随着畜禽养殖规模化和集约化的快速发展,畜禽生产在满足居民对肉、蛋、奶的需求基础上,大量排放的粪污及臭气物质引起的环境污染问题正逐渐成为人们关注的焦点,是制约发展环境友好型畜牧业的主要因素。据统计,2019年,我国禽肉产量达2 239万t[1]。按照料重比1.8、鲜粪/采食量1.2计算,肉禽排放的粪便量高达4 836万t。如果肉禽排放的粪便量占畜禽粪便总排放量按15%计,那么畜禽粪便总排放量约为3.2亿t。伴随着粪便的大量排放,臭味物质的散发和排放对畜禽生产、居民健康、空气质量等也产生了不利影响[2]。2015年1月1日起正式实施的新《环境保护法》加强了对生产过程中臭味物质排放的防治要求[3],明确畜禽生产中臭味物质的生成途径,采取有效的防治措施已然迫在眉睫。
畜禽排放的臭味物质成分复杂、种类多样,如猪粪中的臭味物质高达230多种,鸡粪中的臭味物质高达130多种[4-5]。根据化学性质,畜禽排放的臭味物质主要包括挥发性含氮化合物[如氨气(NH3)、腐胺等]、挥发性含硫化合物[如硫化氢(H2S)、硫醇、硫醚等]、挥发性脂肪酸(如乙酸、丙酸、丁酸等)和芳香族化合物(如吲哚、粪臭素、酚类等)四大类。其中,粪臭素(3-甲基吲哚,3MI)被认为是畜禽排放的仅次于NH3和H2S的第三大有害臭味物质,具有强烈的粪臭气味,嗅阈值低于0.003 mg/m3,在浓度极低的情况下仍可被人和畜禽感知[5-6]。此外,粪臭素具有中等毒性,能够诱发肠上皮细胞功能障碍,引起肠道疾病[7];引起反刍动物急性肺水肿和肺气肿[8];沉积于脂肪组织中,引起肉产生膻味,降低肉品质[9-10]。近年来,国内外学者在研究粪臭素的产生机制及减排措施方面取得了一定的进展,本文就此方面进行综述,旨在为畜禽健康养殖和可持续发展提供新思路。
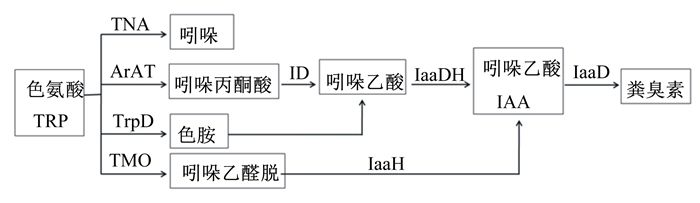
1 粪臭素的产生机制 1.1 粪臭素生成的物质代谢基础畜禽粪臭素的生成是由肠道微生物发酵饲粮中未被消化的色氨酸(Trp)而来的。Trp在肠道微生物的作用下进行分解,有2条途径:1)吲哚生成途径。Trp在色氨酸酶(TNA)的作用下生成吲哚,同时伴随着丙酮酸和NH3的产生;2)吲哚乙酸(IAA)生成途径。Trp生成IAA的途径有3条,一是Trp在芳香族氨基酸转氨酶(ArAT)作用下生成吲哚丙酮酸,在吲哚丙酮酸脱羧酶(ID)作用下生成吲哚乙醛,经吲哚乙醛脱氢酶(IaaDH)作用生成IAA;二是Trp在色氨酸脱羧酶(TrpD)作用下生成色胺,经未知酶的作用下生成吲哚乙醛,经IaaDH作用生成IAA;三是Trp在色氨酸单氧酶(TMO)作用下生成吲哚乙酰胺,经吲哚乙酰胺水解酶(IaaH)作用下生成IAA[11-14]。生成的IAA在吲哚乙酸脱羧酶(IaaD)作用下生成粪臭素[6](图 1)。由此可见,粪臭素和吲哚是Trp经肠道微生物作用下的主要终产物。
|
TNA:色氨酸酶 tryptophanase;ArAT:芳香族氨基酸转氨酶 aromatic amino acid aminotransferase;TrpD:色氨酸脱羧酶 tryptophan decarboxylase;TMO:色氨酸单氧酶 tryptophan monooxygenase;ID:吲哚丙酮酸脱羧酶 indolepyruvate decarboxylase;IaaDH:吲哚乙醛脱氢酶 indoleacetaldehyde dehydrogenase;IaaD:吲哚乙酸脱羧酶 indoleacetate decarboxylase;IaaH:吲哚乙酰胺水解酶 indoleacetamide hydrolase。 图 1 粪臭素的生成途径 Fig. 1 Generative pathway of skatole |
肠道中粪臭素的生成受到多种因素的影响,如代谢酶活性、前体代谢物浓度、微环境(pH、二氧化碳含量)、肠道菌群结构等[12]。由于粪臭素的形成必须经由IaaD作用于IAA实现,因而以IaaD为靶点筛选粪臭素生成菌,或者开发IaaD抑制剂,对阐明畜禽肠道粪臭素的生成机制及研发除臭剂具有重要的理论意义和应用前景。
1.2 粪臭素生成的微生物基础多种微生物能够参与Trp代谢,生成吲哚和IAA,但是很少微生物能够生成粪臭素[15]。到目前为止,已知有9种菌株能够产生粪臭素:粪味梭菌(Clostridium scatologenes)、疾病梭菌(Clostridium nauseum)、假单胞菌(Pseudomonas sp.)、根瘤菌(Rhizobium sp.)、瑞士乳杆菌(Lactobacillus helveticus)、乳酸杆菌11201(Lactobacillus sp. 11201)、产粪臭素奥尔森氏菌(Olsenella scatoligenes)、牙龈奥尔森氏菌DSM 7084(Olsenella uli DSM 7084)和扭曲真杆菌(Faecalicatena contorta)[12],它们分别来源于猪粪肥料[16]、土壤[17]、植物[18]、奶酪[19]、牛瘤胃[20]、猪粪便[21]、人牙龈缝隙[22]和人坏疽性阑尾炎[23]。在这些菌株中,只有Clostridium scatologenes可以直接将Trp降解成粪臭素[24],而其余菌株生成粪臭素的前体代谢物均是IAA。Olsenella scatoligenes是目前已知的唯一从猪粪便中分离到的动物源粪臭素生成菌[25],而Lactobacillus sp. 11201被认为与反刍动物肺水肿和肺气肿及公猪肉膻味有关[26],其余菌株均不是畜禽来源,因此分离和鉴定畜禽肠道粪臭素生成菌需要进一步研究,有助于阐明肠道粪臭素产生的微生物学机制。
1.3 粪臭素生成的时空效应畜禽肠道中粪臭素的生成具有时间-空间效应,且这种时间-空间效应与肠道微生物发育功能不断完善有关[27]。比如肉鸡肠道中粪臭素浓度随着日龄的增加而提高,2周龄肉仔鸡直肠鲜粪中的粪臭素浓度为27 μg/g,到6周龄时浓度上升到65 μg/g[28],这种变化可能与肠道特定菌群数量和微生物多样性有关,而与总菌数量无关。有研究表明,鸡小肠每克内容物中细菌总数为108~109个,盲肠每克内容物中细菌总数为1010~1011个[29-30]。而达到如此数量的细菌,雏鸡所需要的时间仅需3 d(即孵化出壳后3 d),且该数量保持相对稳定[31]。然而,肠道微生物菌群结构和多样性随着日龄的增加而发生改变。例如,肠球菌是鸡肠道中的正常微生物菌群之一,在3周龄前,鸡肠道内的肠球菌是以粪肠球菌和屎肠球菌为主;3周龄后,盲肠肠球菌逐渐成为了优势菌群,这种肠道特定优势菌群数量的增加被认为与粪臭素的生成有关系[32]。此外,肠道微生物多样性的改变也表现出了类似的关联性,1日龄的肉仔鸡盲肠内微生物种类约有50个属,到42日龄时增加到200多个属[33],微生物多样性的提高有助于减少粪臭素的产生[28]。
畜禽不同肠段的微生物区系组成是不同的,反映在肠道各段粪臭素的浓度也是不同的。以鸡为例,整个肠道中占主导地位的细菌是乳杆菌属(Lactobacillus)、肠球菌属(Enterococcus)、拟杆菌属(Bacteroides)和棒状菌属(Corynebacterium)[34]。然而,乳酸杆菌主要分布于消化道前段(十二指肠、空肠、回肠),肠球菌属主要分布于消化道后段,拟杆菌属主要存在于盲肠,这种肠道微生物区系组成的差异化或许是粪臭素浓度不同的原因。Yang等[35]研究发现,42日龄肉鸡回肠、盲肠和直肠内容物中粪臭素浓度分别为28、333和185 ng/g,且与肠道微生物多样性(Shannon-Wiener指数)呈正相关。与肉鸡不同,150日龄的育肥猪肠道中粪臭素浓度排序为盲肠 < 结肠 < 直肠,这种差异源于各肠段微生物区系的不同发酵模式所致[36]。
1.4 粪臭素的代谢肠道中产生的粪臭素由粪便排出体外,但仍有一部分经肠细胞吸收进入血液中,通过门静脉到达肝脏。进入到肝脏中的粪臭素大部分可快速被代谢,由尿液排出;少部分没有被代谢的运送到外周血液,在脂肪和肌肉中沉积,这是导致猪肉膻味的主要原因[37]。猪肉脂肪组织中粪臭素浓度的平均可接受阈值为0.20~0.25 μg/g,甚至低于0.15 μg/g[38-39]。粪臭素在肝脏中的代谢分为2相阶段,在细胞色素P450(CYP450)酶的氧化作用下生成3-羟基-3-甲基吲哚(3-hydroxy-3-methylindolenine)等7种相Ⅰ(phase Ⅰ)代谢物;这些代谢物随后经由硫化和葡萄苷酸化进行相Ⅱ(phase Ⅱ)反应[40-41]。猪CYP450酶具有多个亚型,其中CYP1A、2A19、2C33v4、2C49、2E1和3A是参与粪臭素代谢的主要氧化酶[42],且受到部分相Ⅰ代谢产物, 如吲哚-3-甲醇(indole-3-carbinol)和2-氨基苯乙酮(2-aminoacetophenone)的抑制[43-44]。粪臭素的代谢终产物主要是6-羟基硫酸粪臭素(6-sulfatoxyskatole, MII)、3-羟基-3-甲氧基吲哚(3-hydroxy-3-methyloxindole, MIII)和3-[(N-乙酰半胱氨酸-S-yl)甲基]吲哚(3-[(N-acetylcysteine-S-yl)methyl]indole, MX)。其中,MII和MIII可在猪血液和尿液中检测出,而MX只在尿液中检测出[45]。尿液中的MIII和吲哚-3-羧酸(indole-3-carboxylic acid,ICA)含量与脂肪组织中粪臭素浓度呈高度正相关,可作为脂肪中粪臭素浓度的最佳生物标记物[46]。这些相关酶活性及表达情况、代谢产物浓度变化反映了粪臭素在肝脏中的分解效率和在组织中的沉积能力,对阐明粪臭素的代谢途径及提高肉品质具有重要的参考价值。
2 粪臭素的减排措施虽然臭气防控的重要性得到了人们的重视,然而关于臭气的防控措施存在不少问题。目前,养殖场臭气防控的主要方法有源头减量、过程控制和末端减排,可参考相关文献[47-48]。作为臭气物质之一,粪臭素的减排措施研究相对较少,这里主要从饲粮、饲料添加剂和微生物学方面进行简要概述。
2.1 饲粮饲粮中未被消化的Trp是肠道中粪臭素的直接来源,因此在满足畜禽对Trp需要的基础上,降低饲粮中Trp和粗蛋白质水平是从源头上减少粪臭素生成的主要途径之一。在生长育肥公猪上的试验结果表明,饲粮粗蛋白质水平降低,粪臭素等臭气物质的排放量也随之减少[49]。随着“理想蛋白质”、“低氮饲粮”、“精准饲养”等理念的深入,降低饲粮粗蛋白质水平的同时,合理优化饲粮氨基酸水平及组成不仅可以满足畜禽的需要,而且有利于减少氮的排放和提高养分的转化效率。近期研究表明,氨基酸组成平衡的饲粮不会对生长育肥猪生长性能和饲料转化率产生负面影响,而且猪粪便中粪臭素等臭气物质的水平也随之下降[50]。在蛋鸡上的研究表明,饲粮标准回肠可消化Trp水平过高,盲肠中粪臭素的浓度有升高的趋势[51]。除了调控饲粮粗蛋白质水平和氨基酸组成外,保证肠道后段易发酵碳水化合物的供给也有助于减少粪臭素的生成,且这种调控作用可能与丁酸等生成有关[52]。相反地,也有研究结果表明,丁酸的增加与粪臭素的生成并不存在必然的因果关系,而且易发酵碳水化合物的供给也不是通过减少粪臭素生成菌数量的方式起到降低效果,而是可能与氨基酸的利用途径改变有关,即Trp被细菌利用,合成菌体蛋白,提高微生物活性,从而抑制Trp向粪臭素的转化[53]。因此,维持畜禽后段肠道微生物发酵过程中能量和蛋白质平衡是降低粪臭素产生的有利方式。
2.2 饲料添加剂通过在饲粮中添加1种或几种外源添加剂,影响肠道微生物菌群结构,干扰粪臭素生成菌的活性,是降低粪臭素产生的最根本的方式。目前,在畜禽饲粮中用于减少粪臭素产生的添加剂有酸制剂、酶制剂、寡糖、微生态制剂、植物提取物等[54],而且这些不同类型的饲料添加剂在抑制粪臭素生成方面的研究报道不多。Øverland等[55]在公猪饲粮中添加1.0%甲酸或0.85%苯甲酸可以显著降低血浆粪臭素的浓度,但对结肠和脂肪组织中粪臭素浓度没有显著影响。Pauly等[56]研究结果表明,向燕麦型饲粮中添加0.05 g/kg的酶制剂(葡聚糖酶+木聚糖酶)可以有效地降低公猪结肠中粪臭素的浓度,但对饲喂大麦型饲粮公猪结肠中粪臭素浓度没有显著影响。此外,公猪脂肪组织中粪臭素浓度与饲粮类型无关。体外发酵试验结果显示,添加10 g/L的大豆寡糖或菊糖(按总糖含量计)可以显著降低肉鸡盲肠内容物发酵液中的粪臭素浓度[57]。饲养试验结果表明,在肉仔鸡饲粮中分别添加5 g/kg甘露寡糖、1.2 g/kg菊糖、1.5 g/kg果寡糖或1.25 g/kg大豆寡糖均可以降低肉仔鸡粪便中粪臭素浓度,作用效果为果寡糖>菊糖>大豆寡糖>甘露寡糖[58-59]。Zhu等[60]研究结果进一步证明,向肉仔鸡饲粮中添加0.6%的水苏糖(大豆寡糖核心成分)显著降低了肉仔鸡盲肠中粪臭素的浓度。慕春龙等[61]研究发现,果寡糖改变苯丙氨酸和Trp的体外发酵类型,降低猪后肠发酵液中Trp向粪臭素的转化。Aluwe等[62]在公猪饲粮中添加5%菊糖或5%果寡糖,发现公猪背膘中粪臭素浓度明显降低。Sheng等[63]研究表明,育肥猪饲粮添加0.1%的微生态制剂可以降低粪便中粪臭素浓度。Tavendale等[64]报道,体外添加缩合单宁可抑制IAA转化成粪臭素,其幅度超过85%;Čandek-Potokar等[65]研究发现,添加1%~3%水解单宁降低了猪结肠中粪臭素浓度,且与肝脏CYP450酶活性有关。Schreurs等[66]发现,每天给羔羊饲喂33 g葡萄籽提取物可以降低瘤胃液和血浆中粪臭素浓度。
通过有限的文献资料可看出,饲料添加剂主要调控肠道,尤其是后段肠道的微环境(如pH等),干扰微生物菌群结构(如微生物多样性、特定菌群数量等),从而影响粪臭素的生成。然而,还有若干问题需要进一步解决:1)成本问题,如有机酸等价格较高,添加量过高会导致饲料成本上升;2)抗营养问题,有些添加剂本身属于抗营养因子,影响饲粮中营养成分的消化和吸收;3)稳定性问题,胃的强酸性环境会导致某些添加剂的效价降低,宿主肠道微生物菌群的多样性使得外源特定微生物活性降低,导致功能的不稳定;4)作用机制问题,外源饲料添加剂影响粪臭素的生成是通过调控肠道局部环境和微生物菌群结构实现的,这种作用的实现是直接作用于粪臭素生成菌,还是作用于粪臭素生成的代谢过程等,这些都是未来需要解决的问题。
2.3 生物降解与转化粪臭素在畜禽肠道中是连续产生的,完全阻止其生成似乎是不切实际的。虽然目前采取的一些物理学(如吸附、掩蔽、稀释、扩散等)和化学(如化学吸附、洗涤、氧化等)清除粪臭素的方法有一定的效果,但是这些方法引起的成本、能量消耗、二次污染等问题仍然限制了应用范围[67]。利用微生物进行生物降解与转化是一种可行的和生态友好型的清除粪臭素的方法。自从20世纪90年代以来,已经发现多种菌株能够降解粪臭素。研究发现,粪臭素初始浓度为2 mmol/L时,铜绿假单胞菌Gs(Pseudomonas aeruginosa Gs)和恶臭假单胞菌LPC24(Pseudomonas putida LPC24)可分别在24和30 d内将粪臭素完全降解[68-69];而沼泽红假单胞菌-KDNS3(Rhodopseudomonas palustris WKU-KDNS3)在21 d时对粪臭素的降解率超过93%[70]。Meng等[71]发现,短乳杆菌1.12(Lactobacillus brevis 1.12)在120 h内对粪臭素的降解率可达65%;而Tesso等[72]研究结果表明,当粪臭素浓度 < 200 mg/L时,不动杆菌NTA1-2A(Acinetobacter toweneri NTA1-2A)和不动杆菌TAT1-6A(Acinetobacter gullouiae TAT1-6A)在6 d内对粪臭素的降解率超过85%。最新的研究成果显示,贪铜菌属KK10(Cupriavidus sp. KK10)、伯克霍尔德菌IDO3(Burkholderia sp. IDO3)、红球菌DMU1(Rhodococcus sp. DMU1)和红球菌DMU2(Rhodococcus sp. DMU2)清除粪臭素的速率更快,24 h对粪臭素的降解率可达99%~100%[73-75]。
总得来看,这些菌株降解粪臭素的能力不一,且多是在厌氧环境下完成的。厌氧菌降解粪臭素的过程相对较长,且易受氧气等发酵环境的制约,不利于大面积推广。相应地,分离和培养需氧菌相对容易一些,也易于操作。降解粪臭素的菌株在发挥作用的同时,需要与其他复杂的微生物菌群共存,这种菌株的适应性和稳定性是保证降解效果的关键。此外,目前的研究主要集中于粪臭素降解菌的发现与特性鉴定,对于粪臭素的降解途径及机制问题还需要进一步研究。
3 小结与展望规模化和集约化生产过程中,畜禽排放的粪臭素等臭气物质对畜禽健康和周围环境产生了不利的影响。近年来,关于粪臭素的研究主要集中在3个方面:一是粪臭素产生的微生物学机制及其关键调控位点(基因或酶);二是粪臭素在体内的沉积机制;三是粪臭素的减排措施。尽管以上几个方面的研究取得了一些进展,但还需进一步解决某些问题,如畜禽肠道中产生粪臭素的微生物种类及其影响因素,外源添加剂降低或抑制粪臭素生成的机制,粪臭素减排方法的稳定性及生物安全性评价等。随着畜牧科技的发展,人们对粪臭素产生机制的研究将越来越深入,也必将开发出最佳的除臭技术,为畜禽养殖者提供更好的应用保障。
| [1] |
国家统计局. 中华人民共和国2019年国民经济和社会发展统计公报[EB/OL]. (2020-02-28). http://www.gov.cn/xinwen/2020-02/28/content_5484361.htm. National Bureau of Statistics.National economic and social development statistics bulletin of the People's Republic of China in 2019[EB/OL].(2020-02-28).http://www.gov.cn/xinwen/2020-02/28/content_5484361.htm. (in Chinese) |
| [2] |
THOME P.Industrial livestock production facilities: airborne emissions[M]//NRIAGU J O.Encyclopedia of environmental health.2nd ed.Amsterdam: Elsevier, 2019: 652-660.
|
| [3] |
新华社. 中华人民共和国环境保护法自2015年1月1日起施行[EB/OL]. (2014-04-25). http://www.gov.cn/xinwen/2014-04/25/content_2666328.htm. Xinhua News Agency.Environmental protection law of the People's Republic of China fffective from January 1, 2015.[EB/OL].(2014-04-25)http://www.gov.cn/xinwen/2014-04/25/content_2666328.htm. (in Chinese) |
| [4] |
张信宜, 王燕, 吴银宝, 等. 规模化猪场臭气减排的营养和饲养技术研究进展[J]. 家畜生态学报, 2017, 38(4): 1-7, 14. ZHANG X Y, WANG Y, WU Y B, et al. Research progress on nutrition and feeding technology in reducing odour in large-scale pig farms[J]. Acta Ecologiae Animalis Domastici, 2017, 38(4): 1-7, 14 (in Chinese). |
| [5] |
DUNLOP M, BLACKALL P, STUETZ R. Odour emissions from poultry litter-a review litter properties, odour formation and odorant emissions from porous materials[J]. Journal of Environmental Management, 2016, 177: 306-319. |
| [6] |
LIU D Z, WEI Y F, LIU X Y, et al. Indoleacetate decarboxylase is a glycyl radical enzyme catalysing the formation of malodorant skatole[J]. Nature Communications, 2018, 9: 4224. DOI:10.1038/s41467-018-06627-x |
| [7] |
KURATA K, KAWAHARA H, NISHIMURA K, et al. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38[J]. Biochemical and Biophysical Research Communications, 2019, 510(4): 649-655. DOI:10.1016/j.bbrc.2019.01.122 |
| [8] |
YOKOYAMA M, CARLSON J. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole[J]. The American Journal of Clinical Nutrition, 1979, 32(1): 173-178. DOI:10.1093/ajcn/32.1.173 |
| [9] |
WALSTRA P, CLAUDI-MAGNUSSEN C, CHEVILLON P, et al. An international study on the importance of androstenone and skatole for boar taint: levels of androstenone and skatole by country and season[J]. Livestock Production Science, 1999, 62(1): 15-28. DOI:10.1016/S0301-6226(99)00054-8 |
| [10] |
HAN X F, ZHOU M, CAO X H, et al. Mechanistic insight into the role of immunocastration on eliminating skatole in boars[J]. Theriogenology, 2019, 131: 32-40. DOI:10.1016/j.theriogenology.2019.03.017 |
| [11] |
XU K, LIU H N, BAI M M, et al. Redox properties of tryptophan metabolism and the concept of tryptophan use in pregnancy[J]. International Journal of Molecular Science, 2017, 18(7): 1595-1622. DOI:10.3390/ijms18071595 |
| [12] |
DESLANDES B, GARIÉPY C, HOUDE A. Review of microbiological and biochemical effects of skatole on animal production[J]. Livestock Production Science, 2001, 71(2/3): 193-200. |
| [13] |
ROAGER H M, LICHT T R. Microbial tryptophan catabolites in health and disease[J]. Nature Communications, 2018, 9: 3294. DOI:10.1038/s41467-018-05470-4 |
| [14] |
AGUS A, PLANCHAIS J, SOKOL H. Gut microbiota regulation of tryptophan metabolism in health and disease[J]. Cell Host & Microbe, 2018, 23(6): 716-724. |
| [15] |
LEE J H, WOOD T, LEE J. Roles of indole as an interspecies and interkingdom signaling molecule[J]. Trends in Microbiology, 2015, 23(11): 707-718. DOI:10.1016/j.tim.2015.08.001 |
| [16] |
WHITEHEAD T R, PRICE N P, DRAKE H L, et al. Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure[J]. Applied and Environmental Microbiology, 2008, 74(6): 1950-1953. DOI:10.1128/AEM.02458-07 |
| [17] |
ROSENBERGER R F. Obligate anaerobes which form skatole[J]. Journal of Bacteriology, 1959, 77(4): 517. DOI:10.1128/JB.77.4.517-517.1959 |
| [18] |
PROCTOR M H. Bacterial dissimilation of indoleacetic acid: a new route of breakdown of the indole nucleus[J]. Nature, 1958, 181(4619): 1345. |
| [19] |
ALEKSEEVA I I, SHARAMKO V I. Indolic compounds of the bacteroid membrane and soluble fractions of the yellow lupine nodules[J]. Soviet Plant Physiology, 1977, 24: 114-118. |
| [20] |
KOWALEWSKA J, ZELAZOWSKA H, BABUCHOWSKI A, et al. Isolation of aroma-bearing material from Lactobacillus helveticus culture and cheese[J]. Journal of Dairy Science, 1985, 68(9): 2165-2171. DOI:10.3168/jds.S0022-0302(85)81086-9 |
| [21] |
HONEYFIELD D C, CARLSON J R. Assay for the enzymatic conversion of indoleacetic acid to 3-methylindole in a ruminal Lactobacillus species[J]. Applied and Environmental Microbiology, 1990, 56(3): 724-729. DOI:10.1128/AEM.56.3.724-729.1990 |
| [22] |
OLSEN I, JOHNSON J, MOORE L V H, et al. Lactobacillus uli sp.nov.and Lactobacillus rimae sp. nov. from the human gingival crevice and emended descriptions of Lactobacillus minutus and Streptococcus parvulus[J]. International Journal of Systemtic Bacteriology, 1991, 41(2): 261-266. DOI:10.1099/00207713-41-2-261 |
| [23] |
SAKAMOTO M, ⅡNO T, OHKUMA M. Faecalimonas umbilicata gen.nov., sp.nov., isolated from human faeces, and reclassification of Eubacterium contortum, Eubacterium fissicatena and Clostridium oroticum as Faecalicatena contorta gen. nov., comb. nov., Faecalicatena fissicatena comb. nov. and Faecalicatena orotica comb.nov[J]. International Journal of Systemtic and Evolutionary Microbiology, 2017, 67(5): 1219-1227. DOI:10.1099/ijsem.0.001790 |
| [24] |
JENSEN M T, COX R P, JENSEN B B. 3-methylindole (skatole) and indole production by mixed populations of pig fecal bacteria[J]. Applied and Environmental Microbiology, 1995, 61(8): 3180-3184. DOI:10.1128/AEM.61.8.3180-3184.1995 |
| [25] |
LI X, JENSEN R, HOJBERG O, et al. Olsenella scatoligenes sp.nov., a 3-methylindole-(skatole) and 4-methylphenol-(p-cresol) producing bacterium isolated from pig faeces[J]. International Journal of Systematic and Evolutionary Microbiology, 2015, 65(4): 1227-1233. |
| [26] |
HONEYFIELD D C, CARLSON J. Effect of indoleacetic acid and related indoles on Lactobacillus sp. strain 11201 growth, indoleacetic acid catabolism, and 3-methylindole formation[J]. Applied and Environmental Microbiology, 1990, 56(5): 1373-1377. DOI:10.1128/AEM.56.5.1373-1377.1990 |
| [27] |
TALEB S. Tryptophan dietary impacts gut barrier and metabolic diseases[J]. Frontiers in Immunology, 2019, 10: 2113. DOI:10.3389/fimmu.2019.02113 |
| [28] |
张沛. 肉仔鸡粪臭素产生的基本规律及与肠道微生物组成的变化关系研究[D]. 硕士学位论文. 沈阳: 沈阳农业大学, 2016. ZHANG P.Research on the basic laws of skatole production and its variation with intestinal microbial components in broilers[D].Master's Thesis.Shenyang: Shenyang Agricultural University, 2016.(in Chinese) |
| [29] |
YEOMAN C J, CHIA N, JERALDO P, et al. The microbiome of the chicken gastrointestinal tract[J]. Animal Health Research Reviews, 2012, 13(1): 89-99. DOI:10.1017/S1466252312000138 |
| [30] |
FEYE K M, BAXTER M F A, TELLEZ-ISAIAS G, et al. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes[J]. Poultry Science, 2020, 99(2): 653-659. DOI:10.1016/j.psj.2019.12.013 |
| [31] |
APAJALAHTI J, KETTUNEN A, BEDFORD M, et al. Percent G+C profiling accurately reveals diet-related differences in the gastrointestinal microbial community of broiler chickens[J]. Applied and Environmental Microbiology, 2001, 67(12): 5656-5667. DOI:10.1128/AEM.67.12.5656-5667.2001 |
| [32] |
DEVRIESE L A, HOMMEZ J, WIJFELS R, et al. Composition of the enterococcal and streptococcal intestinal flora of poultry[J]. Journal of Applied Bacteriology, 1991, 71(1): 46-50. DOI:10.1111/j.1365-2672.1991.tb04585.x |
| [33] |
OAKLEY B B, BUHR R J, RITZ C W, et al. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives[J]. BMC Veterinary Research, 2014, 10: 282. DOI:10.1186/s12917-014-0282-8 |
| [34] |
XIAO Y P, XIANG Y, ZHOU W D, et al. Microbial community mapping in intestinal tract of broiler chicken[J]. Poultry Science, 2017, 96(5): 1387-1393. DOI:10.3382/ps/pew372 |
| [35] |
YANG G Q, ZHANG P, LIU H Y, et al. Spatial variations in intestinal skatole production and microbial composition in broiler[J]. Animal Science Journal, 2019, 90(3): 412-422. DOI:10.1111/asj.13164 |
| [36] |
李彩燕. 日粮纤维对猪体粪臭素沉积的调控及分子机理研究[D]. 硕士学位论文. 杭州: 浙江大学, 2009. LI C Y.Effects of dietary fiber on skatole levels in swine body and the underlying regulatory mechanisms[D].Master's Thesis.Hangzhou: Zhejiang University, 2009.(in Chinese) |
| [37] |
MEINERT L, LUND B, BEJERHOLM C, et al. Distribution of skatole and androstenone in the pig carcass correlated to sensory characteristics[J]. Meat Science, 2017, 127: 51-56. DOI:10.1016/j.meatsci.2017.01.010 |
| [38] |
ROWE S J, KARACAÖREN B, DE KONING D J, et al. Analysis of the genetics of boar taint reveals both single SNPs and regional effects[J]. BMC Genomics, 2014, 15: 424. DOI:10.1186/1471-2164-15-424 |
| [39] |
MÖRLEIN D, LUNGERSHAUSEN M, STEINKE K, et al. A single nucleotide polymorphism in the CYP2E1 gene promoter affects skatole content in backfat of boars of two commercial Duroc-sired crossbred populations[J]. Meat Science, 2012, 92(4): 739-744. DOI:10.1016/j.meatsci.2012.06.031 |
| [40] |
DIAZ G J, SKORDOS K W, YOST G S, et al. Identification of phase Ⅰ metabolites of 3-methylindole produced by pig liver microsomes[J]. Drug Metabolism and Disposition, 1999, 27(10): 1150-1156. |
| [41] |
BABOL J, SQUIRES E J, LUNDSTRÖM K. Relationship between oxidation and conjugation metabolism of skatole in pig liver and concentrations of skatole in fat[J]. Journal of Animal Science, 1998, 76(3): 829-838. DOI:10.2527/1998.763829x |
| [42] |
RASMUSSEN M K, ZAMARATSKAIA G. Regulation of porcine hepatic cytochrome P450-implication for boar taint[J]. Computational and Structural Biotechnology Journal, 2014, 11(19): 106-112. DOI:10.1016/j.csbj.2014.09.003 |
| [43] |
ZAMARATSKAIA G, THØGERSEN R, ČANDEK-POTOKAR M, et al. Co-treatment with indole-3-carbinol and resveratrol modify porcine CYP1A and CYP3A activities and expression[J]. Xenobiotica, 2018, 48(3): 232-240. DOI:10.1080/00498254.2017.1300708 |
| [44] |
BURKINA V, ZLABEK V, RASMUSSEN M K, et al. End-product inhibition of skatole-metabolising enzymes CYP1A, CYP2A19 and CYP2E1 in porcine and piscine hepatic microsomes[J]. Toxicology Letters, 2019, 303: 67-71. DOI:10.1016/j.toxlet.2018.12.017 |
| [45] |
BAEK C, HANSEN-MØLLER J, FRⅡS C, et al. Identification of selected metabolites of skatole in plasma and urine from pigs[J]. Journal of Agricultural and Food Chemistry, 1997, 45(6): 2332-2340. DOI:10.1021/jf9605862 |
| [46] |
BRUNIUS C, VIDANARACHCHI J, TOMANKOVA J, et al. Skatole metabolites in urine as a biological marker of pigs with enhanced hepatic metabolism[J]. Animal, 2016, 10(10): 1734-1740. DOI:10.1017/S1751731116000574 |
| [47] |
刘吉喆, 赵歆昀, 杨桂芹. 家禽排泄物臭气化合物的产生及降低途径研究进展[J]. 饲料工业, 2019, 40(7): 58-64. LIU J Z, ZHAO X Y, YANG G Q. Research progress on generation and reduction of odor-causing compounds in excreta of poultry[J]. Feed Industry, 2019, 40(7): 58-64 (in Chinese). |
| [48] |
廖新俤, 吴银宝, 王燕, 等. 畜禽养殖场臭气综合治理技术研究进展[J]. 中国家禽, 2019, 41(17): 1-8. LIAO X D, WU Y B, WANG Y, et al. Research progress on comprehensive treatments of farm odor[J]. China Poultry, 2019, 41(17): 1-8 (in Chinese). |
| [49] |
CHO S, HWANG O, PARK S. Effect of dietary protein levels on composition of odorous compounds and bacterial ecology in pig manure[J]. Asian-Australasian Journal of Animal Sciences, 2015, 28(9): 1362-1370. DOI:10.5713/ajas.15.0078 |
| [50] |
RECHARLA N, KIM K, PARK J, et al. Effects of amino acid composition in pig diet on odorous compounds and microbial characteristics of swine excreta[J]. Journal of Animal Science and Technology, 2017, 59: 28. DOI:10.1186/s40781-017-0153-5 |
| [51] |
KHATTAK F, HELMBRECHT A. Effect of different levels of tryptophan on productive performance, egg quality, blood biochemistry, and caecalmirobiota of hens housed in enriched colony cages under commercial stocking density[J]. Poultry Science, 2019, 98(5): 2094-2104. DOI:10.3382/ps/pey562 |
| [52] |
ØVERLAND M, KJOS N K, FAUSKE A K, et al. Easily fermentable carbohydrates reduce skatole formation in the distal intestine of entire male pigs[J]. Livestock Science, 2011, 140(1/2/3): 206-217. |
| [53] |
LI X Q, JENSEN B B, CANIBE N. The mode of action of chicory roots on skatole production in entire male pigs is neither via reducing the population of skatole-producing bacteria nor via increased butyrate production in the hindgut[J]. Applied and Environmental Microbiology, 2019, 85(6): e02327-18. |
| [54] |
辛娜, 刁其玉, 张乃锋. 粪臭素对动物的作用机理及其减少排放的有效方法[J]. 中国饲料, 2011(8): 10-12, 15. XIN N, DIAO Q Y, ZHANG N F. The mechanism of action of faecan on animals and the effective methods to reduce the emission[J]. Chinese Feed, 2011(8): 10-12, 15 (in Chinese). |
| [55] |
ØVERLAND M, KJOS N P, BORG M, et al. Organic acids in diets for entire male pigs: effects on skatole level, microbiota in digesta, and growth performance[J]. Livestock Science, 2008, 115(2/3): 169-178. |
| [56] |
PAULY C, SPRING P, GAHAN D, et al. The effect of cereal type and enzyme supplementation on carcass characteristics, volatile fatty acids and intestinal microflora and boar taint in entire male pigs[J]. Animal, 2011, 5(3): 378-386. DOI:10.1017/S1751731110001849 |
| [57] |
LIU H Y, HOU R, YANG G Q, et al. In vitro effects of inulin and soya bean oligosaccharide on skatole production and the intestinal microbiota in broilers[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(3): 706-716. DOI:10.1111/jpn.12830 |
| [58] |
YANG G Q, YIN Y, LIU H Y, et al. Effects of dietary oligosaccharide supplementation on growth performance, concentrations of the major odor-causing compounds in excreta, and the cecal microflora of broilers[J]. Poultry Science, 2016, 95(10): 2342-2351. DOI:10.3382/ps/pew124 |
| [59] |
YANG G Q, LIN X J. Effects of dietary oligosaccharides on growth performance, concentration of main compounds producing odor in feces and cecal microflora of broilers[J]. Guangdong Feed, 2016, 25(10): 49 (in Chinese). |
| [60] |
ZHU X, LIU J Z, LIU H Y, et al. Soybean oligosaccharide, stachyose, and raffinose in broilers: effects on odor compound concentration and microbiota in cecal digesta[J]. Poultry Science, 2020, 99(7): 3532-3539. DOI:10.1016/j.psj.2020.03.034 |
| [61] |
慕春龙, 马梅蕾, 何香玉, 等. 体外法探究果寡糖对猪后肠微生物代谢苯丙氨酸和色氨酸的影响[J]. 畜牧兽医学报, 2018, 49(3): 559-564. MU C L, MA M L, HE X Y, et al. Effect of FOS on metabolism of phenylalanine and tryptophan in pig hindgut bacteria fermentation broths in vitro[J]. Acta Veterinaria et ZootechnicaSinica, 2018, 49(3): 559-564 (in Chinese). |
| [62] |
ALUWE M, HEYRMAN E, THEIS S, et al. Chicory fructans in pig diet reduce skatole in back fat of entire male pigs[J]. Research in Veterinary Science, 2017, 115: 340-344. DOI:10.1016/j.rvsc.2017.06.016 |
| [63] |
SHENG Q K, ZHOU K F, HU H M, et al. Effect of Bacillus subtilis natto on meat quality and skatole content in TOPIGS pigs[J]. Asian-Australasian Journal of Animal Science, 2016, 29(5): 716-721. |
| [64] |
TAVENDALE M H, LANE G A, SCHREURS N M, et al. The effects of condensed tannins from Dorycnium rectum on skatole and indole ruminal biogenesis for grazing sheep[J]. Australian Journal of Agricultural Research, 2005, 56(12): 1331-1337. DOI:10.1071/AR04232 |
| [65] |
ČANDEK-POTOKAR M, ŠKRLEP M, LUKAČN B, et al. Hydrolysable tannin fed to entire male pigs affects intestinal production, tissue deposition and hepatic clearance of skatole[J]. The Veterinary Journal, 2015, 204(2): 162-167. DOI:10.1016/j.tvjl.2015.02.012 |
| [66] |
SCHREURS N M, TAVEBDALE M H, LANE G A, et al. The effect of supplementation of a white clover or perennial ryegrass diet with grape seed extract on indole and skatole metabolism and the sensory characteristics of lamb[J]. Journal of the Science of Food and Agriculture, 2007, 87(6): 1030-1041. DOI:10.1002/jsfa.2802 |
| [67] |
GUFFANTI P, PIFFERI V, FALCIOLA L, et al. Analyses of odours from concentrated animal feeding operations: a review[J]. Atmospheric Environment, 2018, 175: 100-108. DOI:10.1016/j.atmosenv.2017.12.007 |
| [68] |
YIN B, HUANG L M, GU J D. Biodegradation of 1-methylindole and 3-methylindole by mangrove sediment enrichment cultures and a pure culture of an isolated Pseudomonas aeruginosa Gs[J]. Water, Air, and Soil Pollution, 2006, 176(1/2/3/4): 185-199. DOI:10.1007/s11270-006-9159-1 |
| [69] |
LI P, TONG L, LIU K, et al. Biodegradation of 3-methylindole by Pseudomonas putida LPC24 under oxygen limited conditions[J]. Fresenius Environmental Bulletin, 2010, 19(2): 238-242. |
| [70] |
SHARMA N, DOERNER K C, ALOK P C, et al. Skatole remediation potential of Rhodopseudomonas palustris WKU-KDNS3 isolated from an animal waste lagoon[J]. Letters in Applied Microbiology, 2015, 60(3): 298-306. DOI:10.1111/lam.12379 |
| [71] |
MENG X, HE Z F, LI H J, et al. Removal of 3-methylindole by lactic acid bacteria in vitro[J]. Experimental and Therapeutic Medicine, 2013, 6(4): 983-988. DOI:10.3892/etm.2013.1251 |
| [72] |
TESSO T A, ZHENG A J, CAI H Y, et al.Isolation and characterization of two Acinetobacter species able to degrade 3-methylindole[J].PLoS One, 14(1): e0211275.
|
| [73] |
FUKUOKA K, OZEKI Y, KANALY R. Aerobic biotransformation of 3-methylindole to ring cleavage products by Cupriavidus sp.strain KK10[J]. Biodegradation, 2015, 26(5): 359-373. DOI:10.1007/s10532-015-9739-0 |
| [74] |
MA Q, QU H, MENG N, et al. Biodegradation of skatole by Burkholderia sp.IDO3 and its successful bioaugmentation in activated sludge systems[J]. Environmental Research, 2020, 182: 109123. DOI:10.1016/j.envres.2020.109123 |
| [75] |
MA Q, LIU S W, LI S Z, et al. Removal of malodorant skatole by two enriched microbial consortia: performance, dynamic, function prediction and bacteria isolation[J]. Science of the Total Environment, 2020, 725: 138416. DOI:10.1016/j.scitotenv.2020.138416 |



