霉菌毒素是由霉菌产生的有毒次级代谢产物,广泛存在于饲料原料和人类食品中,严重威胁着动物健康和人类食品安全。霉菌毒素污染是世界上多个国家都面临的重大问题,全球每年有很多食品或饲料受到不同程度的霉菌毒素污染[1]。在中国,霉菌毒素污染的形势也尤为严峻。Yang等[2]发布的最新报道显示,中国的玉米和玉米制品中霉菌毒素的检出率较高,并且检出率排在前3位的霉菌毒素分别是脱氧雪腐镰刀菌烯醇(deoxynivalenol,DON)、黄曲霉毒素B1(aflatoxin B1,AFB1)和玉米赤霉烯酮(zearalenone,ZEN)。DON是众多霉菌毒素中污染范围最广、检出频率最高的一种霉菌毒素,因此,DON污染是一个重大的全球性食品安全问题。
DON是由禾谷镰刀菌(F. graminearum)和黄色镰刀菌(F. culmorum)等产生的一种具有毒害作用的真菌毒素,其广泛存在于小麦、大麦、燕麦、玉米等谷物中,严重威胁着食品和饲料安全。DON对人和动物有很强的毒性,急性中毒能引起呕吐,而长期接触低浓度DON可引起厌食、体重下降、腹泻、肠黏膜损坏以及免疫系统损伤[3-4]。DON摄入能够使动物产生氧化应激反应,进而导致DNA损伤和细胞凋亡[5]。DON还能够改变神经内分泌信号传导、诱导促炎基因表达、破坏生长激素轴和改变肠道的完整性[4]。此外,动物的食欲中枢也是DON致机体损伤的重要靶点,它可造成动物采食量下降,产生厌食或呕吐反应。厌食和呕吐反应是非常复杂的过程,它由神经中枢、胃肠激素以及肠道微生物等元素共同进行调控。目前在学术界对于DON引起动物厌食和呕吐反应的机制已有一些报道,本文从食欲调控中枢、食欲调节激素、炎症细胞因子以及肠道微生物这4个方面总结了DON诱导动物产生厌食和呕吐反应的可能途径,阐述了肠道微生物与DON诱导的动物厌食和呕吐反应之间的潜在联系,为更深层次研究DON的毒性机制奠定基础,也为寻找DON解毒剂提供新的思路。
1 DON诱导动物产生厌食和呕吐DON具有神经毒性,能够改变神经内分泌信号传导,对脑部产生毒性作用,导致动物产生厌食反应[6]。猪采食受到低浓度DON(1~2 mg/kg)污染的饲粮可导致采食量和日增重下降,引起部分厌食,而当摄入饲粮的DON浓度达到12 mg/kg时就会引起完全厌食反应[7-8]。Meta分析显示,饲粮中含有约3.63 mg/kg的DON可导致生长猪采食量和日增重均下降26%,并且猪的采食量随饲粮中DON浓度(0.02~2.50 mg/kg)的增加而线性降低[9-10]。最新研究显示,28日龄断奶仔猪连续28 d摄入含有1.28或2.89 mg/kg DON的饲粮,可造成其采食量和日增重显著降低[11]。此外,除了以猪为模型研究DON的厌食作用外,部分学者还利用大鼠和小鼠建立模型研究DON的毒性作用。大鼠在摄入0.25~1.00 mg/kg DON污染饲粮持续9周后,可明显观察到大鼠采食量降低以及体重下降[12]。给小鼠连续7~14 d饲喂0.35~3.00 mg/kg DON污染饲粮,可造成小鼠采食量和体重显著降低[13-14]。另外,小鼠腹腔注射和灌胃模型均证明了DON的厌食作用。按体重给小鼠腹腔注射1.0或2.5 mg/kg DON,在注射0.5~3.0 h后小鼠出现厌食反应,并且呈剂量依赖性,但在6 h后小鼠采食量逐渐恢复,至16 h时观察到了剂量依赖性促食欲反应,因此短期DON暴露对动物食物摄入的影响是短暂的,并且短期DON暴露有一定的促食欲作用[15-16],灌胃模型小鼠试验也证实了这一理论,按体重给小鼠灌胃6.25、12.50或25.00 mg/kg的DON,在灌胃后的0~3 h内小鼠采食量显著降低,而在6 h后小鼠采食量逐渐增加,在18~24 h时各试验组小鼠采食量均高于对照组,出现了促食欲的作用;但从整个试验周期的统计数据可知,DON有明显的厌食作用[17]。
呕吐反射是动物在摄取有毒食物后机体产生的自我保护机制。DON摄入对人和动物有着强烈的催吐作用,呕吐是DON致人和动物损伤的主要症状之一[18]。慢性低浓度摄入DON,可使机体产生厌食反应、体重增加缓慢甚至下降以及生长激素失调;而急性高浓度摄入DON可引起机体肠胃炎症,产生呕吐反应甚至是休克[19]。不同动物对DON的敏感性不同,物种的差异导致DON吸收、分布以及代谢有着很大差异,动物对DON敏感性从大到小依次为猪、小鼠、大鼠、家禽、反刍动物[20]。早在1977年,Forsyth等[21]建立了DON致猪呕吐的模型,研究结果显示对于9~10 kg的仔猪,按每千克体重口服0.1~0.2 mg DON或者按照每千克体重腹腔注射0.05 mg DON即可引起仔猪的呕吐反应。而对于15~20 kg的仔猪,按体重静脉注射80 μg/kg DON或口服300 μg/kg DON则可观测到仔猪发生呕吐[22]。除了口服或注射一定剂量DON可使动物产生呕吐反应外,动物采食受到DON污染的饲料也会导致动物发生呕吐。仔猪食用含有约20 mg/kg DON的饲粮即可引起仔猪呕吐,当摄入饲粮中DON浓度为12 mg/kg时会引起仔猪拒食,而饲粮中含有仅1.3 mg/kg DON就会引起仔猪采食量下降和体重增加速度变缓[23]。
2 DON对下丘脑食欲调控中枢的影响动物的采食量或食欲受到外周神经和下丘脑中枢神经系统的调控。来自肠道、胰腺、肝脏或脂肪组织的外周信号直接或间接聚集于下丘脑,形成脏器、组织、脑干和下丘脑之间的通信网络,下丘脑和脑干则整合外周激素和中枢神经递质信号并通过高级皮质中枢调控食欲,从而影响肠胃运动、激素分泌以及机体的能量平衡[24]。下丘脑弓状核(arcuate nucleus,ARC)在食欲调控中发挥着重要作用,其与下丘脑腹内侧核(ventromedial nucleus,VMN)、背中核(dorsomedial nucleus,DMN)、室旁核(paraventricular nucleus,PVN)、外侧下丘脑(lateral hypothalamic area,LHA)、视交叉上核(suprachiasmatic nucleus,SCN)等共同调控动物的食欲。ARC是中枢食欲调节神经网络的核心,其包含2类主要的神经元,一类是促食欲神经元神经肽Y(neuropeptide,NPY)/刺鼠相关蛋白(agouti-related peptide,AGRP),另一类是抑食欲神经元阿片黑素皮质素原(pro-opiomelanocortin,POMC)/可卡因-安菲他明调节转录物(cocaine-amphetamine-regulated transcript,CART)[25]。
DON诱导的动物厌食反应与其对大脑的毒性有关,按体重给小鼠灌服25 mg/kg DON,在5 min后便可在大脑内检测到(0.8±0.1) μg/g DON,这表示DON可迅速转移至大脑并对其产生毒性作用,这也暴露了毒素干扰正常食欲调节的可能性[26]。促食欲神经元和抑食欲神经元共同调控动物食欲。在肥胖小鼠模型中,小鼠连续7周摄入含有10 mg/kg DON的饲粮后,可明显观察到下丘脑中AGRP mRNA表达水平上升,并且POMC mRNA表达水平也呈上升趋势[27]。小鼠按体重急性灌胃12.5 mg/kg DON 3 h后,下丘脑POMC及其下游基因黑皮质素受体4(melanocortin 4 receptor,MC4R)、脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)、酪氨酸蛋白激酶受体B(tyrosine kinase receptor B,TrkB)的mRNA表达水平显著升高,同时促肾上腺皮质激素释放激素(corticotrophin-releasing hormone,CRH)mRNA表达水平呈上升趋势,但对下丘脑NPY和AGRP的mRNA表达水平没有显著影响,这表示DON调节厌食途径而不影响摄食途径[17]。然而,物种差异可能导致DON诱导的厌食反应机制也不一致,给仔猪饲喂含2.89 mg/kg DON的饲粮持续28 d,仔猪下丘脑POMC的蛋白表达水平显著增加,而NPY的蛋白表达水平显著下降,显示DON既能够对厌食途径产生影响,也能够对摄食途径产生影响[28]。然而,以猪为模型研究DON对厌食和摄食中枢调控的报道较少,DON诱导的厌食机制在不同物种之间的差异有待进一步研究。
5-羟色胺(serotonin,5-HT),又称血清素,其作为神经递质参与调控动物的食欲。研究显示,动物的摄食量和5-HT浓度之间呈负相关,并且5-HT还与抑食欲和促食欲的多肽有着紧密的关联,是POMC、α-黑素细胞刺激素(α-melanocyte-stimulating hormone,α-MSH)、NPY以及AGRP的重要上游调节因子[29]。按体重给小鼠灌胃2.5 mg/kg DON,在24 h后小鼠小脑和下丘脑中的5-HT浓度显著增加[30]。此外,按体重急性给药0.25 mg/kg DON 8 h后便可观察到猪下丘脑中的5-HT浓度显著升高[31]。以水貂为模型研究DON诱导呕吐反应机制的研究显示,根据体重给水貂腹腔注射0.10或0.25 mg/kg DON后,在15~30 min内可观察到呕吐反应,并且在60 min后水貂血浆5-HT浓度显著升高,用5-HT3受体拮抗剂进行预处理后可完全抑制DON诱导的呕吐反应[32],并且DON能够激活钙敏感受体(calcium-sensing receptor,CaSR)和瞬时受体电位离子通道1(transient receptor potential ankyrin 1,TRPA1),从而诱导肠嗜铬细胞(enterochromaffin cell,EC细胞)释放5-HT(图 1)[33]。以猪为模型的研究显示,猪摄入DON后血浆中5-HT及其代谢物5-羟基吲哚乙酸的浓度显著增加[34],而5-HT3受体拮抗剂可抑制DON诱导的猪呕吐反应[22],因此DON诱导的呕吐作用与5-HT途径有关,其机制可能是,5-HT3受体受到DON的刺激后引起细胞外钙离子(Ca2+)通过5-HT3Rs/L型Ca2+通道进入细胞内,从而增加细胞内Ca2+浓度。细胞内Ca2+浓度的升高激活了钙调素依赖性蛋白激酶Ⅱa(calmodulin dependent protein kinases Ⅱa,CaMKⅡa)和细胞外信号调节蛋白激酶1/2(extracellular regulated kinase 1/2,ERK1/2),促进动物产生呕吐反应[35]。另外,5-HT调控食欲的相关受体主要有5-HT1BR、5-HT2CR和MC4R,黑素皮质素与MC4R结合后参与5-HT2CR对食欲的调控[36],5-HT与5-HT2CR结合能够激活POMC,促进其表达,并产生α-MSH,从而抑制动物的食欲[37]。5-HT1BR能够抑制促食欲神经元NPY/AGRP的活性,从而抑制动物的食欲[29, 38]。因此,5-HT及其受体均参与DON诱导的动物食欲下降和呕吐反应,进而导致动物拒食和体重降低。
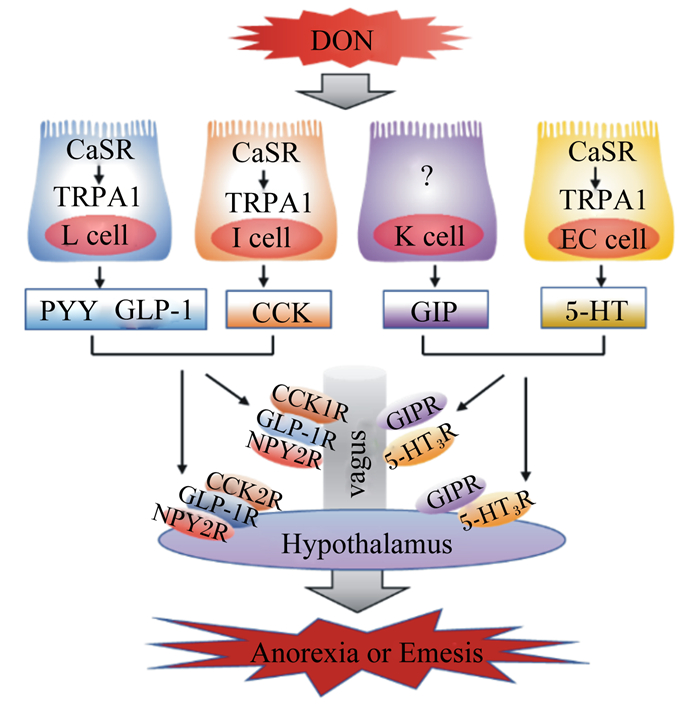
|
DON:脱氧雪腐镰刀菌烯醇 deoxynivalenol;CaSR:钙敏感受体 calcium-sensing receptor;TRPA1:瞬时受体电位离子通道 1 transient receptor potential ankyrin 1;L cell:L细胞;I cell:I细胞;K cell:K细胞;EC cell:肠嗜铬细胞 enterochromaffin cell;PYY:酪酪肽 peptide YY;GLP-1:胰高血糖素样肽-1 glucagon-like peptide-1;CCK:胆囊收缩素 cholecystokinin;GIP:抑胃肽 gastric inhibitory peptide;5-HT:5-羟色胺 serotonin;CCK1R:胆囊收缩素 1 受体 cholecystokinin 1 receptor;CCK2R:胆囊收缩素 2 受体 cholecystokinin 2 receptor;NPY2R:神经肽 Y2 受体 neuropeptide Y2 receptor;GIPR:抑胃肽受体 gastric inhibitory peptide receptor;5-HT3R:5-羟色胺 3 受体 serotonin 3 receptor;GLP-1R:胰高血糖素样肽-1 受体 glucagon-like peptide-1 receptor;vagus:迷走神经;Hypothalamus:下丘脑;Anorexia or Emesis:厌食或呕吐。 图 1 胃肠激素和5-HT在DON诱导厌食反应中的作用 Fig. 1 Role of gastrointestinal hormones and 5-HT in DON-induced anorexia |
胃肠道在调节食欲中起着关键作用。动物的食欲和饱腹感不仅与肠道活动息息相关,还与位于胃肠道内的肠内分泌细胞(enteroendocrine cell,EEC)所分泌的胃肠激素有着密切的联系[39]。EEC分泌的胃肠激素有30多种,其中与食欲调控相关的胃肠激素主要有酪酪肽(peptide YY,PYY)、胆囊收缩素(cholecystokinin,CCK)、胰高血糖素样肽-1(glucagon-like peptide-1,GLP-1)、抑胃肽(gastric inhibitory peptide,GIP)等,这些胃肠激素均参与了DON诱导的厌食反应[40]。
PYY是调节食欲和能量的胃肠饱感激素,同时CCK也作为一种饱感激素调节动物摄食行为。PYY和CCK是DON诱导厌食反应和抑制动物生长的重要介质。按照每千克体重给小鼠腹腔注射或者口服1或5 mg DON 15 min后,血浆PYY和CCK浓度显著增加,小鼠的采食量降低,引起小鼠产生厌食反应,并且NPY2受体拮抗剂能够减弱DON诱导的厌食反应,而CCK受体拮抗剂的作用不明显,这表明在DON诱导的厌食反应中PYY是一个比CCK更为关键的介质[41]。此外,成年小鼠(3个月)和老龄小鼠(22个月)对DON的敏感性不同,在急性DON暴露后成年小鼠和老年小鼠血浆PYY和CCK浓度均显著增加,但老龄小鼠血浆胃肠饱感激素浓度更高,因此年龄是DON诱导胃肠激素改变程度大小的一个相关因素[42]。根据体重给小鼠灌胃2.5 mg/kg DON后的0.5 h便可观察到血浆CCK浓度的显著增加[43],因而DON可能通过刺激EEC增加CCK的浓度而诱导动物食欲降低。研究显示,DON诱导CCK产生的机制可能是通过激活CaSR和TRPA1介导的[44],DON暴露可显著提高胃肠组织CCK和TRPA1的mRNA表达水平[17],并且CCK浓度的增加可通过迷走神经激活下丘脑中的POMC神经元,进而降低动物食欲[45]。除CCK外,PYY的产生机制也与CaSR和TRPA1有着一定的关联(图 1)[46],并且PYY还能够通过迷走神经参与调控下丘脑中抑食欲神经元POMC和促食欲神经元NPY的表达,促进POMC的上调和NPY的下调[47],这说明胃肠激素和下丘脑共同调控动物的食欲,但具体机制仍然未知,有待进一步研究。
由于啮齿动物缺乏呕吐反射,因此很多学者以猪和水貂为研究模型探讨DON对动物产生的厌食和呕吐反应。断奶仔猪连续28 d摄入含1~3 mg/kg DON的饲粮,其血浆PYY浓度显著增加[28]。仔猪血浆PYY和CCK浓度与DON的毒性作用有关,摄入不同浓度的DON污染饲粮,均可观察到血浆PYY和CCK浓度增加,并且与DON摄入的时间和浓度呈线性关系,然而由于DON浓度较低,并未使仔猪产生呕吐反应[48]。另外,按体重给水貂口服0.25 mg/kg DON后,仅15 min便可观察到其开始产生呕吐反应,并且其血浆PYY浓度显著增加,在使用NPY2受体拮抗剂后可降低血浆PYY的浓度,并且产生呕吐反应的概率下降50%[32]。因此,DON诱导的食欲下降和呕吐反应与动物血浆PYY和CCK的浓度有着紧密的联系,DON可促进PYY和CCK的分泌,进而导致动物食欲下降,采食量降低,甚至抑制动物生长。
GLP-1和GIP分别来源于肠内分泌L细胞和K细胞,二者是与能量平衡相关的饱感激素,在食欲调控中起着非常重要的作用。按每千克体重给小鼠灌胃1.0或2.5 mg DON,小鼠血浆GLP-1和GIP的浓度在30 min后显著增加,但在6 h后各组之间血浆GLP-1和GIP的浓度差异不显著,此结果表示DON诱导血浆GLP-1和GIP浓度升高是短暂可逆的,并且用GLP-1受体拮抗剂Exendin9-39和GIP受体拮抗剂Pro3GIP进行预处理可降低DON诱导的食欲下降[16]。研究显示,DON通过激活CaSR以及瞬时受体电位离子通道-5(transient receptor potential ion channel-5,TRPM-5)和TRPA1,促进GLP-1的分泌,进而导致动物食欲降低(图 1)[16, 44]。此外,断奶仔猪摄入受到DON污染的饲粮后,其血浆GLP-1浓度显著升高[28]。因此,GLP-1和GIP在DON诱导的食欲下降和厌食反应中起着重要作用,但DON诱导肠内分泌细胞分泌GLP-1和GPI的具体机制尚不清楚,未来的研究中应更深入探讨DON诱导厌食反应的机制。
4 DON诱导的厌食反应与炎症细胞因子有关DON引起厌食反应的一个潜在原因可能是炎症细胞因子的诱导。细胞因子作为一种多肽分子,能够广泛调节细胞功能,作用于免疫系统、造血系统以及神经、内分泌系统,对动物的生长发育具有重要作用。细胞因子基因的异常表达可引起许多发育、生理和免疫问题,例如白细胞介素-1β(IL-1β)、白细胞介素-6(IL-6)和肿瘤坏死因子-α(TNF-α)等细胞因子的异常表达可导致人类和动物产生疾病[49]。按体重给小鼠灌胃25 μg/kg DON持续10 d便可引起低程度的炎症状态,其血浆、脂肪组织、十二指肠和大脑中IL-1β表达均显著增加[50]。另外,DON暴露可增加小鼠血浆IFN-α、白细胞介素-2(IL-2)、白细胞介素-4(IL-4)和IL-6的浓度,同时DON也不同程度地影响小鼠血浆IL-1β、白细胞介素-10(IL-10)和TNF-α的浓度[51]。已经有研究证实,促炎性细胞因子的分泌可能会降低动物的采食量,血浆IL-1β和IL-6的浓度与DON引起的厌食反应有关[42]。给小鼠灌服DON可诱导厌食反应,并且急性外源腹腔注射TNF-α和IL-1β同样也可引起小鼠食欲下降和厌食,而使用肿瘤坏死因子-α受体拮抗剂(TNFR)和白细胞介素1受体拮抗剂(IL-1RA)对小鼠进行预处理后可缓解DON、TNF-α和IL-1β所引起的厌食反应,因此,TNF-α和IL-1β在DON诱导的厌食反应中发挥着重要作用[52]。小鼠在按照每千克体重口服12.5 mg DON 3 h后下丘脑的炎症细胞因子和POMC的表达水平均显著增加[17],最终诱导小鼠产生厌食反应,而炎症细胞因子诱导厌食反应的中间媒介可能是核转录因子-κB(nuclear factor-kappa B,NF-κB),被证明可以调节炎症相关基因,包括IL-1β、TNF-α和IL-6,并且NF-κB能够激活POMC神经元,进而调节食欲调控中枢,产生厌食反应[53]。
5 肠道微生物在DON诱导厌食反应中的作用肠道中栖居着大量的微生物群落,这些微生物与宿主之间有着紧密的联系,建立了一个稳定共生的动态体系,维持宿主肠道微环境的稳态。肠道微生物可利用宿主肠道的营养物质进行发酵产生发酵产物,进而调控营养物质的吸收、代谢以及机体免疫系统。肠道微生物菌群失调可导致宿主产生相关应激行为,例如抑郁、焦虑以及食欲减退等[54],并且在一些精神类疾病和代谢性疾病中都可观察到肠道微生物菌群的改变[55]。DON可破坏肠道组织完整性,降低肠道绒毛高度,抑制肠道细胞分化,破坏肠道微生物平衡[4, 56]。研究显示,断奶仔猪摄入1或3 mg/kg DON饲粮持续28 d,其肠道微生物结构发生了巨大改变,DON显著降低小肠中厚壁菌门和变形菌门的丰度,增加放线菌门和蓝藻菌门的丰度[57]。另外,给4周龄小鼠按体重隔天灌胃5 mg/kg的DON,持续14 d,小鼠体重和日增重显著降低,并且DON暴露改变了肠道微生物菌群的组成,在门水平上拟杆菌门、壁厚菌门及脱铁杆菌门的物种丰度显著增加,在属水平上拟杆菌属、Mucispirillum和副杆菌属(Parabacteroides)的物种丰度显著增加,在种水平上的物种丰度也发生了改变,这些肠道微生物的改变影响了机体正常的生物合成和降解,并进一步导致代谢途径紊乱[58]。低浓度的DON也可引起肠道微生物的变化,小鼠按体重每日灌胃25 μg/kg的DON持续30 d,可引起肠道微生物菌群的显著改变,在门水平上DON显著增加了变形菌门和疣微菌门的物种丰度,在属水平上DON显著增加了Parabacteroides和肠杆菌属的物种丰度,而乳杆菌属、Odoribacter、未定毛螺旋菌属(Lachnospiracea incertae sedis)的物种丰度显著下降[59]。因此,无论是高浓度还是低浓度的DON均可导致动物肠道微生物群落的改变,这种改变可能是导致动物厌食的一个主要原因。
近年来,新一代测序技术进一步揭示了肠道微生物和宿主的食欲之间的密切联系。肠道微生物可对宿主的中枢神经系统产生影响,从而间接调控宿主的食欲[60]。肠道微生物利用宿主的营养物质进行发酵产生代谢产物,如短链脂肪酸(short chain fatty acids,SCFAs),能通过游离脂肪酸受体2(FFAR2)和游离脂肪酸受体3(FFAR3)刺激肠道内分泌细胞释放PYY和GLP-1[61],而PYY和GLP-1均已被证实在DON诱导的厌食反应中起着重要作用[28]。肠道微生物还可通过影响免疫系统,间接调控宿主食欲。肠道微生物与机体形成的宿主-微生物代谢轴,调控动物机体营养素代谢和免疫应答,从而影响食欲中枢,调控动物的摄食和厌食反应[56, 62]。另外,肠道微生物可能通过调控5-HT的水平进而调控机体食欲,色氨酸是5-HT的前体物质,研究显示肠道微生物能够从头合成色氨酸,进而参与5-HT的合成,调控动物食欲[63]。肠道微生物还可利用色氨酸进行代谢产生吲哚,吲哚能够刺激肠道内分泌L细胞,调节GLP-1的分泌,进而影响动物食欲[64]。肠道微生物调控动物食欲的信号主要通过其代谢产生的SCFAs、5-HT以及吲哚等代谢产物来进行传递,而DON对5-HT以及由吲哚调节分泌的GLP-1有着直接的影响,因此肠道微生物与DON诱导的厌食和呕吐反应有着一定的潜在联系,其深入机制还有待进一步挖掘。
6 小结DON作为较常见的一种霉菌毒素严重威胁着人类的食品安全和畜牧业发展,其主要的毒性特点为诱导动物产生厌食和呕吐反应。目前,对于DON致厌食和呕吐的机制已有大量研究报道,但大部分仅停留在对神经元基因表达和胃肠激素含量的影响层面,缺乏深入细致的研究。另外,DON致厌食和呕吐的可能途径有多个(图 2),如胃肠激素途径、5-HT途径以及免疫系统途径,这些途径均与肠道微生物有着不同程度的联系,因此肠道微生物与DON诱导的厌食和呕吐反应有着潜在的联系,但具体的调控机制未见报道,仍需更多的研究来阐明二者之间的联系,为探究DON的毒性机制奠定理论基础,为寻找更有效的DON抑制剂或解毒剂提供新思路,从而降低霉菌毒素带来的危害,促进畜牧业的发展。
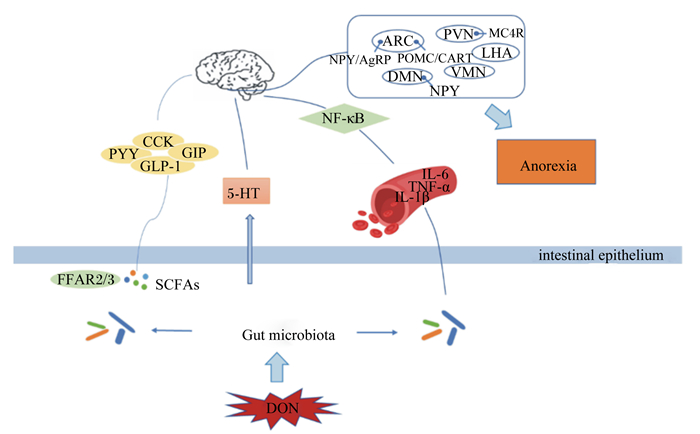
|
ARC:弓状核 arcuate nucleus;PVN:室旁核 paraventricular nucleus;LHA:侧下丘脑 lateral hypothalamic area;VMN:腹内侧核 ventromedial nucleus;DMN:背中核 dorsomedial nucleus;MC4R:黑皮质素受体 4 melanocortin 4 receptor;NPY:神经肽 Y neuropeptide;AGRP:刺鼠相关蛋白 agouti-related peptide;POMC:阿片黑素皮质素原 pro-opiomelanocortin;CART:可卡因-安菲他明调节转录物 cocaine-amphetamine-regulated transcript;NF-κB:核转录因子-κB nuclear factor-kappa B;CCK:胆囊收缩素 cholecystokinin;PYY:酪酪肽 peptide YY;GIP:抑胃肽 gastric inhibitory peptide;GLP-1:胰高血糖素样肽-1 glucagon-like peptide-1;5-HT:5-羟色胺 serotonin;IL-6:白细胞介素-6 interleukin-6;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;IL-1β:白细胞介素-1β interleukin-1β;Anorexia:厌食;FFAR2/3:游离脂肪酸受体2/3 free fatty acid receptor 2/3;SCFAs:短链脂肪酸 short chain fatty acids;intestinal epithelium:肠上皮;Gut microbiota:肠道微生物;DON:脱氧雪腐镰刀菌烯醇 deoxynivalenol。 图 2 DON诱导动物产生厌食反应的途径 Fig. 2 Pathway of DON-induced anorexia in animals |
| [1] |
RODRIGUES I, NAEHRER K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed[J]. Toxins, 2012, 4(9): 663-675. DOI:10.3390/toxins4090663 |
| [2] |
YANG X, GAO J, LIU Q, et al. Co-occurrence of mycotoxins in maize and maize-derived food in China and estimation of dietary intake[J]. Food Additives & Contaminants: Part B, 2019, 12(2): 124-134. |
| [3] |
GEREZ J R, PINTON P, CALLU P, et al. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs[J]. Experimental and Toxicologic Pathology, 2015, 67(2): 89-98. DOI:10.1016/j.etp.2014.10.001 |
| [4] |
LIAO Y X, PENG Z, CHEN L K, et al. Deoxynivalenol, gut microbiota and immunotoxicity: a potential approach?[J]. Food and Chemical Toxicology, 2018, 112: 342-354. DOI:10.1016/j.fct.2018.01.013 |
| [5] |
WU Q H, WANG X, YANG W, et al. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update[J]. Archives of Toxicology, 2014, 88(7): 1309-1326. DOI:10.1007/s00204-014-1280-0 |
| [6] |
BONNET M S, ROUX J, MOUNIEN L, et al. Advances in deoxynivalenol toxicity mechanisms: the brain as a target[J]. Toxins, 2012, 4(11): 1120-1138. DOI:10.3390/toxins4111120 |
| [7] |
TRENHOLM H L, HAMILTON R M, FRIEND D W, et al. Feeding trials with vomitoxin (deoxynivalenol)-contaminated wheat: effects on swine, poultry, and dairy cattle[J]. Journal of the American Veterinary Medical Association, 1984, 185(5): 527-531. |
| [8] |
ABBAS H K, MIROCHA C J, TUITE J. Natural occurrence of deoxynivalenol, 15-acetyl-deoxynivalenol, and zearalenone in refusal factor corn stored since 1972[J]. Applied and Environmental Microbiology, 1986, 51(4): 841-843. DOI:10.1128/AEM.51.4.841-843.1986 |
| [9] |
ANDRETTA I, KIPPER M, LEHNEN C R, et al. Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs[J]. Animal, 2012, 6(9): 1476-1482. DOI:10.1017/S1751731111002278 |
| [10] |
CHAN H M, SHIN S Y, KIM B G. Aflatoxin, deoxynivalenol, and zearalenone in swine diets: predictions on growth performance[J]. Revista Colombiana De Ciencias Pecuarias, 2013, 26(3): 243-254. |
| [11] |
WANG S, YANG J C, ZHANG B Y, et al. Deoxynivalenol impairs porcine intestinal host defense peptide expression in weaned piglets and IPEC-J2 cells[J]. Toxins, 2018, 10(12): 541. DOI:10.3390/toxins10120541 |
| [12] |
ARNOLD D L, KARPINSKI K F, MCGUIRE P F, et al. A short-term feeding study with deoxynivalenol (vomitoxin) using rats[J]. Fundamental and Applied Toxicology, 1986, 6(4): 691-696. DOI:10.1016/0272-0590(86)90182-X |
| [13] |
ROBBANA-BARNAT S, LORIDON-ROSA B, COHEN H, et al. Protein synthesis inhibition and cardiac lesions associated with deoxynivalenol ingestion in mice[J]. Food Additives & Contaminants, 1987, 4(1): 49-56. |
| [14] |
LIN R Q, SUN Y, MU P Q, et al. Lactobacillus rhamnosus GG supplementation modulates the gut microbiota to promote butyrate production, protecting against deoxynivalenol exposure in nude mice[J]. Biochemical Pharmacology, 2020, 175: 113868. DOI:10.1016/j.bcp.2020.113868 |
| [15] |
FLANNERY B M, WU W D, PESTKA J J. Characterization of deoxynivalenol-induced anorexia using mouse bioassay[J]. Food and Chemical Toxicology, 2011, 49(8): 1863-1869. DOI:10.1016/j.fct.2011.05.004 |
| [16] |
JIA H, WU W D, LU X, et al. Role of glucagon-like peptide-1 and gastric inhibitory peptide in anorexia induction following oral exposure to the trichothecene mycotoxin deoxynivalenol (vomitoxin)[J]. Toxicological Sciences, 2017, 159(1): 16-24. DOI:10.1093/toxsci/kfx112 |
| [17] |
TOMINAGA M, MOMONAKA Y, YOKOSE C, et al. Anorexic action of deoxynivalenol in hypothalamus and intestine[J]. Toxicon, 2016, 118: 54-60. DOI:10.1016/j.toxicon.2016.04.036 |
| [18] |
VESONDER R F, CIEGLER A, JENSEN A H. Isolation of the emetic principle from fusarium-infected corn[J]. Applied Microbiology, 1973, 26(6): 1008-1010. DOI:10.1128/AM.26.6.1008-1010.1973 |
| [19] |
PESTKA J J. Deoxynivalenol-induced proinflammatory gene expression: mechanisms and pathological sequelae[J]. Toxins, 2010, 2(6): 1300-1317. DOI:10.3390/toxins2061300 |
| [20] |
PESTKA J J, SMOLINSKI A T. Deoxynivalenol: toxicology and potential effects on humans[J]. Journal of Toxicology and Environmental Health: Part B, 2005, 8(1): 39-69. DOI:10.1080/10937400590889458 |
| [21] |
FORSYTH D M, YOSHIZAWA T, MOROOKA N, et al. Emetic and refusal activity of deoxynivalenol to swine[J]. Applied and Environmental Microbiology, 1977, 34(5): 547-552. DOI:10.1128/AEM.34.5.547-552.1977 |
| [22] |
PRELUSKY D B, TRENHOLM H L. The efficacy of various classes of anti-emetics in preventing deoxynivalenol-induced vomiting in swine[J]. Natural Toxins, 1993, 1(5): 296-302. DOI:10.1002/nt.2620010508 |
| [23] |
YOUNG L G, MCGIRR L, VALLI V E, et al. Vomitoxin in corn fed to young pigs[J]. Journal of Animal Science, 1983, 57(3): 655-664. DOI:10.2527/jas1983.573655x |
| [24] |
SCHWARTZ M W. Central nervous system regulation of food intake[J]. Obesity, 2006, 14(S2): 1S-8S. |
| [25] |
HUSSAIN S S, BLOOM S R. The regulation of food intake by the gut-brain axis: implications for obesity[J]. International Journal of Obesity, 2013, 37(5): 625-633. DOI:10.1038/ijo.2012.93 |
| [26] |
PESTKA J J, ISLAM Z, AMUZIE C J. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse[J]. Toxicology Letters, 2008, 178(2): 83-87. DOI:10.1016/j.toxlet.2008.02.005 |
| [27] |
KOBAYASHI-HATTORI K, AMUZIE C J, FLANNERY B M, et al. Body composition and hormonal effects following exposure to mycotoxin deoxynivalenol in the high-fat diet-induced obese mouse[J]. Molecular Nutrition & Food Research, 2011, 55(7): 1070-1078. |
| [28] |
WANG S, YANG J C, ZHANG B Y, et al. Potential link between gut microbiota and deoxynivalenol-induced feed refusal in weaned piglets[J]. Journal of Agricultural and Food Chemistry, 2019, 67(17): 4976-4986. DOI:10.1021/acs.jafc.9b01037 |
| [29] |
HEISLER L K, JOBST E E, SUTTON G M, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake[J]. Neuron, 2006, 51(2): 239-249. DOI:10.1016/j.neuron.2006.06.004 |
| [30] |
FITZPATRICK D W, BOYD K E, WATTS B M. Comparison of the trichothecenes deoxynivalenol and T-2 toxin for their effects on brain biogenic monoamines in the rat[J]. Toxicology Letters, 1988, 40(3): 241-245. DOI:10.1016/0378-4274(88)90047-1 |
| [31] |
PRELUSKY D B, YEUNG J M, THOMPSON B K, et al. Effect of deoxynivalenol on neurotransmitters in discrete regions of swine brain[J]. Archives of Environmental Contamination and Toxicology, 1992, 22(1): 36-40. DOI:10.1007/BF00213300 |
| [32] |
WU W D, BATES M A, BURSIAN S J, et al. Peptide YY3-36 and 5-hydroxytryptamine mediate emesis induction by trichothecene deoxynivalenol (vomitoxin)[J]. Toxicological Sciences, 2013, 133(1): 186-195. DOI:10.1093/toxsci/kft033 |
| [33] |
WU W D, ZHOU H R, BURSIAN S J, et al. Calcium-sensing receptor and transient receptor ankyrin-1 mediate emesis induction by deoxynivalenol (vomitoxin)[J]. Toxicological Sciences, 2017, 155(1): 32-42. DOI:10.1093/toxsci/kfw191 |
| [34] |
PRELUSKY D B. The effect of deoxynivalenol on serotoninergic neurotransmitter levels in pig blood[J]. Journal of Environmental Science and Health, Part B, 1994, 29(6): 1203-1218. DOI:10.1080/03601239409372923 |
| [35] |
CHEN L K, PENG Z, NÜSSLER A K, et al. Current and prospective sights in mechanism of deoxynivalenol-induced emesis for future scientific study and clinical treatment[J]. Journal of Applied Toxicology, 2017, 37(7): 784-791. DOI:10.1002/jat.3433 |
| [36] |
LAM D D, PRZYDZIAL M J, RIDLEY S H, et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors[J]. Endocrinology, 2008, 149(3): 1323-1328. DOI:10.1210/en.2007-1321 |
| [37] |
TILIGADA E, WILSON J F. Regulation of α-melanocyte-stimulating hormone release from superfused slices of rat hypothalamus by serotonin and the interaction of serotonin with the dopaminergic system inhibiting peptide release[J]. Brain Research, 1989, 503(2): 225-228. DOI:10.1016/0006-8993(89)91668-5 |
| [38] |
SOHN J W, XU Y, JONES J E, et al. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels[J]. Neuron, 2011, 71(3): 488-497. DOI:10.1016/j.neuron.2011.06.012 |
| [39] |
POSOVSZKY C, WABITSCH M. Regulation of appetite, satiation, and body weight by enteroendocrine cells.Part 1:characteristics of enteroendocrine cells and their capability of weight regulation[J]. Hormone Research in Paediatrics, 2015, 83: 1-10. |
| [40] |
TERCIOLO C, MARESCA M, PINTON P, et al. Review article: role of satiety hormones in anorexia induction by trichothecene mycotoxins[J]. Food and Chemical Toxicology, 2018, 121: 701-714. DOI:10.1016/j.fct.2018.09.034 |
| [41] |
FLANNERY B M, CLARK E S, PESTKA J J. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY[J]. Toxicological Sciences, 2012, 130(2): 289-297. DOI:10.1093/toxsci/kfs255 |
| [42] |
CLARK E S, FLANNERY B M, GARDNER E M, et al. High sensitivity of aged mice to deoxynivalenol (vomitoxin)-induced anorexia corresponds to elevated proinflammatory cytokine and satiety hormone responses[J]. Toxins, 2015, 7(10): 4199-4215. DOI:10.3390/toxins7104199 |
| [43] |
WU W D, ZHOU H R, HE K Y, et al. Role of cholecystokinin in anorexia induction following oral exposure to the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol[J]. Toxicological Sciences, 2014, 138(2): 278-289. DOI:10.1093/toxsci/kft335 |
| [44] |
ZHOU H R, PESTKA J J. Deoxynivalenol (vomitoxin)-induced cholecystokinin and glucagon-like peptide-1 release in the STC-1 enteroendocrine cell model is mediated by calcium-sensing receptor and transient receptor potential ankyrin-1 channel[J]. Toxicological Sciences, 2015, 145(2): 407-417. DOI:10.1093/toxsci/kfv061 |
| [45] |
MILLINGTON G W M. The role of proopiomelanocortin (POMC) neurones in feeding behaviour[J]. Nutrition & Metabolism, 2007, 4: 18. |
| [46] |
WU W D, ZHOU H R, PESTKA J J. Potential roles for calcium-sensing receptor (CaSR) and transient receptor potential ankyrin-1(TRPA1) in murine anorectic response to deoxynivalenol (vomitoxin)[J]. Archives of Toxicology, 2017, 91(1): 495-507. DOI:10.1007/s00204-016-1687-x |
| [47] |
CHALLIS B G, PINNOCK S B, COLL A P, et al. Acute effects of PYY3-36 on food intake and hypothalamic neuropeptide expression in the mouse[J]. Biochemical and Biophysical Research Communications, 2003, 311(4): 915-919. DOI:10.1016/j.bbrc.2003.10.089 |
| [48] |
LI R N, LI Y S, SU Y T, et al. Short-term ingestion of deoxynivalenol in naturally contaminated feed alters piglet performance and gut hormone secretion[J]. Animal Science Journal, 2018, 89(8): 1134-1143. DOI:10.1111/asj.13034 |
| [49] |
DANTZER R. Cytokine-induced sickness behavior: mechanisms and implications[J]. Annals of the New York Academy of Sciences, 2001, 933(1): 222-234. |
| [50] |
TARDIVEL C, AIRAULT C, DJELLOUL M, et al. The food born mycotoxin deoxynivalenol induces low-grade inflammation in mice in the absence of observed-adverse effects[J]. Toxicology Letters, 2015, 232(3): 601-611. DOI:10.1016/j.toxlet.2014.12.017 |
| [51] |
ISLAM M R, ROH Y S, KIM J, et al. Differential immune modulation by deoxynivalenol (vomitoxin) in mice[J]. Toxicology Letters, 2013, 221(2): 152-163. DOI:10.1016/j.toxlet.2013.05.656 |
| [52] |
WU W D, ZHANG H B. Role of tumor necrosis factor-α and interleukin-1β in anorexia induction following oral exposure to the trichothecene deoxynivalenol (vomitoxin) in the mouse[J]. Journal of Toxicological Sciences, 2014, 39(6): 875-886. DOI:10.2131/jts.39.875 |
| [53] |
JANG P G, NAMKOONG C, KANG G M, et al. NF-κB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia[J]. Journal of Biological Chemistry, 2010, 285(13): 9706-9715. DOI:10.1074/jbc.M109.070706 |
| [54] |
ALCOCK J, MALEY C C, AKTIPIS C A. Is eating behavior manipulated by the gastrointestinal microbiota?Evolutionary pressures and potential mechanisms[J]. Bioessays, 2014, 36(10): 940-949. DOI:10.1002/bies.201400071 |
| [55] |
LEE W J, HASE K. Gut microbiota-generated metabolites in animal health and disease[J]. Nature Chemical Biology, 2014, 10(6): 416-424. DOI:10.1038/nchembio.1535 |
| [56] |
VIGNAL C, DJOUINA M, PICHAVANT M, et al. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice[J]. Archives of Toxicology, 2018, 92(7): 2327-2338. DOI:10.1007/s00204-018-2228-6 |
| [57] |
LIU M, ZHANG L, CHU X H, et al. Effects of deoxynivalenol on the porcine growth performance and intestinal microbiota and potential remediation by a modified HSCAS binder[J]. Food and Chemical Toxicology, 2020, 141: 111373. DOI:10.1016/j.fct.2020.111373 |
| [58] |
WANG J J, ZHANG R Q, ZHAI Q Y, et al. Metagenomic analysis of gut microbiota alteration in a mouse model exposed to mycotoxin deoxynivalenol[J]. Toxicology and Applied Pharmacology, 2019, 372: 47-56. DOI:10.1016/j.taap.2019.04.009 |
| [59] |
PENG Z, LIAO Y X, CHEN L K, et al. Heme oxygenase-1 attenuates low-dose of deoxynivalenol-induced liver inflammation potentially associating with microbiota[J]. Toxicology and Applied Pharmacology, 2019, 374: 20-31. DOI:10.1016/j.taap.2019.04.020 |
| [60] |
SAMPSON T R, MAZMANIAN S K. Control of brain development, function, and behavior by the microbiome[J]. Cell Host & Microbe, 2015, 17(5): 565-576. |
| [61] |
PSICHAS A, SLEETH M L, MURPHY K G, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents[J]. International Journal of Obesity, 2015, 39(3): 424-429. DOI:10.1038/ijo.2014.153 |
| [62] |
NICHOLSON J K, HOLMES E, KINROSS J, et al. Host-gut microbiota metabolic interactions[J]. Science, 2012, 336(6086): 1262-1267. DOI:10.1126/science.1223813 |
| [63] |
DESBONNET L, GARRETT L, CLARKE G, et al. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat[J]. Journal of Psychiatric Research, 2008, 43(2): 164-174. DOI:10.1016/j.jpsychires.2008.03.009 |
| [64] |
CHIMEREL C, EMERY E, SUMMERS D K, et al. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells[J]. Cell Reports, 2014, 9(4): 1202-1208. DOI:10.1016/j.celrep.2014.10.032 |




