精氨酸(Arg)是20种蛋白质氨基酸中碱性最强的氨基酸,天然存在D型和L型2种精氨酸,1895年首次在哺乳动物蛋白质中发现精氨酸。哺乳动物体内只能代谢L-精氨酸,呈白色晶体或粉末,无臭而味苦,可溶于水,微溶于乙醇,不溶于乙醚。精氨酸是人和大多数哺乳动物的条件性必需氨基酸之一,幼年动物机体不能内源合成精氨酸,属于必需氨基酸[1];对于成年母畜而言,精氨酸属于非必需氨基酸,但在妊娠期、泌乳期或一些应激状态下,机体内源合成的精氨酸不能满足机体代谢的需要,属于必需氨基酸[2]。小肠是非反刍动物精氨酸的主要吸收部位,有40%在肠黏膜上皮细胞中主要被降解为鸟氨酸和脯氨酸,其余60%进入门静脉,经过肝脏后,肝脏中能降解8%精氨酸用于尿素循环和体蛋白质合成,其余进入血液循环发挥生物学功能[3]。精氨酸在动物机体内参与多种组织的多条代谢途径,是动物机体代谢过程中鸟氨酸、尿素、一氧化氮(NO)、多胺、胍基乙酸、肌酸、胍基丁胺等多种物质的前体[3]。NO、多胺、肌酸和胍基丁胺等在细胞增殖分化、内环境稳态的调节中具有广泛的生物学功能。补充精氨酸已被证明可以改善哺乳动物的生殖、心血管、肺脏、肾脏、胃肠、肝脏和免疫等系统功能[3]。NO是哺乳动物主要的血管舒张剂,是胎盘血管生成的主要参与者,母马在妊娠期补喂精氨酸可调控生殖系统血流量,改善胎盘功能,进而改善母马的繁殖力[4-6]。此外,补充精氨酸可调节机体胰岛素分泌和葡萄糖耐受量,改善母体代谢对妊娠的适应[7-9],同时增加妊娠期母体胎盘转移营养物质相关基因的表达[10]。
1 精氨酸的分解代谢精氨酸分解代谢主要有4条途径:精氨酸/肌酸途径、精氨酸/胍基丁胺-多胺途径、精氨酸/NO途径、精氨酸/尿素途径[11-12]。精氨酸在精氨酸-甘氨酸眯基转移酶(AGAT)和胍基乙酸N-甲基转移酶(GAMT)催化下沿精氨酸/肌酸途径代谢合成肌酸;精氨酸沿精氨酸/胍基丁胺-多胺途径分解代谢的产物为胍基丁胺和多胺,胍基丁胺也可转化为尿素和腐胺;精氨酸沿精氨酸/NO途径分解代谢的产物为瓜氨酸和NO,瓜氨酸可经过瓜氨酸/NO循环参与精氨酸的内源合成;精氨酸沿精氨酸/尿素途径分解代谢的产物为尿素和鸟氨酸,鸟氨酸可进一步转化为多胺、脯氨酸和谷氨酰胺等[13-14]。精氨酸的分解代谢途径如图 1所示。
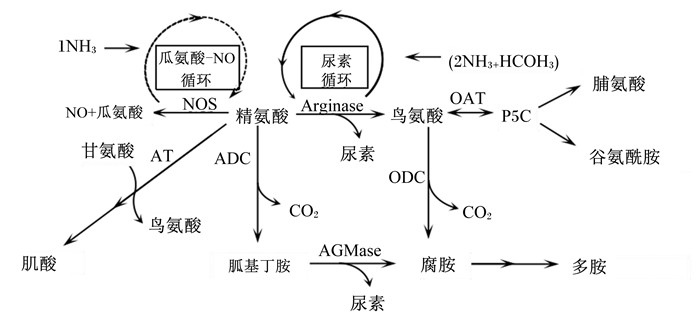
|
NH3:氨气ammonia;NO:一氧化氮nitric oxide;HCO3:碳酸氢根hydrogen carbonate;NOS:一氧化氮合成酶nitric oxide synthase;AT:精氨酸-甘氨酸转移酶arginine-glycine transferase;CO2:二氧化碳carbon dioxide;Arginase:精氨酸脱羧酶arginine decarboxylase;P5C:二氢吡咯-5-羧酸dihydropyrrole-5-carboxylic acid;OAT:鸟氨酸转氨酶ornithine aminotransferase;ADC:精氨酸脱羧酶arginine decarboxylase;ODC:鸟氨酸脱羧酶ornithine decarboxylase;AGMase:胍基丁胺酶agmatinase。 图 1 精氨酸的分解代谢途径 Fig. 1 Arginine catabolism pathway[11] |
AGAT和GAMT是精氨酸/肌酸途径分解的2种限速酶。AGAT主要在肾脏中表达,GAMT主要在肝脏中表达。有研究发现,体外培养的胎盘中可表达并合成AGAT和GAMT[15],胎儿宫内生长受限(IUGR)胎盘中AGAT和GAMT mRNA表达量均显著降低,AGAT、GAMT mRNA表达量均与妊娠期长短、新生儿体重和胎盘重量相关[16]。也有报道孕妇妊娠期间血浆肌酸浓度与胎儿的生长发育有关[17-18],这表明胎盘中AGAT和GAMT的表达可能对胎儿发育至关重要。
2.2 精氨酸/胍基丁胺-多胺途径分解的调节因子精氨酸在精氨酸脱羧酶(ADC)的氧化脱羧作用下生成胍基丁胺,胍基丁胺在胍基丁胺酶(AGMAT)作用下转化为腐胺。精氨酸通过ADC和AGMAT合成多胺为机体组织中多胺合成的次要途径[18]。ADC和AGMAT mRNA表达量的增加能代替绵羊滋养层细胞鸟氨酸脱羧酶(ODC)产生多胺,缺乏ODC会导致ADC/胍基丁胺依赖途径的多胺合成增加[18],表明体内ADC和ODC可以相互补偿另一种途径产生的多胺[19]。由此可见,哺乳动物生殖系统中ADC/AGMAT调节多胺合成对促进胚胎的生长发育具有重要意义。
2.3 精氨酸/NO途径分解的调节因子一氧化氮合成酶(NOS)代谢精氨酸产生NO和瓜氨酸,NOS有3种同工酶:神经元型一氧化氮合成酶(nNOS)、内皮型一氧化氮合成酶(eNOS)和诱导型一氧化氮合成酶(iNOS)[20]。有研究发现,母马受精13 h后,持续性子宫内膜炎(PBIE)母马子宫内膜组织iNOS mRNA表达量显著提高,eNOS和nNOS mRNA表达量无显著差异[21]。PBIE母马子宫内膜组织NOS活性显著高于炎症反应消退的母马,iNOS抑制剂治疗能显著降低PBIE母马子宫内膜组织NOS活性[22],NOS抑制剂对PBIE发病机制的研究具有重要意义。
2.4 精氨酸/尿素途径分解的调节因子精氨酸酶(ARS)是分解机体大部分精氨酸产生尿素和鸟氨酸的主要酶,是由2个不同的基因编码的同工酶:精氨酸酶Ⅰ(ARSⅠ)和精氨酸酶Ⅱ(ARSⅡ)。有研究发现,ARSⅠ可诱导iNOS竞争底物精氨酸,降低ARS对精氨酸的分解,并能抑制iNOS诱导的炎症和氧化应激[23]。在巨噬细胞和其他细胞类型中,ARSⅠ和ARSⅡ之间可能存在相互调控作用[24],且巨噬细胞中ARSⅠ的表达对抑制iNOS表达至关重要[25]。ODC和鸟氨酸转氨酶(OAT)均能分解鸟氨酸生成多胺和二氢吡咯-5-羧酸(P5C)[26],且ODC是催化机体组织精氨酸合成多胺的主要途径[19]。研究也报道多胺合成途径在调节脂质代谢中起重要作用,ODC的表达与甘油三酯储存密不可分[27]。多胺调控细胞增殖和分化[28],胎盘ODC活性降低可能是诱导IUGR的主要原因。
3 精氨酸分解代谢的主要产物 3.1 精氨酸/肌酸途径分解的主要产物精氨酸在AGAT/GAMT催化下沿精氨酸/肌酸途径分解生成胍基乙酸和肌酸。胍基乙酸调节机体生长激素(GH)、胰岛素样生长因子-Ⅰ(IGF-Ⅰ)的分泌;其极性影响胰腺胰岛细胞分泌胰岛素,也通过降低循环血液中的葡萄糖含量来刺激胰岛素分泌[28]。研究表明,胍基乙酸具有间接的抗氧化作用,同时可作为超氧化物,机体内低浓度的胍基乙酸体现抗氧化作用,而高浓度的胍基乙酸可诱导氧化应激[29]。肌酸对妊娠期胎盘和胎儿的发育具有一定的影响,肌酸能改善胎盘线粒体呼吸、缺氧诱导的胎盘线粒体损伤及缺氧条件下胎盘的细胞活力[30]。研究也发现,肌酸对胎盘中ATP的转换起重要作用,可参与维持ATP稳态[15]。
3.2 精氨酸/胍基丁胺-多胺途径分解的主要产物精氨酸在ADC/AGMAT作用下生成胍基丁胺和多胺。研究报道,胍基丁胺是α2-肾上腺素的内源性配体和咪唑啉受体,能刺激肾上腺释放儿茶酚胺,对动物中枢神经系统(CNS)的功能有影响[31]。研究也证明,胍基丁胺是一种新型的神经调节递质,作用于CNS的多个受体[32],对神经类疾病具有很好的治疗效果[33]。在精氨酸及其衍生物的代谢中,胍基丁胺抑制NOS的活性[34],而CNS中NOS的表达上调与抑郁症的发病有关[35]。因此,胍基丁胺能通过对NO信号分子的调节发挥抗抑郁作用[36]。胍基丁胺也作为自由基清除剂,保护线粒体免受氧化应激导致的细胞凋亡[32]。此外,Nissim等[37]研究证明,持续摄入胍基丁胺改善了高脂肪饮食引起的代谢和激素紊乱,这个过程与胍基丁胺诱导腺苷酸环化酶(cAMP)表达上调、激活cAMP依赖的蛋白激酶A(protein kinase A,PKA)信号通路和增加机体对脂肪酸氧化有关。
3.3 精氨酸/NO途径分解的主要产物精氨酸在NOS(eNOS、iNOS、nNOS)作用下生成NO和瓜氨酸。NO是哺乳动物机体组织细胞中至关重要的调节分子。在心血管系统中,NO参与多种细胞因子介导的血管肌层活动,包括子宫肌层、胎盘血管等。研究发现,内源性和外源性NO都参与内皮素-1(ET-1)或精氨酸素(AVP)对非妊娠子宫肌层的调节,ET-1和AVP都能刺激子宫肌层收缩力的增强,具有很强的促血管收缩作用,NO通过降低细胞内钙离子(Ca2+)浓度而缩短ET-1诱导的血管收缩时间,从而诱导血管舒张[38]。研究也发现,NO作用于动物机体组织细胞器线粒体(包括子宫平滑肌),并且是主要的靶细胞器,依赖鸟苷环磷酸(cGMP)通路调节线粒体电子传递链合成ATP,抑制细胞色素c氧化酶活性,维持线粒体pH[39]。利用激光共聚焦显微镜和特异性荧光共定位方法也证实,子宫平滑肌细胞线粒体能合成NO,依赖于Ca2+、还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)和精氨酸浓度[40]。胰岛素、内皮细胞氨基酸转运蛋白-1(amino acid transporter 1,CAT-1)对胎盘血管扩张、胎盘血流灌注等方面的调节主要通过增加精氨酸/NO途径产生NO实现。胰岛素通过上调胎盘内皮细胞质膜CAT-1的表达来提高对精氨酸的转运效率,内皮细胞中精氨酸浓度增加,精氨酸/NO通路活性增强,迅速扩张胎盘血管[41]。同时,胰岛素激活脐静脉内皮细胞中磷脂酰肌醇3-激酶(PI3K)和eNOS表达,也增强精氨酸/NO通路活性,NO含量增加,扩张脐带血管[42]。
3.4 精氨酸/尿素途径分解的主要产物精氨酸在ARS、ODC、OAT等作用下生成尿素、鸟氨酸、多胺等多种物质。结肠中微生物降解饲粮中的蛋白质合成多胺也是机体内多胺的来源之一[43]。细胞多胺水平具有调节真核生物翻译起始因子5A(eukaryotic translation initiation factor 5A,EIF5A)的作用[43-45]。对基因转录的调节可影响巨噬细胞线粒体功能,EIF5A活性降低,线粒体对氧气的需求量和功能降低,导致巨噬细胞向促炎表型方向分化[46];ODC的活性降低和细胞内多胺水平下降可诱导促炎巨噬细胞数量显著增加,导致动物胃和结肠炎症增加[47]。多胺还通过直接改变线粒体功能来影响代谢,线粒体基质高水平的精胺可调节Ca2+进入线粒体影响丙酮酸脱氢酶复合物活性或直接影响线粒体功能[48]。多胺乙酰化过程中,乙酰辅酶A(CoA)都在亚精氨/精氨-N1-乙酰基转移酶(SSAT1)过高表达时被耗尽[43]。多胺乙酰化增加导致ODC活性增强[49],可能提高了精氨酸/尿素途径的代谢量。
多胺也是哺乳动物肠道发育和繁殖过程中的重要物质。早产儿母乳中多胺的浓度明显高于足月出生的婴儿[50];在仔猪饲粮配方中添加多胺可促进肠道发育[51],可见多胺对早期的肠道发育起重要作用。研究表明,在妊娠期,多胺调节血管生成、胚胎形成、胚胎植入和胎盘滋养层生长[52]。在小鼠胚胎发育期,ODC活性随着胚胎进入囊胚期而增加,在子宫上皮细胞中,胚胎滞育时添加腐胺可使胚胎发育正常[53]。多胺影响哺乳动物的繁殖,探讨多胺代谢对胎儿发育及胎盘功能影响的分子机制应是未来研究的重要领域。
4 精氨酸对马生殖系统血流量、血液参数和胎盘功能的影响 4.1 对母马生殖系统血流量的影响老龄的经产母马生殖系统血流量显著低于青年母马;发情周期的母马,第1~10天子宫肌层血流量显著低于第15、20天[6],母马生殖系统血流量下降是造成母马妊娠率低及早期胚胎损失增加的重要因素之一。多普勒超声(doppler ultrasonography, DPL)检测方法是评估母马生殖系统血流量的常用方法,阻力指数(resistance index, RI)和搏动指数(pulsatility index, PI)是其常用指数[54]。RI和PI与组织内血管血流灌注程度有关,RI高表明血流灌注少,RI低表明组织血流量增加;PI升高表明远端组织血流灌注减少[54]。RI和PI相关,其中一个指数就能评估母马机体组织血流灌注程度。补喂适量的精氨酸可影响母马生殖系统血流量。围产期母马补喂100 g/d精氨酸,子宫动脉血流量极显著高于与对照组,且母马可测子宫积液短时间内出现下降[4];母马卵泡壁血管血流灌注显著高于对照组,子宫积液更少[5];饲粮每天添加0.5% BW精氨酸,母马子宫液蓄积减少[55]。发情期间补喂100 g/d精氨酸,母马优势卵泡直径显著增大[56]。此外,在妊娠第15~45天期间每天补喂0.012 5% BW精氨酸,第40~45天胚胎/胎儿的直径增大[57];妊娠期最后4个月期间补喂100 g/d精氨酸,可使出生小马驹体重增加[58]。虽未测量RI或PI,但这与精氨酸分解代谢调控生殖系统血流量从而改善母马繁殖力密不可分。
4.2 对马血液参数的影响饲粮中补喂不同剂量的精氨酸可以影响马的血液参数,主要体现在精氨酸是机体NOS的唯一底物、精氨酸促进尿素循环及精氨酸和其他氨基酸的吸收代谢平衡方面。饲粮添加适量的精氨酸可以改变马体循环血浆NO浓度[59],NO是机体重要的调节分子;精氨酸是尿素循环中最关键的氨基酸之一,饲粮添加适量精氨酸促进尿素循环,同时提高机体氮利用率,促进机体氨基酸代谢平衡,降低血浆氨气(NH3)、尿素氮(UN)含量,促进疲劳恢复,可见精氨酸是维持机体内环境稳态的重要氨基酸[59]。此外,补喂精氨酸可影响马体循环血浆精氨酸浓度和其他氨基酸浓度。每天补喂0.025% BW精氨酸可显著增加血浆精氨酸和鸟氨酸浓度,显著降低赖氨酸和蛋氨酸浓度,补喂后1 h,母马血浆中组氨酸、谷氨酸、脯氨酸、异亮氨酸、苏氨酸、苯丙氨酸、亮氨酸、缬氨酸、丙氨酸和牛磺酸等浓度显著降低[65]。每天补喂0.012 5% BW精氨酸显著增加了血浆精氨酸和鸟氨酸浓度,对其他氨基酸浓度无显著影响[60]。研究也表明,妊娠期母马补喂100 g/d精氨酸,血浆精氨酸和鸟氨酸浓度在补喂后6 h显著或极显著提高(精氨酸浓度增加约1倍,鸟氨酸浓度增加约50%)[10];泌乳期母马补喂50 g/d精氨酸对泌乳母马及小马驹血液精氨酸代谢物浓度无显著影响[61]。由此可见,补喂精氨酸影响母马体循环血浆精氨酸、鸟氨酸浓度,同时影响其他氨基酸代谢,但补喂高剂量精氨酸会损害其他氨基酸的吸收。研究也报道,补喂精氨酸影响妊娠后期母马体循环血浆胰岛素和葡萄糖浓度[10]。因此,精氨酸在马健康养殖应用中的补喂范围仍需进一步探讨。
4.3 对母马胎盘功能的影响初产母马产下的新生马驹比经产母马产下的新生马驹更小或更轻,这与母马胎盘结构、胎盘生长因子、胎盘生长因子受体、胎盘血管生成因子及胎盘对母体营养物质的转运能力有关[62-64]。初产母马妊娠后210 d至分娩期间补喂100 g/d精氨酸,结果表明,精氨酸补喂对胎盘结构、超微结构及胎盘中DNA甲基化无显著影响,但胎盘中营养转运因子[葡萄糖转运蛋白-1(GLUT-1)、CD36]和GAMT基因表达量增加[10]。GLUT-1基因表达量增加可能使母体-胎儿葡萄糖转移效率提高,或补喂精氨酸对初产母马胰岛素分泌起到了调节作用;胎盘CD36基因表达量的增加可能影响胎盘转运脂肪酸或影响脂肪酸在胎盘中的利用和储存。初产母马与经产母马相比,初产母马胎盘尿囊组织成分(结缔组织和血管)体积和表面积下降,血管内皮生长因子(VEGF)基因表达量减少,DNA整体甲基化减少;参与精氨酸降解(ARS、ODC)和转运因子[肠道溶质载体家族7成员1(SLC7A1)]的基因表达量增加[10]。此外,精氨酸作为NO气体信号分子的唯一前体,在胎盘功能稳态维持、促进胎盘形成等方面发挥至关重要的作用[65],同时调节胎盘中多胺的合成[20]。由此可见,精氨酸是完善初产母马胎盘功能的主要氨基酸之一。
5 小结精氨酸作为功能最多的氨基酸之一,与精氨酸在动物机体内具有多条代谢途径生成多种生物活性分子有关。NO和多胺促进子宫、胎盘、卵巢等部位血管化发生,可增加母马生殖系统血流量,同时调节免疫系统功能;胍基乙酸、肌酸、胍基丁胺等对机体神经系统、内分泌系统、能量系统都具有调节作用。补喂适量的精氨酸可调节母马机体新陈代谢、生殖系统功能,从而改善母马的繁殖力。精氨酸对母马生殖功能影响的分子机制研究相对较少,仍需进一步探讨,以便为精氨酸在马健康养殖中的应用提供合理的依据。
| [1] |
BANSAL V, OCHOA J B. Arginine availability, arginase, and the immune response[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2003, 6(2): 223-228. DOI:10.1097/00075197-200303000-00012 |
| [2] |
WU G Y, KNABE D A, KIM S W. Arginine nutrition in neonatal pigs[J]. The Journal of Nutrition, 2004, 134(10): 2783S-2790S. DOI:10.1093/jn/134.10.2783S |
| [3] |
WU G Y, BAZER F W, DAVIS T A, et al. Arginine metabolism and nutrition in growth, health and disease[J]. Amino Acids, 2009, 37(1): 153-168. DOI:10.1007/s00726-008-0210-y |
| [4] |
MORTENSEN C J, KELLEY D E, WARREN L K. Supplemental L-arginine shortens gestation length and increases mare uterine blood flow before and after parturition[J]. Journal of Equine Veterinary Science, 2011, 31(9): 514-520. DOI:10.1016/j.jevs.2011.01.004 |
| [5] |
CHAVATTE-PALMER P, DERISOUD E, ROBLES M, et al. Effects of dietary arginine supplementation in pregnant mares on maternal metabolism and foal birthweight[J]. Journal of Equine Veterinary Science, 2018, 66: 225. DOI:10.1016/j.jevs.2018.05.112 |
| [6] |
MESA A M, WARREN L K, SHEEHAN J M, et al. L-Arginine supplementation 0.5% of diet during the last 90 days of gestation and 14 days postpartum reduced uterine fluid accumulation in the broodmare[J]. Animal Reproduction Science, 2015, 159: 46-51. DOI:10.1016/j.anireprosci.2015.05.011 |
| [7] |
MULLOOL N, VERNON W, SMITH D M, et al. Elevated levels of branched-chain amino acids have little effect on pancreatic islet cells, but L-arginine impairs function through activation of the endoplasmic reticulum stress response[J]. Experimental Physiology, 2014, 99(3): 538-551. DOI:10.1113/expphysiol.2013.077495 |
| [8] |
MONTI L D, SETOLA E, LUCOTTI P C G, et al. Effect of a long-term oral L-arginine supplementation on glucose metabolism:a randomized, double-blind, placebo-controlled trial[J]. Diabetes, Obesity and Metabolism, 2012, 14(10): 893-900. DOI:10.1111/j.1463-1326.2012.01615.x |
| [9] |
BOON M R, HANSSEN M J W, BRANS B, et al. Effect of L-arginine on energy metabolism, skeletal muscle and brown adipose tissue in South Asian and Europid prediabetic men:a randomised double-blinded crossover study[J]. Diabetologia, 2019, 62(1): 112-122. DOI:10.1007/s00125-018-4752-6 |
| [10] |
ROBLES M, COUTURIER-TARRADE A, DERISOUD E, et al. Effects of dietary arginine supplementation in pregnant mares on maternal metabolism, placental structure and function and foal growth[J]. Scientific Reports, 2019, 9: 6461. DOI:10.1038/s41598-019-42941-0 |
| [11] |
MORRIS S M, J r. Recent advances in arginine metabolism[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2004, 7(1): 45-51. DOI:10.1097/00075197-200401000-00009 |
| [12] |
MEESSEN J. Urea synthesis[J]. Chemie Ingenieur Technik, 2014, 86(12): 2180-2189. DOI:10.1002/cite.201400064 |
| [13] |
EDISON E E, BROSNAN M E, MEYER C, et al. Creatine synthesis:production of guanidinoacetate by the rat and human kidney in vivo[J]. American Journal of Physiology-Renal Physiology, 2007, 293(6): F1799-F1804. DOI:10.1152/ajprenal.00356.2007 |
| [14] |
CHOE C U, NABUURS C, STOCKEBRAND M C, et al. L-arginine:glycine amidinotransferase deficiency protects from metabolic syndrome[J]. Human Molecular Genetics, 2013, 22(1): 110-123. DOI:10.1093/hmg/dds407 |
| [15] |
ELLERY S J, GATTA P A D, BRUCE C R, et al. Creatine biosynthesis and transport by the term human placenta[J]. Placenta, 2017, 52: 86-93. DOI:10.1016/j.placenta.2017.02.020 |
| [16] |
ELLERY S J, MURTHI P, DAVIES M L, et al. Placental creatine metabolism in cases of placental insufficiency and reduced fetal growth[J]. Molecular Human Reproduction, 2019, 25(8): 495-505. DOI:10.1093/molehr/gaz039 |
| [17] |
DICKINSON H, DAVIES-TUCK M, ELLERY S J, et al. Maternal creatine in pregnancy:a retrospective cohort study[J]. An International Journal of Obstetrics and Gynaecology, 2016, 123(11): 1830-1838. DOI:10.1111/1471-0528.14237 |
| [18] |
PINTO J, BARROS A S, DOMINGUES M R M, et al. Following healthy pregnancy by NMR metabolomics of plasma and correlation to urine[J]. Journal of Proteome Research, 2015, 14(2): 1263-1274. DOI:10.1021/pr5011982 |
| [19] |
LENIS Y Y, JOHNSON G A, WANG X Q, et al. Functional roles of ornithine decarboxylase and arginine decarboxylase during the peri-implantation period of pregnancy in sheep[J]. Journal of Animal Science and Biotechnology, 2018, 9: 10. DOI:10.1186/s40104-017-0225-x |
| [20] |
KHAN F A, SCHOLTZ E L, CHENIER T S. The nitric oxide system in equine reproduction:current status and future directions[J]. Journal of Equine Veterinary Science, 2015, 35(6): 481-487. DOI:10.1016/j.jevs.2015.02.009 |
| [21] |
ALGHAMDI A S, FOSTER D N, CARLSON C S, et al. Nitric oxide levels and nitric oxide synthase expression in uterine samples from mares susceptible and resistant to persistent breeding-induced endometritis[J]. American Journal of Reproductivgfe Immunology, 2005, 53(5): 230-237. DOI:10.1111/j.1600-0897.2005.00270.x |
| [22] |
KHAN F A, CHENIER T S, FOSTER R A, et al. Endometrial nitric oxide synthase activity in mares susceptible or resistant to persistent breeding-induced endometritis and the effect of a specific iNOS inhibitor in vitro[J]. Reproduction in Domestic Animals, 2018, 53(3): 718-724. DOI:10.1111/rda.13162 |
| [23] |
FOUDA A Y, XU Z M, SHOSHA E, et al. Arginase 1 promotes retinal neurovascular protection from ischemia through suppression of macrophage inflammatory responses[J]. Cell Death & Disease, 2018, 9(10): 1001. |
| [24] |
HARDBOWER D M, ASIM M, MURRAY-STEWART T, et al. Arginase 2 deletion leads to enhanced M1 macrophage activation and upregulated polyamine metabolism in response to Helicobacter pylori infection[J]. Amino Acids, 2016, 48(10): 2375-2388. DOI:10.1007/s00726-016-2231-2 |
| [25] |
SUWANPRADID J, SHIH M, PONTIUS L, et al. Arginase1 deficiency in monocytes/macrophages upregulates inducible nitric oxide synthase to promote cutaneous contact hypersensitivity[J]. The Journal of Immunology, 2017, 199(5): 1827-1834. DOI:10.4049/jimmunol.1700739 |
| [26] |
CALDWELL R W, RODRIGUEZ P C, TOQUE H A, et al. Arginase:a multifaceted enzyme important in health and disease[J]. Physiological Reviews, 2018, 98(2): 641-665. DOI:10.1152/physrev.00037.2016 |
| [27] |
LEON K E, FRUIN A M, NOWOTARSKI S L, et al. The regulation of triglyceride storage by ornithine decarboxylase (Odc1) in Drosophila[J]. Biochemical and Biophysical Research Communications, 2020, 523(2): 429-433. DOI:10.1016/j.bbrc.2019.12.078 |
| [28] |
XIE Y F, DONG C D, WU Q, et al. Ornithine decarboxylase inhibition downregulates multiple pathways involved in the formation of precancerous lesions of esophageal squamous cell cancer[J]. Molecular Carcinogenesis, 2020, 59(2): 215-226. DOI:10.1002/mc.23144 |
| [29] |
王誉杰, 张进威, 王讯, 等. 胍基乙酸及代谢产物肌酸的研究进展[J]. 畜牧兽医学报, 2018, 49(8): 1577-1584. WANG Y J, ZHANG J W, WANG X, et al. The research progress of guanidinoacetic acid and creatine[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(8): 1577-1584 (in Chinese). |
| [30] |
MUCCINI A M, COX A G, MARSHALL S, et al. Creatine as a treatment for hypoxic-induced mitochondrial injury in the human placenta[J]. Journal of Paediatrics and Child Health, 2019, 55: 38. |
| [31] |
AKASAKA N, FUJIWARA S. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae[J]. Amino Acids, 2020, 52(2): 181-197. DOI:10.1007/s00726-019-02720-7 |
| [32] |
BARUA S, KIM J Y, KIM J Y, et al. Therapeutic effect of agmatine on neurological disease:focus on ion channels and receptors[J]. Neurochemical Research, 2019, 44(4): 735-750. DOI:10.1007/s11064-018-02712-1 |
| [33] |
KANG S, KIM C H, JUNG H, et al. Agmatine ameliorates type 2 diabetes induced-Alzheimer's disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling[J]. Neuropharmacology, 2017, 113: 467-479. DOI:10.1016/j.neuropharm.2016.10.029 |
| [34] |
SATRIANO J. Arginine pathways and the inflammatory response:interregulation of nitric oxide and polyamines:review article[J]. Amino Acids, 2004, 26(4): 321-329. DOI:10.1007/s00726-004-0078-4 |
| [35] |
KUDLOW P, CHA D S, CARVALHO A F, et al. Nitric oxide and major depressive disorder:pathophysiology and treatment implications[J]. Current Molecular Medicine, 2016, 16(2): 206-215. DOI:10.2174/1566524016666160126144722 |
| [36] |
GAWALI N B, BULANI V D, GURSAHANI M S, et al. Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviours and cognitive impairment by modulating nitrergic signalling pathway[J]. Brain Research, 2017, 1663: 66-77. DOI:10.1016/j.brainres.2017.03.004 |
| [37] |
NISSIM I, HOEYN O, DAIKHIN Y, et al. The molecular and metabolic influence of long term agmatine consumption[J]. Journal of Biological Chemistry, 2014, 289(14): 9710-9729. DOI:10.1074/jbc.M113.544726 |
| [38] |
MODZELEWSKA B, JÓŹWIK M, JÓŹWIK M, et al. The effects of extended nitric oxide release on responses of the human non-pregnant myometrium to endothelin-1 or vasopressin[J]. Pharmacological Reports, 2019, 71(5): 892-898. DOI:10.1016/j.pharep.2019.05.003 |
| [39] |
GHIMIRE K, ALTMANN H M, STRAUB A C, et al. Nitric oxide:what's new to NO?[J]. American Journal of Physiology Cell Physiology, 2017, 312(3): C254-C262. DOI:10.1152/ajpcell.00315.2016 |
| [40] |
DANYLOVYCH H V, DANYLOVYCH Y V, GULINA M O, et al. NO-synthase activity in mitochondria of uterus smooth muscle:identification and biochemical properties[J]. General Physiology and Biophysics, 2019, 38(1): 39-50. DOI:10.4149/gpb_2018034 |
| [41] |
CABRERA L, SAAVEDRA A, ROJAS S, et al. Insulin induces relaxation and decreases hydrogen peroxide-induced vasoconstriction in human placental vascular bed in a mechanism mediated by calcium-activated potassium channels and L-arginine/nitric oxide pathways[J]. Frontiers in Physiology, 2016, 7: 529. |
| [42] |
GONZÁLEZ M, FLORES C, PEARSON J D, et al. Cell signalling-mediating insulin increase of mRNA expression for cationic amino acid transporters-1 and -2 and membrane hyperpolarization in human umbilical vein endothelial cells[J]. Pflügers Archiv, 2004, 448(4): 383-394. DOI:10.1007/s00424-004-1261-x |
| [43] |
BEKEBREDE A F, KEIJER J, GERRITS W J J, et al. The molecular and physiological effects of protein-derived polyamines in the intestine[J]. Nutrients, 2020, 12(1): 197. DOI:10.3390/nu12010197 |
| [44] |
SCHULLER A P, WU C C C, DEVER T E, et al. eIF5A functions globally in translation elongation and termination[J]. Molecular Cell, 2017, 66(2): 194-205. DOI:10.1016/j.molcel.2017.03.003 |
| [45] |
IVAYLO P I, SHIN B S, LOUGHRAN G, et al. Polyamine control of translation elongation regulates start site selection on antizyme inhibitor mRNA via ribosome queuing[J]. Molecular Cell, 2018, 70(2): 254-264. DOI:10.1016/j.molcel.2018.03.015 |
| [46] |
PULESTON D J, BUCK M D, KLEIN G R I, et al. Polyamines and eIF5A hypusination modulate mitochondrial respiration and macrophage activation[J]. Cell Metabolism, 2019, 30(2): 352-363. DOI:10.1016/j.cmet.2019.05.003 |
| [47] |
HARDBOWER D M, ASIM M, LUIS P B, et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(5): E751-E760. DOI:10.1073/pnas.1614958114 |
| [48] |
PEZZATO E, BATTAGLIA V, BRUNATI A M, et al. Ca2+-independent effects of spermine on pyruvate dehydrogenase complex activity in energized rat liver mitochondria incubated in the absence of exogenous Ca2+ and Mg2+[J]. Amino Acids, 2009, 36(3): 449-456. DOI:10.1007/s00726-008-0099-5 |
| [49] |
JELL J, MERALI S, HENSEN M L, et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA[J]. Journal of Biological Chemistry, 2007, 282(11): 8404-8413. DOI:10.1074/jbc.M610265200 |
| [50] |
PLAZA-ZAMORA J, SABATER-MOLINA M, RODRÍGUEZ-PALMERO M, et al. Polyamines in human breast milk for preterm and term infants[J]. British Journal of Nutrition, 2013, 110(3): 524-528. DOI:10.1017/S0007114512005284 |
| [51] |
FANG T T, LIU G M, CAO W, et al. Spermine:new insights into the intestinal development and serum antioxidant status of suckling piglets[J]. RSC Advances, 2016, 6(37): 31323-31335. DOI:10.1039/C6RA05361K |
| [52] |
HUSSAIN T, TAN B E, REN W K, et al. Exploring polyamines:Functions in embryo/fetal development[J]. Animal Nutrition, 2017, 3(1): 7-10. DOI:10.1016/j.aninu.2016.12.002 |
| [53] |
FENELON J C, BANERJEE A, LEFEVRE P, et al. Polyamine-mediated effects of prolactin dictate emergence from mink obligate embryonic diapause[J]. Biology of Reproduction, 2016, 95(1): 6. DOI:10.1095/biolreprod.116.139204 |
| [54] |
BOLLWEIN H, MAIERL J, MAYER R, et al. Transrectal color Doppler Sonography of the A.uterina in cyclic mares[J]. Theriogenology, 1998, 49(8): 1483-1488. DOI:10.1016/S0093-691X(98)00094-6 |
| [55] |
BARR F.O.J. Ginther (Ed.), ultrasonic imaging and animal reproduction:color-doppler ultrasonography-book 4, equiservices publishing, cross plains 2007, ISBN:0964007282, 258pp; £60.00(hard)[J]. The Veterinary Journal, 2008, 175(2): 284-285. |
| [56] |
KELLEY D E, WARREN L K, MORTENSEN C J. Oral L-arginine supplementation impacts several reproductive parameters during the postpartum period in mares[J]. Animal Reproduction Science, 2013, 138(3/4): 233-240. |
| [57] |
KELLEY D, LEBLANC M M, WARREN L K, et al. Influence of L-arginine supplementation on reproductive blood flow and embryo recovery rates in mares[J]. Theriogenology, 2014, 81(5): 752-757. DOI:10.1016/j.theriogenology.2013.12.012 |
| [58] |
AURICH J, KÖHNE M, WULF M, et al. Effects of dietary L-arginine supplementation to early pregnant mares on conceptus diameter-preliminary findings[J]. Reproduction in Domestic Animals, 2019, 54(5): 772-778. DOI:10.1111/rda.13422 |
| [59] |
张仕琦, 李晓斌, 张文杰, 等. 补喂L-精氨酸或N-氨甲酰谷氨酸对伊犁马运动性能和血浆生化指标的影响[J]. 动物营养学报, 2019, 31(7): 3188-3196. ZHANG S Q, LI X B, ZHANG W J, et al. Effects of Supplemented with L-arginine or N-carbamylglutamate on athletic performance and plasma biochemical indexes of Yili horses[J]. Chinese Journal of Animal Nutrition, 2019, 31(7): 3188-3196 (in Chinese). DOI:10.3969/j.issn.1006-267x.2019.07.030 |
| [60] |
KELLEY D E, WARREN L K, MORTENSEN C J. Orally supplemented L-arginine impairs amino acid absorption depending on dose in horses[J]. Journal of Animal Science, 2014, 92(12): 5560-5566. DOI:10.2527/jas.2014-7690 |
| [61] |
HUNKA M M, DE SILVA E R R, KUTSCHENKO M, et al. Effects of L-arginine supplementation on lactating mares and the development of foals[J]. Acta Scientiae Veterinariae, 2016, 44(1): 1352. |
| [62] |
ROBLES M, DUBOIS C, GAUTIER C, et al. Maternal parity affects placental development, growth and metabolism of foals until 1 year and a half[J]. Theriogenology, 2018, 108: 321-330. DOI:10.1016/j.theriogenology.2017.12.019 |
| [63] |
KLEWITZ J, STRUEBING C, ROHN K, et al. Effects of age, parity, and pregnancy abnormalities on foal birth weight and uterine blood flow in the mare[J]. Theriogenology, 2015, 83(4): 721-729. DOI:10.1016/j.theriogenology.2014.11.007 |
| [64] |
ROBLES M, PEUGNET P M, VALENTINO S A, et al. Placental structure and function in different breeds in horses[J]. Theriogenology, 2018, 108: 136-145. DOI:10.1016/j.theriogenology.2017.11.007 |
| [65] |
GUERRA D D, HURT K J. Gasotransmitters in pregnancy:from conception to uterine involution[J]. Biology of Reproduction, 2019, 101(1): 4-25. DOI:10.1093/biolre/ioz038 |




