2. 中国农业科学院北京畜牧兽医研究所, 农业农村部奶产品质量安全风险评估实验室, 北京 100193;
3. 中国农业科学院北京畜牧兽医研究所, 农业农村部奶及奶制品质量监督检验测试中心, 北京 100193;
4. 中国农业科学院北京畜牧兽医研究所, 动物营养学国家重点实验室, 北京 100193
2. Laboratory of Quality and Safety Risk Assessment for Dairy Products of Ministry of Agriculture and Rural Affairs, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China;
3. Milk and Milk Products Inspection Center of Ministry of Agriculture and Rural Affairs, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China;
4. State Key Laboratory of Animal Nutrition, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193, China
霉菌毒素是由曲霉菌、青霉菌和镰刀菌属的丝状真菌产生的有毒代谢产物,通常存在于谷物和新鲜蔬菜中。据估计,全世界1/4的粮食会受到霉菌毒素的污染[1]。由于全球范围内大量农产品受到污染,且有研究显示霉菌毒素在食品和饲料中的含量仍在不断上升,因此食物摄入被认为是人类暴露于霉菌毒素的主要途径,由此所引发的健康问题不容小觑,日益引起人们的关注[2-3]。根据毒理学数据显示,玉米赤霉烯酮(zenralenone, ZEN)、伏马菌素(fumonisin, FBs)、黄曲霉毒素(aflatoxins, AFs)、赭曲霉毒素A(ochratoxin A, OTA)、脱氧雪腐镰刀菌烯醇(deoxynivalenol, DON)等被认为是最重要的霉菌毒素[4-5]。
肠道作为第1个接触食物污染物的组织,被认为是霉菌毒素作用的靶器官[6]。越来越多的研究表明,肠道上皮经常被霉菌毒素污染,且浓度高于其他组织[7-8]。之前的研究揭示了肠道紧密连接(tight junction, TJ)在维持肠道屏障功能中的重要性,因此当肠道TJ发生功能障碍时,会大大增加机体暴露于外源性化学物质和病原菌的几率,从而引起肠道炎症的发生[7]。霉菌毒素通过改变肠道TJ蛋白的表达水平、定位,导致肠道机械屏障功能受损,从而诱导肠道功能损伤和肠道炎症等疾病的发生。基于肠道TJ蛋白在维持肠道屏障功能中发挥的重要作用,本文就霉菌毒素对肠道TJ蛋白产生的影响及作用机制进行综述。
1 肠道TJ蛋白概述肠道黏膜屏障由机械屏障、生物屏障和化学屏障组成,其中机械屏障是保护肠道免受病原体或有害物质侵袭肠道黏膜的关键,并为维持肠道上皮的选择透过性及其屏障功能提供了结构基础。肠道上皮与细胞间形成的完整连接构成了机械屏障的结构基础,构成机械屏障结构基础的细胞间连接方式主要包括TJ、黏附连接(adherens junction)、桥粒(desmosome)以及缝隙连接(gap junction)(图 1),其中TJ是最主要的连接方式[9]。TJ附着在上皮细胞以维持其结构和细胞极性,从而维持上皮细胞的生物学和屏障功能。而霉菌毒素可以通过破坏肠道黏膜机械屏障的完整性来发挥毒性作用[10-11]。TJ蛋白是构成肠道上皮机械屏障的重要组成部分,不仅能维持上皮屏障功能、阻止有害物质和病原体,而且能选择性地调节营养物质、离子和水分进入体内。因此,TJ蛋白表达或分布的改变会破坏肠道屏障的通透性,从而影响肠道功能[12]。TJ蛋白包括:1)跨膜蛋白,其胞外域跨过质膜并与相邻细胞相互作用;2)胞质支架蛋白,其完全位于质膜的胞内侧。跨膜TJ蛋白在肠道上皮细胞的顶外侧膜上形成由咬合蛋白(Occludin)、闭合蛋白(Claudins)、连接黏附分子(junctional adhesion molecule)和Tricellulin组成的水平屏障。胞质支架蛋白,例如带状闭合蛋白(zona occludens,ZOs),提供了跨膜TJ蛋白与肌动蛋白细胞骨架之间的直接联系[13]。研究表明,Occludin、Claudins和ZOs是最重要的3类TJ蛋白,在维持细胞形态和形成阻止病原体入侵的屏障方面发挥着重要的作用[14-15]。
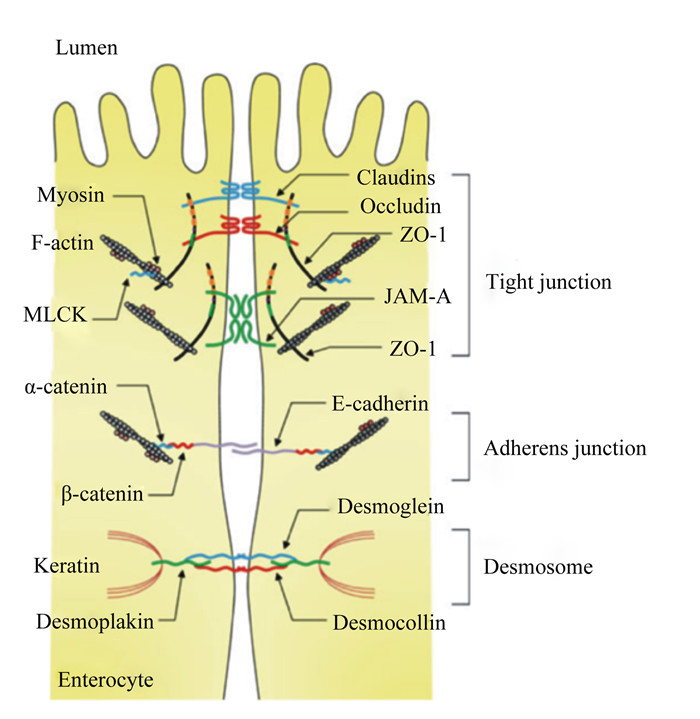
|
Tight junction:紧密连接;Adherens junction:黏附连接;Desmosome:桥粒;Myosin:肌球蛋白;F-actin:纤维形肌动蛋白fibrous actin;MLCK:肌球蛋白轻链激酶myosin light chain kinase;α-catenin:α-连环蛋白;β-catenin:β-连环蛋白;Keratin:角蛋白;Desmoplakin:桥粒斑蛋白;Enterocyte:肠上皮细胞;Claudins:闭合蛋白;Occludin:咬合蛋白;ZO-1:带状闭合蛋白-1 zona occluden-1;JAM-A:连接黏附分子-A junctional adhesion molecule-A;E-cadherin:钙黏附蛋白E;Desmoglein:桥粒芯蛋白;Desmocollin:桥粒胶蛋白。 图 1 肠道上皮细胞的TJ结构 Fig. 1 TJ structure of intestinal epithelial cells[16] |
Occludin是最早发现的TJ蛋白,是一种四聚体膜蛋白,具有4个跨膜结构域、2个细胞外环和1个细胞内环。它不仅在TJ的组装、稳定性和屏障功能中发挥重要作用,而且具有转移各种旁细胞位置的能力,从而改变上皮细胞的通透性,改变其屏障功能。有研究表明,肠道通透性受损与Occludin的蛋白表达量下降以及Occludin的蛋白移位有关[17]。其中,Tamura等[18]的研究结果表明,Occludin敲除小鼠的肠道旁细胞通透性会显著增加。且有研究指出Occludin从TJ处进入细胞质囊泡是肠道屏障功能丧失的主要原因[19]。Cani等[20]研究表明,Occludin的蛋白表达量与异硫氰酸荧光素(FITC)从胃肠道向血液的转运呈负相关,强调Occludin在维持屏障功能中的重要性。
Claudins是四聚体膜蛋白,具有1个胞内和2个胞外环以及C端和N端胞质结构域[21]。作为TJ的另一类重要组成蛋白,Claudins在肠道屏障功能中发挥了重要的作用。基于Claudins之间的链形成,TJ建立了调节肠道上皮细胞间分子流动的阀门。并且,Claudins分子的胞外环与相邻细胞发生相互作用,这些相互作用在细胞旁通路中为选择性分子的通过制造了屏障或通道[22]。肠道上皮完整性对于维持外部和内部环境之间的物理屏障至关重要,而霉菌毒素可以通过降低特定亚型Claudin-3和Claudin-4的蛋白表达量来破坏人结肠癌细胞(Caco-2)的机械屏障性能[17]。进一步使用Claudin-15和Claudin-2敲除小鼠的研究证实了Claudins在肠道屏障形成和细胞旁渗透选择性中发挥的关键作用[18]。
ZOs位于细胞质表面,共有3种类型:ZO-1、ZO-2和ZO-3,与ZO-2和ZO-3相比,ZO-1在肠道上皮细胞TJ的形成中发挥主要作用[23-25]。根据序列分析,ZOs被归类为膜相关的鸟苷激酶同系物(MAGUK)家族的成员,它们携带3个PDZ结构域、1个Src同源-3(SH3)结构域和1个与N端鸟苷酸激酶(GUK)同源区域的多域蛋白。这些多域结构为TJ提供了1个调节和维持TJ结构所必需的细胞内支架。此外,ZO-1是TJ和肌动蛋白细胞骨架之间的重要连接分子,是TJ发挥功能的重要组成成分。ZO-1的N端与许多TJ蛋白半区结合,C端与肌动蛋白的细胞骨架和细胞骨架相关蛋白相互作用[26]。ZO-1敲除细胞虽然能形成正常的TJ结构同时不影响细胞通透性,但却观察到Occludin和Claudins等其他TJ蛋白对TJ的装配存在明显的延迟,这证实了ZO-1在TJ组装调控中的重要作用[27]。
2 霉菌毒素对肠道TJ蛋白的影响 2.1 霉菌毒素对肠道TJ蛋白表达量的影响 2.1.1 霉菌毒素对细胞模型中肠道TJ蛋白表达量的影响霉菌毒素对细胞模型中肠道TJ蛋白表达量的影响如表 1所示。伏马毒素B1(FB1)处理Caco-2细胞后,其对Caco-2细胞屏障的损伤程度随着Claudin-3、Claudin-4和Occludin的mRNA表达量的显著降低而加深;黄曲霉毒素B1(AFB1)在降低了Caco-2细胞跨上皮膜电阻(transepithelial electrical resistance,TEER)值的同时,3、10和30 mmol/L的AFB1以浓度依赖的方式降低了Claudin-3和Occludin的mRNA表达量;OTA降低了Caco-2细胞中Claudin-3、Claudin-4和Occludin的mRNA表达量[28]。McLaughlin等[17]结果表明,OTA(100 μmol/L)在显著降低Caco-2细胞Claudin-3、Claudin-4的蛋白表达量的同时,显著降低细胞的TEER值,并且指出这种屏障性能的降低与Claudins-3和Claudin-4的消失是同时发生的。虽然12 μmol/L黄曲霉毒素M1(AFM1)对Caco-2细胞中Claudin-3、Claudin-4和ZO-1的蛋白表达量没有显著影响,但当相同浓度的AFM1和OTA(20 μmol/L)联合处理后,会显著降低Caco-2细胞的Claudin-3、Claudin-4和ZO-1的蛋白表达量[29]。与此同时,大量研究表明DON可增加多种细胞的通透性并降低其TJ蛋白的表达量,从而损伤肠道屏障功能[30-36]。1 000 ng/mL DON处理猪空肠上皮细胞(IPEC-J2)24 h后,Claudin-3的蛋白表达量降低,当浓度增加、处理时间延长(4 000 ng/mL,48 h)后,Claudin-1、Claudin-3、Claudin-4和Occludin的蛋白表达量均显著降低[27]。用2 000 ng/mL DON处理IPEC-J2细胞48 h后,ZO-1的蛋白表达量显著降低[34, 37]。而另一研究则表明,4 μmol/L DON处理IPEC-J2细胞12 h后,细胞Claudin-1、Claudin-4和Occludin的mRNA表达量升高,而Claudin-3的mRNA表达量下降[36]。
|
|
表 1 霉菌毒素对细胞模型中肠道TJ蛋白表达量的影响 Table 1 Effects of mycotoxin on expression level of TJ protein in cell models |
霉菌毒素引起TJ蛋白mRNA表达量及蛋白表达量的变化并不完全一致。研究报道,在暴露于DON的Caco-2细胞中观察到Claudin-3、Claudin-4、Occludin和ZO-1的mRNA表达量均升高,而蛋白表达量却显著降低[38]。此外,1 μmol/L DON处理小鼠直肠癌细胞(CMT93-Ⅱ)36 h后,DON显著降低了Claudin-4的mRNA表达量,蛋白表达量却显著降低[39]。因此,推测TJ蛋白是DON的主要靶向蛋白,并且mRNA表达量升高被认为是蛋白表达量降低的一种补偿机制[12, 36, 38, 40]。而且,有研究指出Claudins的蛋白表达量下降与Claudins的mRNA表达量升高有关,ZO-1的mRNA表达量升高与ZO-1蛋白表达量的降低有关[38, 41]。总而言之,霉菌毒素对TJ蛋白表达量的影响因细胞的种类、霉菌毒素的种类和浓度而异,但都对肠道屏障功能具有一定程度地损伤作用。
2.1.2 霉菌毒素对动物模型中肠道TJ蛋白表达量的影响霉菌毒素对动物模型中肠道TJ蛋白表达量的影响如表 2所示。大鼠暴露于ZEN(0.3~146.0 mg/kg)后,空肠中Claudin-4和Occludin的mRNA表达量显著下降[42]。当用发霉的小麦配制的DON污染饲粮(3.5 mg/kg)饲喂仔猪后,发现仔猪肠道屏障和免疫功能明显受损,且回肠中的TJ蛋白Claudin-3、Claudin-4和Occludin的mRNA表达量显著下降[43]。在另一项研究中,DON(2 mg/kg)显著降低了仔猪空肠Claudin-3和回肠Claudin-4、ZO-1以及结肠Claudin-1的mRNA表达量[27]。Alizadeh等[44]的研究结果显示,DON(0.28 mg/kg)会显著降低生长猪空肠ZO-1和Occludin的mRNA表达量。当仔猪暴露于FB1污染的饲粮(3 mg/kg)后,FB1会显著降低其回肠中Occludin的蛋白表达量[30]。用AFB1(218.1 μg/kg)和FB1(592.1 μg/kg)污染的饲粮饲喂断奶仔猪后,仔猪空肠通透性增加、结构受损,且ZO-l的mRNA表达量显著下降[45]。但在其他研究中却得到了矛盾的结果,Chen等[46]研究表明,AFB1(1.5 mg/kg)在影响肉鸡肠道屏障功能的基础上,增加了空肠中Claudin-1和Claudin-2的mRNA表达量。此外,用DON污染的饲粮[(7.54±2.20) mg/kg]喂养肉鸡会增加其空肠Claudin-5以及回肠Claudin-1、Claudin-5和ZO-l的mRNA表达量[47]。且DON(0.28 mg/kg)会显著增加生长猪回肠Claudins以及回肠、结肠和盲肠中Occludin的mRNA表达量[44]。而且,有研究表明,DON(25 mg/kg)显著增加了小鼠小肠Claudin-2、Claudin-3和Claudin-4的mRNA表达量[38]。针对上述所提到的DON对TJ蛋白表达量产生的不同影响,有研究指出出现这种差异性的原因之一是由不同TJ的表达方式以及其不同肠段对DON的敏感性差异和受试动物的差异造成的[44]。
|
|
表 2 霉菌毒素对动物模型中肠道TJ蛋白表达量的影响 Table 2 Effects of mycotoxin on expression level of TJ protein in intestine of animal models |
研究表明,霉菌毒素会显著改变细胞及动物模型中肠道TJ蛋白原有的结构和定位。与对照组相比,AFM1+OTA+ZEN组的Caco-2细胞中TJ结构受损,细胞连接松散,细胞间隙增宽,TJ蛋白数量几乎低于检测限[48]。并且,OTA在增加肠道通透性的同时,诱导Claudin-4在Caco-2细胞中的重新分布[11, 49]。Gao等[29]研究表明,正常Caco-2细胞中Claudin-3、Claudin-4、Occludin和ZO-1定位于质膜且呈鹅卵石状分布,而OTA和AFM1+OTA处理却破坏了原有的鹅卵石结构的完整性。OTA和DON显著破坏了Claudin-2在CMT93-Ⅱ细胞接触中定位的完整性[39]。此外,包晓宇[50]使用AFM1和AFB1单独或联合处理Caco-2细胞48 h后,细胞中TJ蛋白网格状分布受到破坏,并出现一定程度的内化——由膜定位变为胞质定位。另一项研究用30 μmol/L的OTA处理24 h后,Caco-2细胞中ZO-1和Claudin-1网络结构的荧光信号分布不均,荧光强度减弱,且连接处出现破裂[51]。当用DON(4.17 μmol/L)处理Caco-2细胞24 h后,与对照组相比,DON破坏了Claudin-1、Claudin-3、Claudin-4、Occludin和ZO-1连续的带状结构,且所有TJ蛋白在细胞膜上均呈现不规则分布[38]。而且,有研究表明低浓度AFM1(1~10 μg/kg)虽使Caco-2细胞TEER值具有降低的趋势,但Occludin和ZO-1的亚细胞定位却不受影响[52]。除此之外,霉菌毒素还能改变动物肠道中TJ蛋白的分布和定位。CD-1小鼠暴露于单独AFB1或AFM1后,回肠Claudin-1在细胞质内的分布增加,且部分细胞顶端膜上不再分布该蛋白,相邻细胞间细胞侧膜上分布也明显减少,细胞间隙不清晰;在AFB1和AFM1联合处理下,大量Claudin-1弥散在细胞质中,细胞膜上的分布明显减少,网格状屏障结构瓦解严重[53]。当用DON(25 mg/kg)灌胃雄性B6C3F1小鼠6 h后,远端小肠中Claudin-1、Claudin-2和Claudin-3呈现不规则分布,而结肠中Claudin-1、Claudin-2、Claudin-3、Claudin-4、Occludin和ZO-1的TJ分布模式并未有明显差异[38]。
2.3 霉菌毒素调控肠道TJ蛋白的相关作用机制研究指出,TJ蛋白的表达主要受几种细胞内途径调控(图 2),包括肌球蛋白轻链激酶(MLCK)、丝裂原活化蛋白激酶(MAPK)和蛋白激酶C(PKC)[54]。其中,MLCK途径是调控肠道TJ蛋白最常见的途径之一,也是通过多种外部刺激(例如细胞因子和病原体)调节TJ通透性的关键途径[54]。有研究表明,OTA通过活性氧/钙离子介导的MLCK蛋白通路显著降低了IPEC-J2细胞中TJ蛋白的表达量[55]。ZEN通过p38MAPK和MLCK相关信号通路破坏了鱼肠道中的TJ,并改变了TJ蛋白的表达量[56]。MAPK主要负责细胞内信号通路,在上皮细胞的炎症反应中发挥着重要作用,包括3个亚家族:p44/42细胞外信号调节激酶(ERK)、p38和c-Jun氨基末端激酶(JNK)。p44/42 MAPK信号传导可以通过上调或下调几种TJ蛋白的表达量来调节肠上皮细胞间的旁细胞运输,从而改变肠道的屏障功能[57]。Gao等[29]研究表明,p44/42 MAPK的活化部分参与了AFM1和OTA单独及联合作用下诱导的TJ蛋白表达量的下降。而且有研究表明,DON诱导IPEC-J2细胞TEER值和Claudin-3的蛋白表达量的降低是由p44/42 MAPK介导而造成的[57]。且DON诱导MAPK活化可以调节小肠上皮细胞系中Claudin-4的表达,ERK1/2磷酸化能调控CMT93-Ⅱ细胞Claudin-2的表达[32, 36, 39]。此外,DON能够迅速激活人以及猪肠道细胞系中ERK1/2和JNK,从而显著降低Claudins的蛋白表达量,而抑制ERK1/2磷酸化可恢复分化的猪小肠上皮细胞(IPEC-1)细胞的屏障功能[32, 33, 58-59]。另一项研究却得到了矛盾的结果,暴露于DON的猪空肠及其外植体中ERK1/2、JNK和p38等MAPK却未被显著激活[33]。这说明DON对不同系细胞产生毒性的作用机制可能存在差异,但均能表明肠上皮的TJ是DON的主要靶点之一,且DON改变TJ蛋白的表达量与肠道上皮屏障通透性增加密切相关。此外,有研究表明PKC在连接蛋白的调节中起到了重要的作用,并指出PKC可能是缓解由霉菌毒素引起的肠道屏障损伤潜在的治疗靶点[60]。Willis等[61]根据PKC抑制试验推测PKC通路可能部分诱导了DON引起的Claudins蛋白表达量下降,且有研究证实了抑制PKCζ的活性可以缓解内皮生长因子引起的人视网膜微血管内皮细胞的TJ蛋白表达量的降低[62]。
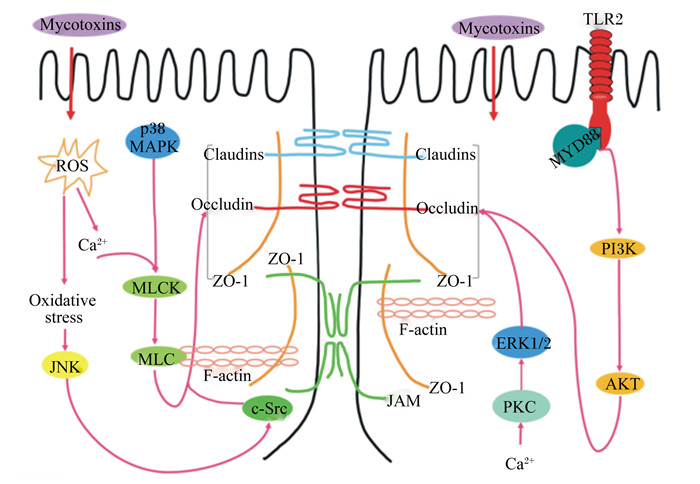
|
Mycotoxins:霉菌毒素;TLR2:Toll样受体2 Toll-like receptor 2;ROS:活性氧reactive oxygen species;p38MAPK:p38丝裂原活化蛋白激酶p38 mitogen-activated protein kinase;Claudins:闭合蛋白;Occludin:咬合蛋白;ZO-1:带状闭合蛋白-1 zona occluden-1;MYD88:髓样分化因子88 myeloid differentiation factor 88;Ca2+:钙离子calcium ion;Oxidative stress:氧化应激;MLCK:肌球蛋白轻链激酶myosin light chain kinase;MLC:肌球蛋白轻链myosin light chain;JNK:c-Jun氨基末端激酶c-Jun NH2-terminalprotein kinas;F-actin:纤维形肌动蛋白fibrous actin;JAM:连接黏附分子junctional adhesion molecule;ERK:细胞外信号调节激酶1/2 extracellular signal regulated kinase 1/2;PKC:蛋白激酶C protein kinase C;PI3K:磷脂酰肌醇3-激酶phosphoinositide 3-kinase;Akt:蛋白激酶B protein kinase B。 图 2 霉菌毒素调控细胞TJ的作用机制 Fig. 2 Mechanism of mycotoxin regulating cell TJ |
除了上述通路外,一些研究已经发现氧化应激参与调节由细胞外刺激引起的TJ和肠道屏障功能[63]。为了确定氧化应激与TJ破坏和肠道屏障功能障碍之间的关系,Wang等[55]在IPEC-J2细胞中使用了活性氧(ROS)清除剂N-乙酰半胱氨酸(NAC),结果表明NAC显著阻断了OTA诱导的细胞通透性的增加,且有效抑制了OTA诱导的ZO-1蛋白表达量下降以及ZO-1和Occludin的重新分布。而且,另一项研究表明OTA介导的肠道屏障毒性是由于OTA降低了Claudin-3和Claudin-4的蛋白表达量,这种作用在ROS清除剂存在时会失效[64]。还有研究指出DON破坏IPEC-1细胞的TJ蛋白与Toll样受体2(TLR2)和磷脂酰肌醇3-激酶(PI3K)-蛋白激酶B(Akt)信号通路调控有关[65]。同时,蛋白质合成受到抑制也可能影响TJ蛋白的表达。Walle等[12]研究证实DON主要通过抑制蛋白质的合成来降低Claudin-4的蛋白表达量,而且,也有研究表明DON可能通过抑制的蛋白质合成降低了IPEC-J2细胞中TJ蛋白的表达量[27]。DON和蛋白质合成抑制剂试验验证了DON通过抑制蛋白质的合成来改变TJ蛋白的表达量,从而影响肠道屏障的通透性[66]。
2.4 TJ破坏对疾病的影响TJ蛋白表达的改变、完整性的破坏以及功能的紊乱会导致多种疾病的发生[67-69]。霉菌毒素作为肠道TJ网络的特异性干扰因子,可能参与炎症性肠道疾病的发病机制[70]。TJ引起的肠道屏障受损会导致黏膜发炎,并已被证实与各种慢性肠炎性疾病的发病机理相关。TJ蛋白作为肠道屏障最重要的功能元件之一,在主要的慢性肠炎性疾病中均观察到TJ蛋白表达量的改变和TJ蛋白的重新分布[9, 71-73]。研究表明,坏死性小肠结肠炎与肠道通透性和Claudin-2的蛋白表达量增加有关[74]。此外,TJ功能障碍与其他疾病有关,例如破坏与Claudins结合的ZO-2突变会引起胆道疾病[75-76]。
3 小结作为肠道机械屏障的重要组成部分,TJ蛋白在维持肠道屏障功能中发挥了重要的作用。由于霉菌毒素的广泛分布,对全世界的人类和动物的健康构成了严重的威胁。近年来,霉菌毒素对肠道屏障的影响特别是对TJ蛋白的损伤作用引起了广泛的关注。之前对霉菌毒素的研究主要集中在肝脏毒性、肾脏毒性、基因毒性、致癌性等,但是对TJ蛋白及其损害肠道屏障完整性的研究仍然有限。由本文上述内容所述,TJ蛋白在基因水平上的表达量因霉菌毒素的种类和浓度以及细胞本身的种类而异,但都对肠道屏障功能具有一定程度地损伤作用。霉菌毒素主要通过MLCK、MAPK、PKC和氧化应激通路以及抑制蛋白质的合成来改变TJ蛋白在mRNA和蛋白水平的表达量,从而影响肠道屏障功能,其中,DON对不同系细胞和不同品种试验动物TJ蛋白调控的作用机制存在差异。因此,针对不同种类的霉菌毒素和细胞/动物模型,应选择不同的预防靶点来促进TJ蛋白相关的肠道屏障功能。
就目前来说,霉菌毒素对肠道TJ损伤如对肠道TJ蛋白的调节相关的机制研究还较少,且大都集中于体外模型的研究,缺乏体内研究的相关数据。今后的研究重点应主要放在霉菌毒素对肠道TJ的机制研究上,可以结合使用组学技术评估相关蛋白及基因功能的变化,为降低和修复霉菌毒素所引起的肠道屏障损伤提供理论依据。此外,我们应该把霉菌毒素对肠道屏障以及TJ蛋白的影响纳入霉菌毒素的总体风险评估中,为人类和动物的健康提供更大的保障。
| [1] |
KABAK B, DOBSON A D W, VAR I. Strategies to prevent mycotoxin contamination of food and animal feed:a review[J]. Critical Reviews in Food Science and Nutrition, 2006, 46(8): 593-619. DOI:10.1080/10408390500436185 |
| [2] |
WU F, GROOPMAN J D, PESTKA J J. Public health impacts of foodborne mycotoxins[J]. Annual Review of Food Science and Technology, 2014, 5: 351-372. DOI:10.1146/annurev-food-030713-092431 |
| [3] |
RODRIGUES I, NAEHRER K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed[J]. Toxins (Basel), 2012, 4(9): 663-675. DOI:10.3390/toxins4090663 |
| [4] |
SHEPHARD G S. Determination of mycotoxins in human foods[J]. Chemical Society Reviews, 2008, 37(11): 2468-2477. DOI:10.1039/b713084h |
| [5] |
杨雪, 高亚男, 王加启, 等. 亚洲和欧美地区霉菌毒素及其暴露风险分析[J]. 食品工业科技, 2020, 41(5): 311-318. |
| [6] |
BOUHET S, OSWALD I P. The intestine as a possible target for fumonisin toxicity[J]. Molecular Nutrition & Food Research, 2007, 51(8): 925-931. |
| [7] |
GRENIER B, APPLEGATE J T. Modulation of intestinal functions following mycotoxin ingestion:meta-analysis of published experiments in animals[J]. Toxins, 2013, 5(2): 396-430. |
| [8] |
BOUHET S, OSWALD I P. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response[J]. Veterinary Immunology and Immunopathology, 2005, 108(1/2): 199-209. |
| [9] |
SUZUKI T. Regulation of intestinal epithelial permeability by tight junctions[J]. Cellular and Molecular Life Sciences, 2013, 70(4): 631-659. DOI:10.1007/s00018-012-1070-x |
| [10] |
REN Z H, GUO C Y, YU S M, et al. Progress in mycotoxins affecting intestinal mucosal barrier function[J]. International Journal of Molecular Sciences, 2019, 20(11): 2777. DOI:10.3390/ijms20112777 |
| [11] |
AKBARI P, BRABER S, VARASTEH S, et al. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins[J]. Archives of Toxicology, 2017, 91(3): 1007-1029. |
| [12] |
WALLE J, SERGENT T, PIRONT N, et al. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis[J]. Toxicology and Applied Pharmacology, 2010, 245(3): 291-298. DOI:10.1016/j.taap.2010.03.012 |
| [13] |
CHIBA H, OSANAI M, MURATA M, et al. Transmembrane proteins of tight junctions[J]. Biochimica et Biophysica Acta:Biomembranes, 2008, 1778(3): 588-600. DOI:10.1016/j.bbamem.2007.08.017 |
| [14] |
蒋宗勇, 朱翠, 陈庄, 等.紧密连接蛋白及其对断奶仔猪肠道健康的影响[C]//中国畜牧兽医学会动物营养学分会第十届全国代表大会暨十二届学术研讨会论文集.北京: 中国农业大学出版社, 2016.
|
| [15] |
YU L C H, WANG J T, WEI S C, et al. Host-microbial interactions and regulation of intestinal epithelial barrier function:from physiology to pathology[J]. World Journal of Gastrointestinal Pathophysiology, 2012, 3(1): 27-43. DOI:10.4291/wjgp.v3.i1.27 |
| [16] |
HAN X F, ZHANG E S, SHI Y J, et al. Biomaterial-tight junction interaction and potential impacts[J]. Journal of Materials Chemistry B, 2019, 7(41): 6310-6320. DOI:10.1039/C9TB01081E |
| [17] |
MCLAUGHLIN J, PADFIELD P J, BURT J P H, et al. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms[J]. American Journal of Physiology-Cell Physiology, 2004, 287(5): C1412-C1417. DOI:10.1152/ajpcell.00007.2004 |
| [18] |
TAMURA A, HAYASHI H, IMASATO M, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine[J]. Gastroenterology, 2011, 140(3): 913-923. DOI:10.1053/j.gastro.2010.08.006 |
| [19] |
SHEN L, WEBER C R, TURNER J R. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state[J]. The Journal of Cell Biology, 2008, 181(4): 683-695. DOI:10.1083/jcb.200711165 |
| [20] |
CANI P D, POSSEMIERS S, VAN DE WIELE T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability[J]. Gut, 2009, 58(8): 1091-1103. DOI:10.1136/gut.2008.165886 |
| [21] |
FURUSE M, FUJITA K, HIIRAGI T, et al. Claudin-1 and -2:novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin[J]. Journal of Cell Biology, 1998, 141(7): 1539-1550. DOI:10.1083/jcb.141.7.1539 |
| [22] |
VAN ITALLIE C M, ANDERSON J M. Claudins and epithelial paracellular transport[J]. Annual Review of Physiology, 2006, 68: 403-429. DOI:10.1146/annurev.physiol.68.040104.131404 |
| [23] |
STEVENSON B R, SILICIANO J D, MOOSEKER M S, et al. Identification of ZO-1:a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia[J]. Journal of Cell Biology, 1986, 103(3): 755-766. DOI:10.1083/jcb.103.3.755 |
| [24] |
HUNZIKER W, KIENER T K, XU J L. Vertebrate animal models unravel physiological roles for zonula occludens tight junction adaptor proteins[J]. Annals of the New York Academy of Sciences, 2009, 1165(1): 28-33. DOI:10.1111/j.1749-6632.2009.04033.x |
| [25] |
TSUKITA S, FURUSE M, ITOH M. Multifunctional strands in tight junctions[J]. Nature Reviews Molecular Cell Biology, 2001, 2(4): 285-293. |
| [26] |
FURUSE M, HATA M, FURUSE K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier:a lesson from claudin-1-deficient mice[J]. Journal of Cell Biology, 2002, 156(6): 1099-1111. DOI:10.1083/jcb.200110122 |
| [27] |
赵珩伊.脱氧雪腐镰刀菌烯醇影响仔猪肠上皮屏障的机制及蒙脱石的保护效果和用量评估[D].博士学位论文.雅安: 四川农业大学, 2017: 1-104. http://cdmd.cnki.com.cn/Article/CDMD-10626-1018025321.htm
|
| [28] |
ROMERO A, ARES I, RAMOS E, et al. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms:protective effect of illite mineral clay[J]. Toxicology, 2016, 353-354: 21-33. DOI:10.1016/j.tox.2016.05.003 |
| [29] |
GAO Y A, LI S L, WANG J Q, et al. Modulation of intestinal epithelial permeability in differentiated Caco-2 cells exposed to aflatoxin M1 and ochratoxin A individually or collectively[J]. Toxins, 2018, 10(1): 13. |
| [30] |
BRACARENSE A P F L, LUCIOLI J, GRENIER B, et al. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets[J]. British Journal of Nutrition, 2012, 107(12): 1776-1786. DOI:10.1017/S0007114511004946 |
| [31] |
PINTON P, NOUGAYRÈDE J P, DEL RIO J C, et al. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression[J]. Toxicology and Applied Pharmacology, 2009, 237(1): 41-48. DOI:10.1016/j.taap.2009.03.003 |
| [32] |
PINTON P, BRAICU C, NOUGAYREDE J P, et al. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism[J]. The Journal of Nutrition, 2010, 140(11): 1956-1962. DOI:10.3945/jn.110.123919 |
| [33] |
PINTON P, TSYBULSKYY D, LUCIOLI J, et al. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine:differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases[J]. Toxicological Sciences, 2012, 130(1): 180-190. DOI:10.1093/toxsci/kfs239 |
| [34] |
DIESING A K, NOSSOL C, PANTHER P, et al. Mycotoxin deoxynivalenol (DON) mediates biphasic cellular response in intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2[J]. Toxicology Letters, 2011, 200(1/2): 8-18. |
| [35] |
GUA M J, SONGA S K, PARK S M, et al. Bacillus subtilis protects porcine intestinal barrier from deoxynivalenol via improved zonula occludens-1 expression[J]. Asian-Australasian Journal of Animal Sciences, 2014, 27(4): 580-586. DOI:10.5713/ajas.2013.13744 |
| [36] |
LING K H, WAN M L Y, EL-NEZAMI H, et al. Protective capacity of resveratrol, a natural polyphenolic compound, against deoxynivalenol-induced intestinal barrier dysfunction and bacterial translocation[J]. Chemical Research in Toxicology, 2016, 29(5): 823-833. DOI:10.1021/acs.chemrestox.6b00001 |
| [37] |
DIESING A K, NOSSOL C, DÄNICKE S, et al. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application[J]. PLoS One, 2011, 6(2): e17472. DOI:10.1371/journal.pone.0017472 |
| [38] |
AKBARI P, BRABER S, GREMMELS H, et al. Deoxynivalenol:a trigger for intestinal integrity breakdown[J]. The FASEB Journal, 2014, 28(6): 2414-2429. DOI:10.1096/fj.13-238717 |
| [39] |
NAKAYAMA H, KITAGAWA N, OTANI T, et al. Ochratoxin A, citrinin and deoxynivalenol decrease claudin-2 expression in mouse rectum CMT93-Ⅱ cells[J]. Microscopy, 2018, 67(2): 99-111. DOI:10.1093/jmicro/dfy005 |
| [40] |
BOL-SCHOENMAKERS M, BRABER S, AKBARI P, et al. The mycotoxin deoxynivalenol facilitates allergic sensitization to whey in mice[J]. Mucosal Immunology, 2016, 9(6): 1477-1486. DOI:10.1038/mi.2016.13 |
| [41] |
MAIERS J L, PENG X, FANNING A S, et al. ZO-1 recruitment to α-catenin-a novel mechanism for coupling the assembly of tight junctions to adherens junctions[J]. Journal of Cell Science, 2013, 126(17): 3904-3915. DOI:10.1242/jcs.126565 |
| [42] |
LIU M, GAO R, MENG Q W, et al. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats[J]. PLoS One, 2014, 9(9): e106412. DOI:10.1371/journal.pone.0106412 |
| [43] |
LESSARD M, SAVARD C, DESCHENE K, et al. Impact of deoxynivalenol (DON) contaminated feed on intestinal integrity and immune response in swine[J]. Food and Chemical Toxicology, 2015, 80: 7-16. DOI:10.1016/j.fct.2015.02.013 |
| [44] |
ALIZADEH A, BRABER S, AKBARI P, et al. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs[J]. Toxins, 2015, 7(6): 2071-2095. DOI:10.3390/toxins7062071 |
| [45] |
高亿清.不同复合添加剂对采食含霉菌毒素饲粮断奶仔猪生长性能和肠道健康的影响[D].硕士学位论文.雅安: 四川农业大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10626-1016052923.htm
|
| [46] |
CHEN X, NAEHRER K, APPLEGATE T J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks[J]. Poultry Science, 2016, 95(6): 1312-1325. DOI:10.3382/ps/pew022 |
| [47] |
OSSELAERE A, SANTOS R, HAUTEKIET V, et al. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine[J]. PLoS One, 2013, 8(7): e69014. DOI:10.1371/journal.pone.0069014 |
| [48] |
WU C Q, GAO Y N, LI S L, et al. Modulation of intestinal epithelial permeability and mucin mRNA (MUC2, MUC5AC, and MUC5B) expression and protein secretion in Caco-2/HT29-MTX co-cultures exposed to aflatoxin M1, ochratoxin A, and zearalenone individually or collectively[J]. Toxicology Letters, 2019, 309: 1-9. DOI:10.1016/j.toxlet.2019.03.010 |
| [49] |
RANALDI G, MANCINI E, FERRUZZA S, et al. Effects of red wine on ochratoxin A toxicity in intestinal Caco-2/TC7 cells[J]. Toxicology in Vitro, 2007, 21(2): 204-210. |
| [50] |
包晓宇.黄曲霉毒素M1及其与黄曲霉毒素B1共存时对人肠道上皮细胞Caco-2的毒性作用研究[D].硕士学位论文.武汉: 华中农业大学, 2019.
|
| [51] |
PENG M X, LIU J W, LIANG Z H. Probiotic Bacillus subtilis CW14 reduces disruption of the epithelial barrier and toxicity of ochratoxin A to Caco-2 cells[J]. Food and Chemical Toxicology, 2019, 126: 25-33. DOI:10.1016/j.fct.2019.02.009 |
| [52] |
CALONI F, CORTINOVIS C, PIZZO F, et al. Transport of aflatoxin M1 in human intestinal Caco-2/TC7 cells[J]. Frontiers in Pharmacology, 2012, 3: 111. |
| [53] |
张牧臣.黄曲霉毒素B1与M1对小鼠肠道的联合毒性作用机理[D].硕士学位论文.北京: 中国农业科学院, 2018.
|
| [54] |
SCOT K G E, MEDDINGS J B, KIRK D R, et al. Intestinal infection with Giardia spp.reduces epithelial barrier function in a myosin light chain kinase-dependent fashion[J]. Gastroenterology, 2002, 123(4): 1179-1190. DOI:10.1053/gast.2002.36002 |
| [55] |
WANG H, ZHAI N H, CHEN Y, et al. OTA induces intestinal epithelial barrier dysfunction and tight junction disruption in IPEC-J2 cells through ROS/Ca2+-mediated MLCK activation[J]. Environmental Pollution, 2018, 242: 106-112. DOI:10.1016/j.envpol.2018.06.062 |
| [56] |
WANG Y L, ZHOU X Q, JIANG W D, et al. Effects of dietary zearalenone on oxidative stress, cell apoptosis, and tight junction in the intestine of juvenile grass carp (Ctenopharyngodon idella)[J]. Toxins, 2019, 11(6): 333. DOI:10.3390/toxins11060333 |
| [57] |
SPRINGLER A, HESSENBERGER S, SCHATZMAYR G, et al. Early activation of MAPK p44/42 is partially involved in DON-induced disruption of the intestinal barrier function and tight junction network[J]. Toxins, 2016, 8(9): 264. DOI:10.3390/toxins8090264 |
| [58] |
PESTKA J J. Deoxynivalenol:mechanisms of action, human exposure, and toxicological relevance[J]. Archives of Toxicology, 2010, 84(9): 663-679. DOI:10.1007/s00204-010-0579-8 |
| [59] |
SERGENT T, PARYS M, GARSOU S, et al. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations[J]. Toxicology Letters, 2006, 164(2): 167-176. |
| [60] |
GAO Y N, LI S L, BAO X Y, et al. Transcriptional and proteomic analysis revealed a synergistic effect of aflatoxin M1 and ochratoxin A mycotoxins on the intestinal epithelial integrity of differentiated human Caco-2 cells[J]. Journal of Proteome Research, 2018, 17(9): 3128-3142. DOI:10.1021/acs.jproteome.8b00241 |
| [61] |
WILLIS C L, MESKE D S, DAVIS T P. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation[J]. Journal of Cerebral Blood Flow & Metabolism, 2010, 30(11): 1847-1859. |
| [62] |
SONG H B, JUN H O, KIM J H, et al. Suppression of protein kinase C-ζ attenuates vascular leakage via prevention of tight junction protein decrease in diabetic retinopathy[J]. Biochemical and Biophysical Research Communications, 2014, 444(1): 63-68. DOI:10.1016/j.bbrc.2014.01.002 |
| [63] |
刘洁, 熊正国, 顿耀艳. 氧化应激对肠屏障功能障碍发病的影响[J]. 生命的化学, 2020, 40(2): 166-172. |
| [64] |
LAMBERT D, PADFIELD P J, MCLAUGHLIN J, et al. Ochratoxin A displaces claudins from detergent resistant membrane microdomains[J]. Biochemical and Biophysical Research Communications, 2007, 358(2): 632-636. DOI:10.1016/j.bbrc.2007.04.180 |
| [65] |
GU M J, SONG S K, LEE I K, et al. Barrier protection via Toll-like receptor 2 signaling in porcine intestinal epithelial cells damaged by deoxynivalnol[J]. Veterinary Research, 2016, 47: 25. DOI:10.1186/s13567-016-0309-1 |
| [66] |
AWAD W A, ZENTEK J. The feed contaminant deoxynivalenol affects the intestinal barrier permeability through inhibition of protein synthesis[J]. Archives of Toxicology, 2015, 89(6): 961-965. DOI:10.1007/s00204-014-1284-9 |
| [67] |
ZEISEL M B, DHAWAN P, BAUMERT T F. Tight junction proteins in gastrointestinal and liver disease[J]. Gut, 2019, 68(3): 547-561. DOI:10.1136/gutjnl-2018-316906 |
| [68] |
TANAKA H, TAKECHI M, KIYONARI H, et al. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice[J]. Gut, 2015, 64(10): 1529-1538. DOI:10.1136/gutjnl-2014-308419 |
| [69] |
BRANDNER J M, ZORN-KRUPPA M, YOSHIDA T, et al. Epidermal tight junctions in health and disease[J]. Tissue Barriers, 2015, 3(1/2): e974451. |
| [70] |
AKBARI P, BRABER S, ALIZADEH A, et al. Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell monolayers and B6C3F1 mice[J]. The Journal of Nutrition, 2015, 145(7): 1604-1613. DOI:10.3945/jn.114.209486 |
| [71] |
BERTIAUX-VANDAËLE N, YOUMBA S B, BELMONTE L P, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype[J]. American Journal of Gastroenterology, 2011, 106(12): 2165-2173. DOI:10.1038/ajg.2011.257 |
| [72] |
HERING N A, FROMM M, SCHULZKE J D. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics[J]. The Journal of Physiology, 2012, 590(5): 1035-1044. DOI:10.1113/jphysiol.2011.224568 |
| [73] |
SZAKÁL D N, GYÖRFFY H, ARATÓ A, et al. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease[J]. Virchows Archiv, 2010, 456(3): 245-250. DOI:10.1007/s00428-009-0879-7 |
| [74] |
ARES G, BUONPANE C, SINCAVAGE J, et al. Caveolin 1 is associated with upregulated claudin 2 in necrotizing enterocolitis[J]. Scientific Reports, 2019, 9: 4982. DOI:10.1038/s41598-019-41442-4 |
| [75] |
BUCKLEY A, TURNER J R. Cell biology of tight junction barrier regulation and mucosal disease[J]. Cold Spring Harbor Perspectives in Biology, 2017, 10(1): a029314. |
| [76] |
CARLTON V E H, HARRIS B Z, PUFFENBERGER E G, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT[J]. Nature Genetics, 2003, 34(1): 91-96. DOI:10.1038/ng1147 |




