2. 扬州大学农业科技发展研究院, 扬州 225009;
3. 扬州大学教育部农业与农产品安全国际合作联合实验室, 扬州 225009;
4. 江西省农业科学院畜牧兽医研究所, 南昌 330200
2. Institutes of Agricultural Science and Technology Development, Yangzhou University, Yangzhou 225009, China;
3. Joint International Research Laboratory of Agriculture and Agri-Product Safety of Ministry of Education of China, Yangzhou University Yangzhou 225009, China;
4. Institute of Animal Husbandry and Veterinary, Jiangxi Academy of Agricultural Sciences, Nanchang 330200, China
亚急性瘤胃酸中毒(SARA)是奶牛营养相关的代谢性疾病,发病率高。商业化养殖场中,高产奶牛饲喂高精料从而提高产奶量,但会降低瘤胃pH和微生物活性,提高SARA的风险[1]。国外研究表明,在集约化奶牛养殖场中,超过19%的早期泌乳奶牛和26%的中期泌乳奶牛患有SARA[2]。SARA降低了产奶量、乳脂率、乳蛋白率,使动物过早的淘汰,造成较大经济损失[2-3]。患有SARA的奶牛的瘤胃中脂多糖(LPS)含量升高引起瘤胃炎症[4]。Nyati等[5]研究小鼠胚胎成纤维细胞表明,LPS激活炎症信号核因子-κB(NF-κB)通路,进一步促进炎症因子[如肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)、白细胞介素-6(IL-6)]的表达。Wellnitz等[6]报道,LPS作用于奶牛乳腺上皮细胞会刺激炎症因子TNF-α、IL-1β和IL-6生成。Zhang等[7]报道,LPS可诱导荷斯坦奶牛瘤胃上皮细胞多种炎症细胞因子mRNA表达量升高。LPS对于炎症起到重要的介质作用,并且可以诱导瘤胃组织的炎症。茶树油(TTO)是互叶白千层叶子经水蒸气蒸馏得到的无色至淡黄色轻油状液体[8]。TTO大约包含100种化合物,主要包括松油醇-4、α-松油醇、α-松油烯等[9-12]。TTO对小鼠淋巴细胞产生的促炎细胞因子有抑制作用[13-14]。Nogueira等[15]报道,TTO可抑制LPS诱导巨噬细胞中IL-1β、TNF-α、IL-8的表达。TTO良好的杀菌、抗炎作用得到了科学的验证[8]。但目前TTO在LPS刺激奶牛瘤胃上皮细胞是否有抗炎作用尚未有明确定论。因此,本研究旨在研究TTO对LPS诱导的奶牛瘤胃上皮细胞炎症因子表达的影响,为TTO的应用提供科学的参考。
1 材料和方法 1.1 试验材料TTO:由无锡市某生物科技有限公司提供,其主要成分为松油醇-4>60%、对伞花烃5%~10%、桉叶素2%~10%、α-松油醇>3%、α-松油烯<10%、α-派烯<0.5%。
奶牛瘤胃上皮细胞:由扬州大学动物培养物保藏应用研究所(IACCA)提供。
试验试剂和仪器:DMEM/F12培养基、非必需氨基酸(NEAA)、磷酸盐缓冲液(PBS)和胰蛋白酶(Gibco,美国);LPS、青霉素、链霉素、L-谷氨酰胺溶液、乙二胺四乙酸(Sigma,美国);Gemini胎牛血清、荧光定量96孔板和8连管(Bio-rad,美国);PrimeScriptTM RT Master Mix和SYBR® Premix Ex TaqTM Ⅱ(TaKaRa,中国);总RNA提取试剂盒(Tiangen,中国);CCK-8(Dojindo,中国);玻璃板、塑料槽、盖玻片、梳子、电泳槽等。
1.2 试验方法 1.2.1 TTO对奶牛瘤胃上皮细胞毒性的影响将TTO用0.1%的二甲基亚砜(DMSO)溶解,收集对数生长期的奶牛瘤胃上皮细胞,在96孔板中每孔接种5×103个奶牛瘤胃上皮细胞。培养12 h后,在96孔板中分别加入含有0(对照)、0.006 25%、0.012 5%、0.025%、0.05%、0.1% TTO的DMEM/F12培养基,每组6个重复。培养24 h后,用CCK-8试剂盒测定TTO对奶牛瘤胃上皮细胞的毒性。
1.2.2 TTO对炎症细胞因子表达的影响将培养到一定密度的奶牛瘤胃上皮细胞收集于6孔板中,每孔1×105个细胞,培养12 h后,按下述处理继续培养:对照组只添加DMEM/F12培养基;TTO组添加0.05% TTO的DMEM/F12培养基;LPS组添加1 μg/mL LPS的DMEM/F12培养基;LPS+TTO组添加1 μg/mL LPS和0.05% TTO的DMEM/F12培养基,每组6个重复。培养24 h后,分别收集各组细胞,提取总RNA。总RNA提取参照Tiangen公司总RNA提取试剂盒说明书方法进行。反转录采用Roche公司反转录试剂盒合成cDNA,操作方法、反应体系和反应液配制参照本课题组占今舜等[16]的报道。
每个样品3个重复,以磷酸甘油醛脱氢酶(GAPDH)作为内参基因。引物设计参照本课题组Gong等[17]以及占今舜等[16]研究报道,详见表 1。基因相对表达量采用2-ΔΔCt方法进行计算。
|
|
表 1 引物序列及参数 Table 1 Primer sequences and parameters |
将培养到一定密度的奶牛瘤胃上皮细胞收集于6孔板中,每孔1×106个细胞,培养12 h后,按1.2.2中的处理培养24 h后收集各组细胞,每组6个重复。提取蛋白及蛋白质印迹(Western blot)法参照本课题组詹康[18]研究报道。
1.3 数据统计分析运用SPSS 16.0统计软件中的one-way ANOVA模块进行单因素方差分析,LSD法进行多重比较。P<0.05表示差异显著。
2 结果与分析 2.1 TTO对奶牛瘤胃上皮细胞毒性的影响由图 1可知,与对照组相比,添加0.05% TTO显著提高奶牛瘤胃上皮细胞活力(P<0.05);添加0.1% TTO显著抑制奶牛瘤胃上皮细胞活力(P<0.05)。因此,选择0.05% TTO来研究其对LPS诱导的奶牛瘤胃上皮细胞炎症因子表达的影响。
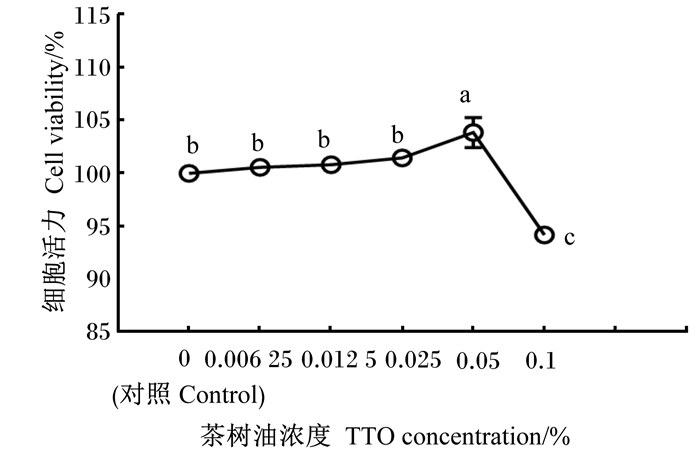
|
不同小写字母表示差异显著(P<0.05)。图 2同。Different small letters mean significant difference (P<0.05). The same as Fig. 2. 图 1 TTO对奶牛瘤胃上皮细胞毒性的影响 Fig. 1 Effects of TTO on cytotoxicity in BRECs |
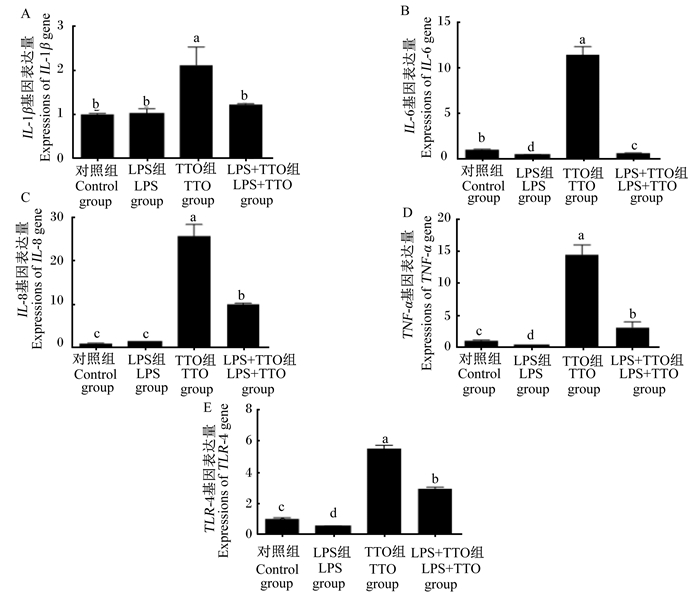
|
图 2 TTO对奶牛瘤胃上皮细胞炎症细胞因子基因表达量的影响 Fig. 2 Effects of TTO on expression of inflammatory cytokine genes in BRECs |
由图 2可知,与对照组相比,LPS组IL-1β、IL-8、IL-6、TNF-α、TLR-4基因表达量显著增加(P<0.05);TTO组IL-6、TNF-α、TLR-4基因表达量显著下降(P<0.05)。
与LPS组相比,LPS+TTO组IL-1β、IL-8、TLR-4、IL-6、TNF-α基因表达量显著下降(P<0.05)。
2.3 TTO对NF-κB信号通路的影响由图 3可知,与对照组相比,LPS组可以提高NF-κB信号通路p65、p-p65的蛋白表达量。与LPS组相比,LPS+TTO组NF-κB信号通路p65、p-p65的蛋白表达量降低。
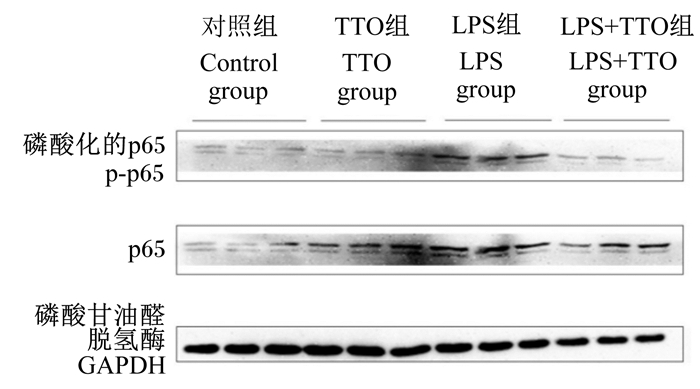
|
图 3 TTO对奶牛瘤胃上皮细胞NF-κB信号通路中p65、p-p65蛋白表达量的影响 Fig. 3 Effects of TTO on expression levels of p65 and p-p65 protein in NF-κB signaling pathway in BRECs |
SARA是一种泌乳奶牛常见的疾病。瘤胃pH降至5.6以下且每天超过3 h是SARA的特征[19]。当瘤胃pH较低时,革兰氏阴性菌裂解较快,增加了瘤胃内LPS的浓度[20]。瘤胃上皮持续暴露在高浓度的LPS中会引起SARA。Zhang等[7]研究表明, LPS可加剧SARA炎症的病情。已有研究证明TTO具有抗炎作用,但未有研究证明TTO对调控SARA引起瘤胃上皮炎症反应是否有影响。本试验结果表明,低浓度TTO对LPS诱导的奶牛瘤胃上皮细胞炎症因子具有抑制作用。
3.1 TTO对奶牛瘤胃上皮细胞毒性的影响据报道,人类中毒试验研究中5% TTO不会产生过敏反应,2.5% TTO不会出现皮肤中毒,2% TTO不会产生肝损伤[21]。因此,低剂量的TTO不会产生不良反应。早期Hayes等[22]研究报道,0.005%~0.030% TTO能够诱导黑色素肿瘤细胞半胱氨酸酶依赖性凋亡,能够明显抑制变异性细胞群。Ninomiya等[23]用TTO治疗口腔念珠菌引起的感染,当TTO浓度为900 μg/mL时可显著抑制白色念珠菌细胞巨噬细胞产生细胞因子。本试验结果显示,0.05% TTO可以提高奶牛瘤胃上皮细胞活力,因此,低浓度的TTO对细胞无害。
3.2 TTO对炎症细胞因子表达的影响瘤胃炎症是SRAR直接引起的症状。TLR-4是脂多糖的受体[24],LPS可以激活TLR-4并引发全身炎症。LPS可以引起奶牛瘤胃上皮细胞的炎性损伤[25]。Wang等[24]研究表明,增多的LPS会激活奶牛乳腺上皮细胞表面的TLR-4,增加炎症细胞因子的表达,并破坏乳腺上皮功能,最终导致全身炎症。重要的是,Zhang等[7]发现,LPS在体外刺激奶牛瘤胃上皮细胞可以显著的提高IL-1β和IL-8 mRNA表达。这些研究表明,奶牛瘤胃上皮细胞能够对炎症细胞因子的释放做出反应。LPS水平增加会导致一系列的免疫应答,如促炎细胞因子的产生和释放,包括TNF-α、IL-1β和IL-6等,LPS诱导这些因子的释放对于激活免疫系统,引起免疫应答具有重要的作用[26]。因此本试验使用奶牛瘤胃上皮细胞探讨TTO体外抗炎的机制。本试验结果表明,LPS诱导奶牛瘤胃上皮细胞可以显著提高炎症因子IL-1β、IL-6、IL-8、TLR-4的基因表达量,但是低浓度TTO可以显著降低炎症细胞因子的表达量,因此TTO可以改善瘤胃上皮由LPS引发的炎症反应。
3.3 TTO对NF-κB通路的影响由于高精料饲喂过量可能导致SARA的发生,致使瘤胃内营养物质过剩,导致瘤胃微生物大量繁殖,其代谢物可使TNF-α、IL-1β等炎症因子的表达量增加,并且NF-κB信号通路激活后炎症因子进一步增加,从而加剧炎症反应[27-29]。王婷婷[30]研究表明,NF-κB蛋白的磷酸化和入核过程反映信号通路的传导抑制,可以增加转录因子NF-κB p65的入核与转录活性,并进一步增加炎症细胞因子TNF-α、IL-6和IL-1β等的表达和与释放,从而引起瘤胃上皮细胞的炎症损伤。NF-κB信号通路参与许多组织炎症疾病的发病机制[31]。Western Blot结果表明,TTO会降低LPS诱导奶牛瘤胃上皮细胞中的p65蛋白表达量,从而抑制奶牛瘤胃上皮细胞炎症因子的表达。
4 结论① 低浓度的TTO可以提高奶牛瘤胃上皮细胞的活力。
② 低浓度TTO对炎症因子IL-1β、IL-6、IL-8、TNF-α和TLR-4的基因表达有显著抑制作用。
③ 低浓度TTO可以抑制NF-κB信号通路p65、p-p65蛋白表达量,进而抑制促炎细胞因子表达。
| [1] |
PAN X H, YANG L, XUE F G, et al. Relationship between thiamine and subacute ruminal acidosis induced by a high-grain diet in dairy cows[J]. Journal of Dairy Science, 2016, 99(11): 8790-8801. DOI:10.3168/jds.2016-10865 |
| [2] |
ENEMARK J M D. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA):a review[J]. The Veterinary Journal, 2008, 176(1): 32-43. DOI:10.1016/j.tvjl.2007.12.021 |
| [3] |
PLAIZIER J C, KRAUSE D O, GOZHO G N, et al. Subacute ruminal acidosis in dairy cows:the physiological causes, incidence and consequences[J]. The Veterinary Journal, 2008, 176(1): 21-31. DOI:10.1016/j.tvjl.2007.12.016 |
| [4] |
GOZHO G N, KRAUSE D O, PLAIZIER J C. Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows[J]. Journal of Dairy Science, 2007, 90(2): 856-866. DOI:10.3168/jds.S0022-0302(07)71569-2 |
| [5] |
NYATI K K, KAZUYA M, ZAMAN M M U, et al. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a[J]. Nucleic Acids Research, 2017, 45(5): 2687-2703. DOI:10.1093/nar/gkx064 |
| [6] |
WELLNITZ O, KERR D E. Cryopreserved bovine mammary cells to model epithelial response to infection[J]. Veterinary Immunology and Immunopathology, 2004, 101(3/4): 191-202. |
| [7] |
ZHANG R Y, ZHU W Y, MAO S Y. High-concentrate feeding upregulates the expression of inflammation-related genes in the ruminal epithelium of dairy cattle[J]. Journal of Animal Science and Biotechnology, 2016, 7(1): 42. |
| [8] |
刘博铭.茶树油肺吸入制剂的研究[D].硕士学位论文.郑州: 河南大学, 2014.
|
| [9] |
CARSON C F, HAMMER K A, RILEY T V. Melaleuca alternifolia (tea tree) oil:a review of antimicrobial and other medicinal properties[J]. Clinical Microbiology Reviews, 2006, 19(1): 50-62. DOI:10.1128/CMR.19.1.50-62.2006 |
| [10] |
COX S D, MANN C M, MARKHAM J L. Interactions between components of the essential oil of Melaleuca alternifolia[J]. Journal of Applied Microbiology, 2001, 91(3): 492-497. DOI:10.1046/j.1365-2672.2001.01406.x |
| [11] |
KESZEI A, HASSAN Y, FOLEY W J. A biochemical interpretation of terpene chemotypes in Melaleuca alternifolia[J]. Journal of Chemical Ecology, 2010, 36(6): 652-661. |
| [12] |
SHELTON D, ZABARAS D, CHOHAN S, et al. Isolation and partial characterisation of a putative monoterpene synthase from Melaleuca alternifolia[J]. Plant Physiology and Biochemistry, 2004, 42(11): 875-882. DOI:10.1016/j.plaphy.2004.10.010 |
| [13] |
BRAND C, GRIMBALDESTON M A, GAMBLE J R, et al. Tea tree oil reduces the swelling associated with the efferent phase of a contact hypersensitivity response[J]. Inflammation Research, 2002, 51(5): 236-244. DOI:10.1007/PL00000299 |
| [14] |
BRAND C, TOWNLEY S L, FINLAY-JONES J J, et al. Tea tree oil reduces histamine-induced oedema in murine ears[J]. Inflammation Research, 2002, 51(6): 283-289. DOI:10.1007/PL00000305 |
| [15] |
NOGUEIRA M N M, AQUINO S G, ROSSA C, et al. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages[J]. Inflammation Research, 2014, 63(9): 769-778. DOI:10.1007/s00011-014-0749-x |
| [16] |
占今舜, 谷德平, 胡利珍, 等. 苜蓿素对脂多糖刺激下体外培养奶牛乳腺上皮细胞活性和炎症相关基因表达的影响[J]. 动物营养学报, 2018, 30(2): 641-648. |
| [17] |
GONG X X, SU X S, ZHAN K, et al. The protective effect of chlorogenic acid on bovine mammary epithelial cells and neutrophil function[J]. Journal of Dairy Science, 2018, 101(11): 10089-10097. DOI:10.3168/jds.2017-14328 |
| [18] |
詹康.SCFAs通过GPR41调控奶牛瘤胃上皮细胞炎症反应的研究[D].博士学位论文.扬州: 扬州大学, 2018.
|
| [19] |
GOZHO G N, PLAIZIER J C, KRAUSE D O, et al. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response[J]. Journal of Dairy Science, 2005, 88(4): 1399-1403. DOI:10.3168/jds.S0022-0302(05)72807-1 |
| [20] |
KHAFIPOUR E, PLAIZIER J C, AIKMAN P C, et al. Population structure of rumen Escherichia coli associated with subacute ruminal acidosis (SARA) in dairy cattle[J]. Journal of Dairy Science, 2011, 94(1): 351-360. DOI:10.3168/jds.2010-3435 |
| [21] |
HAMMER K A, CARSON C F, RILEY T V, et al. A review of the toxicity of Melaleuca alternifolia (tea tree) oil[J]. Food and Chemical Toxicology, 2006, 44(5): 616-625. DOI:10.1016/j.fct.2005.09.001 |
| [22] |
HAYES A J, LEACH D N, MARKHAM J L, et al. In vitro cytotoxicity of Australian tea tree oil using human cell lines[J]. Journal of Essential Oil Research, 1997, 9(5): 575-582. DOI:10.1080/10412905.1997.9700780 |
| [23] |
NINOMIYA K, HAYAMA K, ISHIJIMA S A, et al. Suppression of inflammatory reactions by terpin-4-ol, a main constituent of tea tree oil, in a murine model of oral candidiasis and its suppressive activity to cytokine production of macrophages in vitro[J]. Biological and Pharmaceutical Bulletin, 2013, 36(5): 838-844. DOI:10.1248/bpb.b13-00033 |
| [24] |
WANG J F, ZHANG X, HE X J, et al. LPS-induced reduction of triglyceride synthesis and secretion in dairy cow mammary epithelial cells via decreased SREBP1 expression and activity[J]. Journal of Dairy Research, 2018, 85(4): 439-444. DOI:10.1017/S0022029918000547 |
| [25] |
JANSSENS S, BEYAERT R. Role of toll-like receptors in pathogen recognition[J]. Clinical Microbiology Reviews, 2003, 16(4): 637-646. DOI:10.1128/CMR.16.4.637-646.2003 |
| [26] |
于子阳.奶牛SARA相关内环境参数变化及黄连素对瘤胃上皮细胞炎症的影响[D].硕士学位论文.长春: 吉林大学, 2016.
|
| [27] |
WULLAERT A, BONNET M C, PASPARAKIS M. NF-κB in the regulation of epithelial homeostasis and inflammation[J]. Cell Research, 2011, 21(1): 146-158. |
| [28] |
任建聪.AEBP1通过NF-κB通路促进腹主动脉瘤发生发展的研究[D].博士学位论文.沈阳: 中国医科大学, 2019.
|
| [29] |
BOULANGER D, BUREAU F, MÉLOTTE D, et al. Increased nuclear factor κb activity in milk cells of mastitis-affected cows[J]. Journal of Dairy Science, 2003, 86(4): 1259-1267. DOI:10.3168/jds.S0022-0302(03)73710-2 |
| [30] |
王婷婷.亚急性瘤胃酸中毒病牛瘤胃组胺对瘤胃上皮细胞炎性通路的影响[D].硕士学位论文.长春: 吉林大学, 2015.
|
| [31] |
TIAN M Y, FAN J H, ZHUANG Z W, et al. Effects of silymarin on p65 NF-κB, p38 MAPK and CYP450 in LPS-induced hoof dermal inflammatory cells of dairy cow[J]. BMC Veterinary Research, 2019, 15(1): 127. DOI:10.1186/s12917-019-1868-y |




