atg24蛋白结合到膜上,形成前自噬体结构[6]。吞噬膜泡扩大,包裹住底物形成自噬体。在吞噬泡膜延伸的过程中,泛素化蛋白结合系统Atg4-Atg12-Atg16和自噬微管相关蛋白1轻链3(autophagy microtubule-associated protein-1 light chain 3, LC3)协同作用,被Atg4同源体切割,暴露出LC3的氨基酸残基,产生位于胞浆的可溶性残片LC3-Ⅰ,LC3-Ⅰ与磷脂酰乙醇胺(phosphatidyl ethanolamine, PE)共价连接形成片段LC3-Ⅱ参与自噬泡膜延伸。当自噬体与溶酶体融合,LC3-Ⅱ即被降解,内囊泡被释放。自噬溶酶体膜裂解及其内容物在溶酶体水解酶作用下降解[4]。
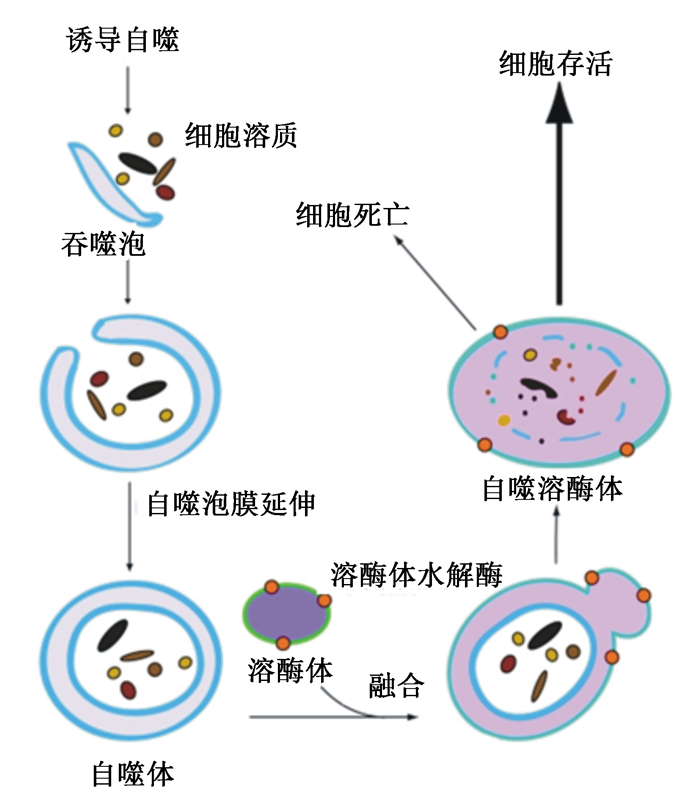
|
图 1 细胞自噬的基本过程及其在细胞存活与死亡中的作用 Fig. 1 The basic process of autophagy and its roles in cell survival and death[4] |
自噬的溶酶体降解途径在维持细胞、组织和器官稳态过程中扮演重要角色,受到进化保守的Atg调控。自噬会选择性地靶向功能失调的细胞器、细胞内的微生物和致病蛋白,但是这些过程中的缺陷可能导致疾病[7]。一部分选择性自噬的类型及其在生理和疾病中的可能作用见表 1[8-16]。
|
|
表 1 选择性自噬的类型及其在生理和疾病中的可能作用 Table 1 Types of selective autophagy and its possible roles in physiology and disease |
细胞自噬在所有真核细胞中都受到严密的调控,自噬缺陷或活性过高对机体都可能造成伤害。自噬的调控机制非常复杂,受体内多种信号途径调控,例如mTOR、腺苷酸活化的蛋白激酶(AMP-activated protein kinase, AMPK)、核因子-κB(nuclear factor kappa-B, NF-κB)和低氧诱导因子(hypoxia inducible factor, HIF)-1α通路等[6]。mTOR通路是调节细胞内蛋白质合成和降解、能量代谢、细胞增殖与凋亡以及自噬的核心信号通路[17]。mTORC1和mTORC2是mTOR的2种复合物形式,其中mTORC1对雷帕霉素敏感,接头蛋白是Raptor,与自噬的关系更为密切。mTORC1能够响应细胞外能量水平、氨基酸及应激等刺激因素。正常状态下,活化状态下的mTOR能够磷酸化下游的p70核糖体蛋白S6激酶1(p70 ribosomal S6 kinase 1, p70S6K1)和真核生物翻译起始因子4E结合蛋白1(eukaryotic translational initiation factor 4E-binding protein 1, 4E-BP1),促进转录合成蛋白质,并通过磷酸化ULK1和Atg13负向调控自噬[18]。与mTOR负调控自噬相比,AMPK对自噬有正调控作用[6]。当细胞内能量水平不足时,ATP/AMP值降低,AMPK通过磷酸化激活肿瘤结节状复合物2(tuberous sclerosis complex 2, TSC2)和Raptor,从而抑制脑中富含的Ras同系物(Ras homologue enriched in brain,Rheb)活化,进而抑制mTORC1的活性,启动自噬[17]。mTOR通路可以负调控NF-κB通路,自噬通路上的关键蛋白p62的表达依赖于NF-κB通路。NF-κB通路的激活会抑制由肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)诱导的自噬[19]。应对感染,HIF-1α的转录激活需要依赖于活化的NF-κB通路,mTOR也正调控HIF-1α的转录激活[20]。HIF可通过抑制B淋巴细胞瘤-2(B-cell lymphoma-2, Bcl-2)与Beclin-1的结合导致胞内游离的Beclin-1增多,从而激活自噬维持细胞稳态[6](图 2)。
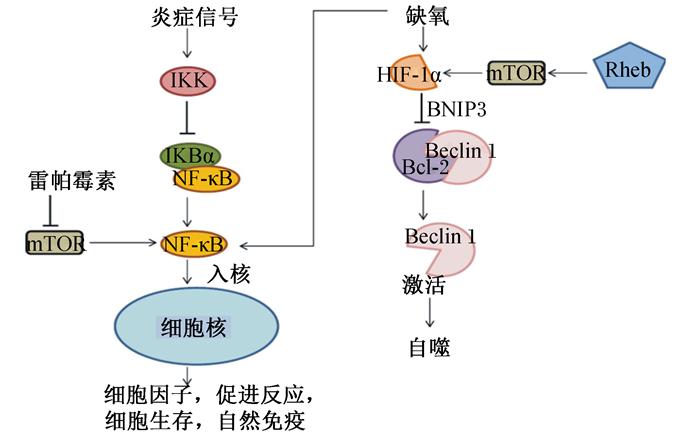
|
IKK:核因子-κB抑制物激酶inhibitor of nuclear factor-κB kinase;NF-κB:核因子-κB nuclear factor-κB;IKBα:核因子-κB抑制剂α inhibitor of nuclear factor-κB α;mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;Rheb:脑中富含的Ras同系物Ras homologue enriched in brain;HIF-1α:低氧诱导因子-1α hypoxia inducible factor-1α;BNIP3:B淋巴细胞瘤-2/腺病毒E1B 19 ku蛋白相互作用蛋白3 Bcl-2/adenovirus E1B 19 ku protein-interacting protein 3;Bcl-2:B淋巴细胞瘤-2 B-cell lymphoma-2。 图 2 缺氧、炎症和自噬的相互关系 Fig. 2 Interrelationship among hypoxia, inflammation and autophagy[6] |
ROS主要来源于线粒体电子传递链和酶促反应,还会由外界刺激(如TNF-α和脂多糖)诱导产生。自噬是细胞反应中氧化还原信号的一个主要传感器。氧化应激状态下,ROS能通过自噬形成过程中的各种通路诱导自噬的产生[21](图 3)。自噬相关蛋白Atg7、Atg3和Atg10在其催化位点上利用半胱氨酸残基催化泛素转移,半胱氨酸残基上的巯基特别容易受到ROS的氧化修饰,所以这些蛋白可能对氧化还原信号敏感。激酶和磷酸酶的活性可以通过氧化还原介导的翻译后修饰来改变,信号通路中间产物的氧化还原依赖修饰可能引起活性、定位或底物特异性的改变[22]。在饥饿状态下,细胞内H2O2增加,抑制Atg4蛋白酶活性,抑制LC3-Ⅱ的去脂化,促使自噬体的形成[23]。有研究表明,ROS可以通过破坏线粒体膜电位,抑制Akt-mTOR通路或B淋巴细胞瘤-2/腺病毒E1B 19 ku蛋白相互作用蛋白3(Bcl-2/adenovirus E1B 19 ku protein-interacting protein 3, BNIP3)-mTOR通路诱导自噬[21-22]。此外,在自噬诱导中,丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)已被发现是ROS的下游效应因子,ROS可以通过激活细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)和c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK)来诱导自噬[1]。在A357细胞中,甘露糖结合凝集素可通过线粒体依赖的ROS-p38-p53通路诱导自噬[24]。抑制p53的靶基因——TP53诱导的糖酵解和凋亡的调控子(TP53-induced glycolysis and apoptosis regulator, TIGAR)表达可以提高ROS水平,从而引发自噬[21]。还有研究报道,重金属镉在系膜细胞中通过ROS-糖原合成酶激酶-3β(glycogen synthase kinase 3β,GSK-3β)途径诱导自噬[25]。ROS还可以通过抑制磷酸酯酶与张力蛋白同源物(phosphatase and tensin homolog,PTEN),进而抑制蛋白激酶B(protein kinase B, PKB)-Akt通路激活自噬;通过影响钙离子通道提高胞内钙离子水平,激活自噬[2, 26]。
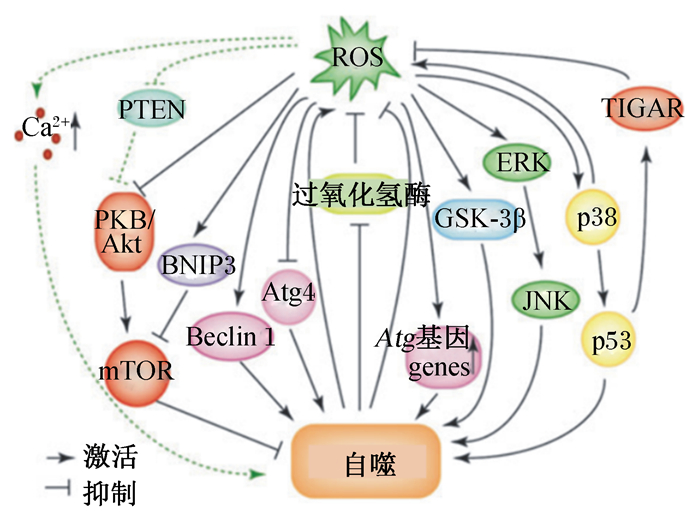
|
ROS:活性氧reactive oxygen species;PTEN:磷酸酯酶与张力蛋白同源物phosphatase and tensin homolog;PKB:蛋白激酶B protein kinase B;BNIP3:B淋巴细胞瘤-2/腺病毒E1B 19 ku蛋白相互作用蛋白3 Bcl-2/adenovirus E1B 19 ku protein-interacting protein 3;mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;Atg4:自噬相关蛋白4 autophagy-related protein 4;GSK-3β:糖原合成酶激酶-3β glycogen synthase kinase 3β;ERK:细胞外信号调节激酶extracellular signal-regulated kinase;JNK:c-Jun氨基末端激酶c-Jun N-terminal kinase;TIGAR:TP53诱导的糖酵解和凋亡的调控子TP53-induced glycolysis and apoptosis regulator。 图 3 ROS诱导自噬的机制 Fig. 3 Mechanisms of autophagy induced by ROS[21] |
线粒体是真核细胞进行能量转化的主要场所,线粒体氧化呼吸过程中会产生适量ROS,但是线粒体的损伤则会导致过多ROS的积累,造成氧化应激,引起细胞凋亡。自噬则可以适时清除老化或损伤的线粒体,有利于细胞正常生长[27]。线粒体自噬可通过2种途径缓解线粒体氧化应激:一是当线粒体膜电位降低时,线粒体膜蛋白PTEN诱导激酶1 (PTEN-induced kinase 1, PINK1)招募具有E3泛素化连接酶活性的帕金森蛋白Parkin聚集到功能受损的线粒体上,随后泛素化标记后的线粒体被自噬性降解;二是应对缺氧或线粒体氧化应激,哺乳动物细胞的线粒体自噬受体NIP3样蛋白X(NIP3-like protein X,NIX)/BNIP3L、BNIP3和FUN14结构域包含蛋白1(FUNDC1)通过转录和翻译后修饰激活自噬[28-30]。
ROS是造成DNA损伤的主要原因之一,当ROS损伤DNA时,感应蛋白可以传导信号激活DNA损伤修复,同时信号也会传导到自噬信号通路[31]。介导自噬参与DNA损伤修复的2种蛋白是:聚腺苷酸二磷酸核糖转移酶-1(polyADP-ribose polymerase 1, PARP1)和共济失调毛细血管扩张症突变蛋白(ataxia-telangiectasia mutated protein, ATM)[32-33]。有研究表明,自噬可以通过维持DNA损伤修复的能量需要来延缓细胞凋亡。自噬基因缺失(如Beclin 1、Atg5等)会引起DNA损伤的积累并促进肿瘤的发生[7]。PARP1在ROS诱导的DNA损伤时被过度激活,消耗烟酰胺腺嘌呤二核苷酸(NAD+)和ATP,这种能量失衡状态通过激活AMPK通路诱导自噬,降解利用ATP的代谢前体,并为DNA损伤修复提供能量[32]。细胞质中的ATM通过肝激酶B1(liver kinase B1,LKB1)/AMPK通路,激活结节性硬化症复合物2(tuberous sclerosis complex 2,TSC2),抑制mTOR通路诱导自噬[33]。此外,p53作为调控DNA损伤修复的重要蛋白,可通过靶向自噬的上游调控因子(如PTEN、TSC2等)以及自噬体形成相关蛋白(如ULK1、Atg2等)的表达来诱导自噬[21]。
5 自噬调节炎症反应许多炎症性疾病都与过高的ROS水平有关,导致氧化应激。有很多研究表明氧化应激在炎症中起着重要的致病作用[34]。而自噬在适应性免疫、无菌和感染相关的炎症、细胞因子和天然免疫细胞过程中,可以清除破坏细胞的无菌刺激物或入侵的病原体,从而缓解因炎症导致的氧化应激[35](图 4)。
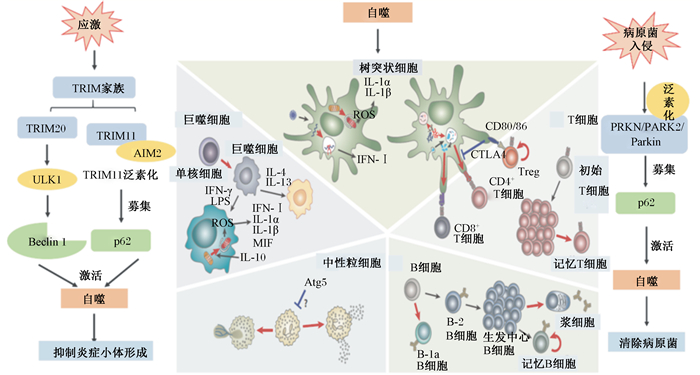
|
TRIM:三结构域蛋白tripartite motif;AIM2:黑色素瘤缺乏因子2 absent in melanoma 2;ULK1:Unc-51样自噬激活激酶1 Unc-51 like autophagy activating kinase 1;CTLA4:细胞毒性T淋巴细胞相关蛋白4 cytotoxic T-lymphocyte-associated protein 4;Treg:调节性T细胞regulatory T cells;ROS:活性氧reactive oxygen species;IFN:干扰素interferon;IL:白细胞介素interleukin;LPS:脂多糖lipopolysaccharide;MIF:巨噬细胞迁移抑制因子macrophage migration inhibitory factor;Atg5:自噬相关蛋白autophagy-related protein 5。 图 4 自噬调节免疫反应的机制 Fig. 4 Mechanisms of immune responses regulated by autophagy[40] |
炎症小体是当机体受到病原菌和内源性激动剂刺激时的胞浆应答的产物,一旦被激活,会促使蛋白裂解过程中关键的促炎细胞因子,包括白细胞介素(IL)-1β的产生。很多研究已表明,自噬可以通过抑制炎症小体的形成来缓解炎症。缺乏自噬相关蛋白Atg16L1的小鼠表现出炎症小体的激活,关键促炎细胞因子IL-1β和IL-18的显著升高[36]。线粒体自噬可以通过抑制去极化线粒体的累积,减少内源性炎症小体激动剂,如ROS和氧化线粒体DNA的产生[21]。缺乏选择性自噬基因——范可尼贫血补体C组蛋白基因(Fanconi anaemia complementation group C,FANCC)的骨髓源性巨噬细胞的小鼠,会积累受损的线粒体,增加线粒体ROS依赖性炎症小体的激活[37]。三结构域蛋白(tripartite motif,TRIM)家族中的自噬受体可以直接调控炎症小体的自噬降解过程。例如TRIM20蛋白可以靶向核苷酸结合寡聚化结构域样受体含pyrin结构域蛋白(nucleotide-binding and oligomerization domain-like receptor family pyrin domain containing,NLRP)1和NLRP3启动自噬降解[38]。TRIM11是典型自噬小体黑色素瘤缺乏因子2(absent in melanoma 2,AIM2)的受体,AIM2与TRIM11的PRY-SPRY结构域结合,泛素化的TRIM11募集p62蛋白通过p62依赖的选择性自噬清除AIM2[39]。
5.2 调控免疫细胞分化自噬蛋白在巨噬细胞中起着调节吞噬物质命运的作用,自噬可以使单核细胞向巨噬细胞进行有效分化,维持细胞增殖和代谢稳态[40]。缺乏Atg7的巨噬细胞倾向于进行糖酵解,并且衰老细胞的吞噬能力降低和炎性细胞因子增加[41]。自噬缺陷的巨噬细胞的代谢紊乱伴随着促炎细胞因子干扰素-γ(IFN-γ)和巨噬细胞迁移抑制因子(macrophage migration inhibitory factor, MIF)等的分泌[42-43]。
自噬蛋白在树突状细胞(dendritic cells, DCs)中通过主要组织相容性复合体Ⅱ(major histocompatibility complex Ⅱ,MHC-Ⅱ)递呈抗原的过程,调控适应性免疫。当机体被病菌感染时,DCs中Atg5和Atg7是CD4+ T细胞增殖和IFN-γ生产的关键[44]。在Toll样受体4(Toll like receptor 4,TLR4)和核苷酸结合寡聚化结构域2(nucleotide-binding and oligomerization domain 2,NOD2)下游的自噬诱导下,DCs刺激T细胞的能力进一步增强[45],而在氧化应激状态下,自噬缺陷增加DCs中IL-1α和IL-1β的产生,从而介导产生IL-17的T细胞亚群的分化[46]。自噬可以在活化的中性粒细胞中诱导产生,会促进中性粒细胞细胞外陷阱的形成,增强捕获和杀死微生物的能力[47-48]。
自噬通过维持线粒体稳态和防止细胞ROS的产生来维持自然杀伤T细胞、记忆T细胞和固有淋巴样细胞存活[49-51]。在T细胞受体参与下,泛素编辑酶A20通过抑制和去泛素化mTOR复合物活性诱导自噬[52]。另外,自噬通过选择性降解细胞周期的主要负调节因子细胞周期蛋白依赖性激酶抑制剂1B(cyclin dependent kinase inhibitor 1B,CDKN1B)来促进CD8+ T细胞的增殖,而自噬缺陷则会在应对病毒侵入时,抑制CD8+ T细胞的形成[40, 53]。自噬是维持天然免疫B-1a B细胞所必需的,但通常在稳态条件下对于更常见的B-2 B细胞中非必需[40]。自噬对抗体分泌的浆细胞的分化和存活至关重要。在流感感染期间,Atg7缺失会导致线粒体ROS的积累和二次抗体反应所需的记忆B细胞的长期缺失[54]。
5.3 抵抗病原菌感染自噬能够将病原体相关分子模式递呈细胞,杀死病原菌[35]。线粒体自噬可通过泛素化E3连接酶PRKN/PARK2/Parkin和p62受体自噬性清除结核分枝杆菌侵入导致的受损溶酶体或线粒体[55-56]。在线粒体自噬中,细胞器通过让内部的线粒体自噬受体PHB2与LC3-Ⅱ结合,靶向线粒体进行自噬清除[57]。自噬缺陷小鼠无法有效抑制鼠伤寒沙门氏菌的扩散,增加鼠伤寒沙门氏菌的感染[58]。
6 营养物质调控细胞自噬氨基酸被很多研究者证实是调节自噬的主要调控物质[17]。细胞溶质内的精氨酸和亮氨酸可通过胞内重要的Rag-GTPases复合物来提高mTORC1活性:激活的Rag-GTPases复合物促使mTORC1与溶酶体相连,并募集大量Rheb蛋白激活mTORC1,导致ULK1活性复合物解离,自噬溶酶体形成受阻,抑制自噬[18],其中,GATOR1/2是重要的氨基酸感应器,可调控Rag活性。细胞内亮氨酸的感应器是Sestrin2,而精氨酸的感应器包括CASTOR1和溶酶体氨基酸感应器溶质载体家族38成员9(solute carrier family 38 member 9,SLC38A9)[17]。氨基酸代谢产物也可以通过SAMTOR来调控mTORC1通路:当蛋氨酸或S-腺苷蛋氨酸缺乏时,SAMTOR通过集合GATOR1和KICSTOR抑制mTORC1[59]。
能量水平可直接通过调控mTORC1活性启动或抑制自噬,葡萄糖是细胞基本的能量来源。当葡萄糖缺乏时,AMPK被激活,mTORC1被抑制,启动自噬。脂质与自噬的调控关系目前也有研究。体内或体外脂质含量升高可以抑制自噬溶酶体融合;而棕榈酸在内的游离脂肪酸通过非依赖性mTORC1激活蛋白激酶C促进自噬[60-61]。
此外,有研究发现植物提取物如黄酮类化合物、槲皮素、甘氨香豆素、姜黄素等也可通过抑制磷脂酰肌醇-3-羟激酶(phosphatidylinositol 3-hydroxy kinase, PI3K)-Akt-mTOR通路增加自噬水平来缓解机体炎症反应[62-65]。芹黄素和荭草素可分别通过调控AMPK和Akt-mTOR信号通路激活自噬,降低细胞凋亡水平[66-67]。二十二碳酸可通过提高核因子E2相关因子2(nuclear factor E2-related factor 2, Nrf2)、p62等蛋白表达诱导自噬[68]。一些益生菌如枯草芽孢杆菌可以通过提高p38 MAPK依赖的自噬缓解大鼠因氧化应激导致的肠道损伤[69]。抗氧化物如维生素D、白藜芦醇、虾青素等大多能通过调节自噬信号通路和自噬基因的表达诱导自噬产生,缓解氧化应激,减少细胞凋亡[70-72]。因此,系统地研究不同营养物质对自噬的调控作用,可为其在畜牧生产过程中缓解氧化应激提供新的研究方向。
7 小结氧化应激是造成畜禽机体损伤发生和发展过程中的关键因素,维持细胞氧化和抗氧化的平衡对缓解氧化应激非常重要。近年来研究人员不断探索从营养学角度来缓解氧化应激的调节机制。自噬的研究进展也为氧化应激的研究提供了新的思路,氧化应激可以激活自噬,而自噬可以通过清除ROS造成的细胞损伤,降低炎症反应,维持细胞内环境稳态。如何通过添加外源营养物质调控自噬来缓解氧化应激,将为缓解畜禽机体氧化还原状态紊乱、促进损伤修复、发挥生长潜力提供营养调控手段。
| [1] |
SÁNCHEZ-ÁLVAREZ M, STRIPPOLI R, DONADELLI M, et al. Sestrins as a therapeutic bridge between ROS and autophagy in cancer[J]. Cancers, 2019, 11(10): E1415. DOI:10.3390/cancers11101415 |
| [2] |
WANG T, WANG Q W, SONG R L, et al. Autophagy plays a cytoprotective role during cadmium-induced oxidative damage in primary neuronal cultures[J]. Biological Trace Element Research, 2015, 168(2): 481-489. DOI:10.1007/s12011-015-0390-8 |
| [3] |
TANG Y L, LI J J, LI F N, et al. Autophagy protects intestinal epithelial cells against deoxynivalenol toxicity by alleviating oxidative stress via IKK signaling pathway[J]. Free Radical Biology and Medicine, 2015, 89: 944-951. DOI:10.1016/j.freeradbiomed.2015.09.012 |
| [4] |
CHEN Y Q, KLIONSKY D. The regulation of autophagy-unanswered questions[J]. Journal of Cell Science, 2011, 124(2): 161-170. |
| [5] |
HOSOKAWA N, HARA T, KAIZUKA T, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy[J]. Molecular Biology of the Cell, 2009, 20(7): 1981-1991. DOI:10.1091/mbc.e08-12-1248 |
| [6] |
RAVANAN P, SRIKUMAR I F, TALWAR P. Autophagy:the spotlight for cellular stress responses[J]. Life Science, 2017, 188: 53-67. DOI:10.1016/j.lfs.2017.08.029 |
| [7] |
LEVINE B, KROEMER G. Biological functions of autophagy genes:a disease perspective[J]. Cell, 2019, 176(1/2): 11-42. |
| [8] |
GATICA D, LAHIRI V, KLIONSKY D. Cargo recognition and degradation by selective autophagy[J]. Nature Cell Biology, 2018, 20(3): 233-242. DOI:10.1038/s41556-018-0037-z |
| [9] |
DRAKE L E, SPRINGER M Z, POOLE L P, et al. Expanding perspectives on the significance of mitophagy in cancer[J]. Seminars in Cancer Biology, 2017, 47: 110-124. DOI:10.1016/j.semcancer.2017.04.008 |
| [10] |
WYANT G, ABU-REMAILEH M, FRENKEL E, et al. NUFIP1 is a ribosome receptor for starvation-induced ribophagy[J]. Science, 2018, 360(6390): 751-758. DOI:10.1126/science.aar2663 |
| [11] |
CHAUHAN S, KUMAR S, JAIN A, et al. TRIMs and galectins globally cooperate and TRIM16 and galectin-3 co-direct autophagy in endomembrane damage homeostasis[J]. Developmental Cell, 2016, 39(1): 13-27. DOI:10.1016/j.devcel.2016.08.003 |
| [12] |
YOSHIDA Y, YASUDA S, FUJITA T, et al. Ubiquitination of exposed glycoproteins by SCFFBXO27 directs damaged lysosomes for autophagy[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(32): 8574-8579. DOI:10.1073/pnas.1702615114 |
| [13] |
CIPOLLA C M, LODHI I J. Peroxisomal dysfunction in age-related diseases[J]. Trends in Endocrinology and Metabolism, 2017, 28(4): 297-308. DOI:10.1016/j.tem.2016.12.003 |
| [14] |
ZECHNER R, MADEO F, KRATKY D. Cytosolic lipolysis and lipophagy:two sides of the same coin[J]. Nature Reviews Molecular Cell Biology, 2017, 18(11): 671-684. DOI:10.1038/nrm.2017.76 |
| [15] |
FRANCO L H, NAIR V R, SCHARN C R, et al. The ubiquitin ligase SMURF1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense[J]. Cell Host and Microbe, 2017, 21(1): 59-72. DOI:10.1016/j.chom.2016.11.002 |
| [16] |
MITCHELL G, ISBERG R R. Innate immunity to intracellular pathogens:balancing microbial elimination and inflammation[J]. Cell Host and Microbe, 2017, 22(2): 166-175. DOI:10.1016/j.chom.2017.07.005 |
| [17] |
LIU G, SABATINI D M. mTOR at the nexus of nutrition, growth, ageing and disease[J]. Nature Reviews Molecular Cell Biology, 2020. DOI:10.1038/s41580-019-0199-y |
| [18] |
ZACHARI M, GANLEY I G. The mammalian ULK1 complex and autophagy initiation[J]. Essays in Biochemistry, 2017, 61(6): 585-596. DOI:10.1042/EBC20170021 |
| [19] |
LAND S C, TEE A R. Hypoxia-inducible factor 1α is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif[J]. The Journal of Biological Chemistry, 2007, 282(28): 20534-20543. DOI:10.1074/jbc.M611782200 |
| [20] |
GÖRLACH A, BONELLO S. The cross-talk between NF-κB and HIF-1:further evidence for a significant liaison[J]. Biochemical Journal, 2008, 412(3): e17-e19. |
| [21] |
HUANG J, LAM G Y, BRUMELL J H. Autophagy signaling through reactive oxygen species[J]. Antioxidants & Redox Signaling, 2011, 14(11): 2215-2231. |
| [22] |
SU Z D, BURCHFIELD J G, YANG P Y, et al. Global redox proteome and phosphoproteome analysis reveals redox switch in Akt[J]. Nature Communication, 2019, 10: 5486. DOI:10.1038/s41467-019-13114-4 |
| [23] |
SCHERZ-SHOUVAL R, SHVETS E, FASS E, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4[J]. EMBO Journal, 2007, 26(7): 1749-1760. DOI:10.1038/sj.emboj.7601623 |
| [24] |
LIU B, CHENG Y, ZHANG B, et al. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS-p38-p53 pathway[J]. Cancer Letters, 2009, 275(1): 54-60. DOI:10.1016/j.canlet.2008.09.042 |
| [25] |
WANG S H, SHIH Y, KUO T C, et al. Cadmium toxicity toward autophagy through ROS-activated GSK-3β in mesangial cells[J]. Toxicological Sciences, 2009, 108(1): 124-131. DOI:10.1093/toxsci/kfn266 |
| [26] |
CHO S H, LEE C H, AHN Y, et al. Redox regulation of PTEN and protein tyrosine phosphatases in H2O2-mediated cell signaling[J]. FEBS Letters, 2004, 560(1/2/3): 7-13. DOI:10.1016/S0014-5793(04)00112-7 |
| [27] |
COOKE M S, EVANS M D, DIZDAROGLU M, et al. Oxidative DNA damage:mechanisms, mutation, and disease[J]. The FASEB Journal, 2003, 17(10): 1195-1214. DOI:10.1096/fj.02-0752rev |
| [28] |
NARENDRA D, TANAKA A, SUEN D F, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy[J]. The Journal of Cell Biology, 2008, 183(5): 795-803. DOI:10.1083/jcb.200809125 |
| [29] |
WU H, CHEN Q. Hypoxia activation of mitophagy and its role in disease pathogenesis[J]. Antioxidants and Redox Signaling, 2015, 22(12): 1032-1046. DOI:10.1089/ars.2014.6204 |
| [30] |
ROOS W P, KAINA B. DNA damage-induced cell death:from specific DNA lesions to the DNA damage response and apoptosis[J]. Cancer Letters, 2013, 332(2): 237-248. DOI:10.1016/j.canlet.2012.01.007 |
| [31] |
DOTIWALA F, EAPEN V V, HARRISON J C, et al. DNA damage checkpoint triggers autophagy to regulate the initiation of anaphase[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(1): E41-E49. DOI:10.1073/pnas.1218065109 |
| [32] |
RODRÍGUEZ-VARGAS J, RUIZ-MAGAÑA M, RUIZ-RUIZ C, et al. ROS-induced DNA damage and PARP-1 are required for optimal induction of starvation-induced autophagy[J]. Cell Research, 2012, 22(7): 1181-1198. DOI:10.1038/cr.2012.70 |
| [33] |
ALEXANDER A, CAI S L, KIM J, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(9): 4153-4158. DOI:10.1073/pnas.0913860107 |
| [34] |
CHEN T W, LUO W, WU G J, et al. A novel MyD88 inhibitor LM9 prevents atherosclerosis by regulating inflammatory responses and oxidative stress in macrophages[J]. Toxicology and Applied Pharmacology, 2019, 370: 44-55. DOI:10.1016/j.taap.2019.03.012 |
| [35] |
DERETIC V, LEVINE B. Autophagy balances inflammation in innate immunity[J]. Autophagy, 2018, 14(2): 243-251. DOI:10.1080/15548627.2017.1402992 |
| [36] |
SAITOH T, FUJITA N, JANG M H, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production[J]. Nature, 2008, 456(7219): 264-268. DOI:10.1038/nature07383 |
| [37] |
SUMPTER R, J r, SIRASANAGANDLA S, FERNÁNDEZ Á F, et al. Fanconi anemia proteins function in mitophagy and immunity[J]. Cell, 2016, 165(4): 867-881. DOI:10.1016/j.cell.2016.04.006 |
| [38] |
KIMURA T, JAIN A, CHOI S W, et al. TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity[J]. The Journal of Cell Biology, 2015, 210(6): 973-989. DOI:10.1083/jcb.201503023 |
| [39] |
LIU T, TANG Q, LIU K P, et al. TRIM11 suppresses AIM2 inflammasome by degrading AIM2 via p62-dependent selective autophagy[J]. Cell Reports, 2016, 16(7): 1988-2002. DOI:10.1016/j.celrep.2016.07.019 |
| [40] |
MATSUZAWA-ISHIMOTO Y, HWANG S, CADWELL K. Autophagy and inflammation[J]. Annual Review of Immunology, 2018, 36: 73-101. DOI:10.1146/annurev-immunol-042617-053253 |
| [41] |
STRANKS A J, HANSEN A L, PANSE I, et al. Autophagy controls acquisition of aging features in macrophages[J]. Journal of Innate Immunity, 2015, 7(4): 375-391. DOI:10.1159/000370112 |
| [42] |
LEE J P W, FOOTE A, FAN H P, et al. Loss of autophagy enhances MIF/macrophage migration inhibitory factor release by macrophages[J]. Autophagy, 2016, 12(6): 907-916. DOI:10.1080/15548627.2016.1164358 |
| [43] |
TAL M C, SASAI M, LEE H K, et al. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(8): 2770-2775. DOI:10.1073/pnas.0807694106 |
| [44] |
RAVINDRAN R, KHAN N, NAKAYA H I, et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation[J]. Science, 2014, 343(6168): 313-317. DOI:10.1126/science.1246829 |
| [45] |
KHAN N, VIDYARTHI A, PAHARI S, et al. Signaling through NOD-2 and TLR-4 bolsters the T cell priming capability of dendritic cells by inducing autophagy[J]. Scientific Reports, 2016, 6: 19084. DOI:10.1038/srep19084 |
| [46] |
CASTILLO E F, DEKONENKO A, ARKO-MENSAH J, et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(46): E3168-E3176. DOI:10.1073/pnas.1210500109 |
| [47] |
MITROULIS I, KOURTZELIS I, KAMBAS K, et al. Regulation of the autophagic machinery in human neutrophils[J]. European Journal of Immunology, 2010, 40(5): 1461-1472. DOI:10.1002/eji.200940025 |
| [48] |
REMIJSEN Q, BERGHE T V, WIRAWAN E, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation[J]. Cell Research, 2011, 21(2): 290-304. DOI:10.1038/cr.2010.150 |
| [49] |
PEI B, ZHAO M, MILLER B C, et al. Invariant NKT cells require autophagy to coordinate proliferation and survival signals during differentiation[J]. The Journal of Immunology, 2015, 194(12): 5872-5884. DOI:10.4049/jimmunol.1402154 |
| [50] |
O'SULLIVAN T E, JOHNSON L R, KANG H H, et al. BNIP3- and BNIP3L-mediated mitophagy promotes the generation of natural killer cell memory[J]. Immunity, 2015, 43(2): 331-342. DOI:10.1016/j.immuni.2015.07.012 |
| [51] |
O'SULLIVAN T E, GEARY C D, WEIZMAN O E, et al. Atg5 is essential for the development and survival of innate lymphocytes[J]. Cell Reports, 2016, 15(9): 1910-1919. DOI:10.1016/j.celrep.2016.04.082 |
| [52] |
MATSUZAWA Y, OSHIMA S, TAKAHARA M, et al. TNFAIP3 promotes survival of CD4 T cells by restricting MTOR and promoting autophagy[J]. Autophagy, 2015, 11(7): 1052-1062. DOI:10.1080/15548627.2015.1055439 |
| [53] |
JIA W, HE M X, MCLEOD I X, et al. Autophagy regulates T lymphocyte proliferation through selective degradation of the cell-cycle inhibitor CDKN1B/p27Kip1[J]. Autophagy, 2015, 11(12): 2335-2345. DOI:10.1080/15548627.2015.1110666 |
| [54] |
CHEN M, HONG M J, SUN H H, et al. Essential role for autophagy in the maintenance of immunological memory against influenza infection[J]. Nature Medicine, 2014, 20(5): 503-510. DOI:10.1038/nm.3521 |
| [55] |
PAPADOPOULOS C, KIRCHNER P, BUG M, et al. VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy[J]. EMBO Journal, 2017, 36(2): 135-150. DOI:10.15252/embj.201695148 |
| [56] |
YOSHII S R, KISHI C, ISHIHARA N, et al. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane[J]. The Journal of Biological Chemistry, 2011, 286(22): 19630-19640. DOI:10.1074/jbc.M110.209338 |
| [57] |
WEI Y J, CHIANG W C, SUMPTER R, et al. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor[J]. Cell, 2017, 168(1/2): 224-238. |
| [58] |
ZHOU H L, WERTZ I, O'ROURKE K, et al. Bcl10 activates the NF-κB pathway through ubiquitination of NEMO[J]. Nature, 2003, 427(6970): 167-171. |
| [59] |
GU X, OROZCO J M, SAXTON R A, et al. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway[J]. Science, 2017, 358(6364): 813-818. DOI:10.1126/science.aao3265 |
| [60] |
TAN S H, SHUI G, ZHOU J, et al. Induction of autophagy by palmitic acid via protein kinase C-mediated signaling pathway independent of mTOR (mammalian target of rapamycin)[J]. The Journal of Biological Chemistry, 2012, 287(18): 14364-14376. DOI:10.1074/jbc.M111.294157 |
| [61] |
KOGA H, KAUSHIK S, CUERVO A M. Altered lipid content inhibits autophagic vesicular fusion[J]. The FASEB Journal, 2010, 24(8): 3052-3065. DOI:10.1096/fj.09-144519 |
| [62] |
CHU Q, YU X, JIA R Y, et al. Flavonoids from Apios americana Medikus leaves protect RAW264.7 cells against inflammation via inhibition of MAPKs, Akt-mTOR pathways, and Nfr2 activation[J]. Oxidative Medicine and Cellular Longevity, 2019, 2019: 1563024. |
| [63] |
ASHRAFIZADEH M, AHMADI Z, FARKHONDEH T, et al. Autophagy as a molecular target of quercetin underlying its protective effects in human diseases[J]. Archives of Physiology and Biochemistry, 2019, 28: 1-9. |
| [64] |
ZHANG E X, YIN S T, ZHAO S, et al. Protective effects of glycycoumarin on liver diseases[J]. Phytotherapy Research, 2019, 1-7. DOI:10.1002/ptr.6598 |
| [65] |
XUAN H Z, OU A Q, HAO S Y, et al. Galangin protects against symptoms of dextran sodium sulfate-induced acute colitis by activating autophagy and modulating the gut microbiota[J]. Nutrients, 2020, 12(2): 347. DOI:10.3390/nu12020347 |
| [66] |
BRIDGEMAN B B, WANG P, YE B P, et al. Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes:a new implication of skin cancer prevention[J]. Cellular Signalling, 2016, 28(5): 460-468. DOI:10.1016/j.cellsig.2016.02.008 |
| [67] |
LIU L Y, WU Y X, HUANG X L. Orientin protects myocardial cells against hypoxia-reoxygenation injury through induction of autophagy[J]. European Journal of Pharmacology, 2016, 776: 90-98. DOI:10.1016/j.ejphar.2016.02.037 |
| [68] |
JOHANSON I, MONSEN V T, PETTERSEN K, et al. The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells[J]. Autophagy, 2015, 11(9): 1636-1651. DOI:10.1080/15548627.2015.1061170 |
| [69] |
WU Y P, WANG B K, XU H, et al. Probiotic Bacillus attenuates oxidative stress-induced intestinal injury via p38-mediated autophagy[J]. Frontiers in Microbiology, 2019, 10: 2185. DOI:10.3389/fmicb.2019.02185 |
| [70] |
YANG J H, CHEN Q, TIAN S Y, et al. The role of 1, 25-dyhydroxyvitamin D3 in mouse liver ischemia reperfusion injury:regulation of autophagy through activation of MEK/ERK signaling and PTEN/PI3K/Akt/mTORC1 signaling[J]. American Journal of Translational Research, 2015, 7(12): 2630-2645. |
| [71] |
SEBORI R, KUNO A, HOSODA R, et al. Resveratrol decreases oxidative stress by restoring mitophagy and improves the pathophysiology of dystrophin-deficient mdx mice[J]. Oxidative Medicine and Cellular Longevity, 2018, 2018: 9179270. DOI:10.1155/2018/9179270 |
| [72] |
KIM S H, KIM H. Astaxanthin modulation of signaling pathways that regulate autophagy[J]. Marine Drugs, 2019, 17(10): 546. DOI:10.3390/md17100546 |




