肠道作为机体抵御外来异源物质的第一道屏障,对机体内环境稳态的维持起重要作用。肠道上皮组织是肠道防御屏障中最大的一类,一旦肠上皮细胞或免疫细胞的稳态受到破坏,肠道菌群失调,会使得机体容易感染病菌,引发炎症性肠病。近年来,由病毒入侵、细菌感染、霉菌毒素中毒、饲料脂肪氧化酸败以及恶劣饲养环境等导致的肠道性疾病日益严重。同时,长期不规范使用抗生素和化学药物导致药物残留及抗生素耐药等给畜禽治疗带来困难,并危害人类健康。目前,随着我国在畜禽饲料中全面禁止使用抗生素条例的实施,研发饲用抗生素的替代品是必然趋势。姜黄素是一种天然植物多酚类物质,来源广泛,具有抗氧化、抗细胞凋亡、抗炎、免疫调节和代谢调控等生物学功能[1];其生物活性多样,被认为是绿色、安全、高效的饲料添加剂,具有良好的应用前景。近年来,研究表明,姜黄素可剂量依赖性抑制肠上皮细胞雷帕霉素靶蛋白复合体1(mammalian target of rapamycin complex 1,mTORC1)的活化,并降低肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)诱导的环氧合酶2表达,从而缓解肠道炎症[2]。姜黄素可抑制由白细胞介素(interluekin,IL)-1α诱导的人结肠癌细胞Caco-2上皮屏障通透性增加[3]。姜黄素通过调节肠道屏障系统的细胞旁路通透性来恢复肠道屏障功能[4]。此外,有研究发现,姜黄素对肠道菌群结构的调节以及肠道菌群对姜黄素的生物转化是其发挥功能作用的关键[5-6]。因此,本文主要介绍姜黄素对动物疾病的治疗作用,并通过对动物肠道健康、应用小鼠等动物模型的相关研究,综述了姜黄素的生物活性及其调节动物肠道黏膜屏障功能的分子机制,以期为畜禽产业的健康可持续发展提供理论依据。
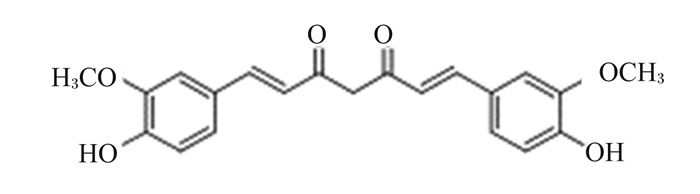
1 姜黄素及其代谢产物 1.1 姜黄素的结构和理化性质姜黄素是从姜科植物姜黄(Curcumin longa)和天南星科(Araceae)植物根茎中提取的一种天然多酚类或二酮类化合物。姜黄素的化学结构如图 1所示,其分子式为C21H20O6,相对分子质量为368.37。姜黄素为橙黄色结晶粉末,有特殊辛辣味,熔点为183 ℃,难溶于水和乙醚,易溶于乙醇和二甲基亚砜等有机溶剂。姜黄素是由2个邻甲基化的酚以及1个β-二酮基组成,在酸性介质中呈淡黄色,在碱性介质中呈红褐色。姜黄素具有光敏感性,见光很容易降解。姜黄中的主要成分为姜黄素约占姜黄总量的70%,除了姜黄素之外,还包含2种衍生物:去甲氧基姜黄素和双去甲氧基姜黄素[7]。
|
图 1 姜黄素的化学结构 Fig. 1 Chemical structure of curcumin |
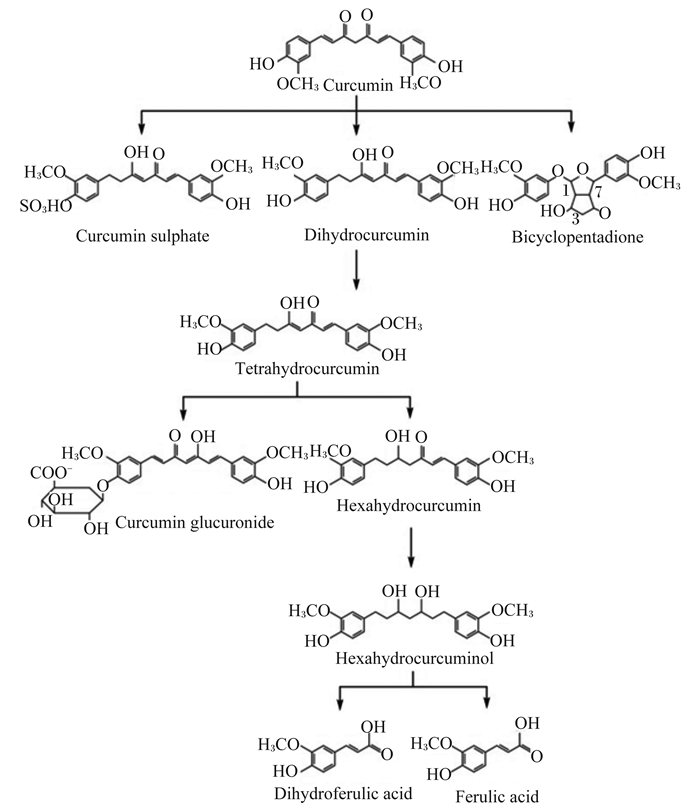
姜黄素的吸收、代谢及组织分布对其活性的发挥至关重要。姜黄素作为亲脂性的多酚类物质,极不溶于水,口服生物利用率低,容易迅速代谢成非活性代谢物。采用胶束、脂质体、磷脂复合物、微乳剂、纳米乳剂、固体脂质纳米载体、无定形纳米颗粒、生物聚合纳米颗粒和微凝胶等传递系统能提高姜黄素的生物学利用率[8-11]。姜黄素主要以生物还原作用生成四氢姜黄素、六氢姜黄素和八氢姜黄素。姜黄素可与氧缀合成姜黄素葡萄糖苷酸和姜黄素硫酸盐,或六氢姜黄素醇化形成六氢姜黄醇,最后通过胆汁排泄形成少量二氢阿魏酸和微量阿魏酸[12](图 2)。近年来研究发现,姜黄素的潜在生物学功能可能不一定取决于其生物利用率,相反可能来自于它对胃肠道健康和功能的积极影响。姜黄素会影响肠道菌群结构、调节肠道通透性、减少胃肠道的炎症和氧化应激;并对细菌、寄生虫和真菌感染产生影响[5-6, 13-15]。同时,姜黄素受动物肠道菌群的生物转化,许多代谢产物具有比亲本姜黄素更强的药理活性和更高的生物利用率[14]。与亲本姜黄素相比,四氢姜黄素和八氢姜黄素可与细胞色素酶CYP2E1活性位点结合抑制其活性,同时激活抗氧化信号通路,具有更优异的抗氧化活性[16]。四氢姜黄素和八氢姜黄素能减少小鼠结肠核因子-κB(nuclear factor-κB,NF-κB)以及环氧合酶2和诱导型一氧化氮合酶的表达,抑制作用效果更好;六氢姜黄素对环氧合酶2的抑制活性明显低于亲本姜黄素[17-18];而去甲氧基姜黄素具有更强的体内稳定性和抗炎特性[19]。因此,肠道菌群转化的代谢产物及其降解产物应被视为鉴定姜黄素生物活性分子的重要来源。这可以进一步解释姜黄素的低生物利用率与通常报道的治疗效果之间的矛盾。鉴于不同物种之间肠道微生物结构的不同,因此,研究确定姜黄素在不同动物不同生理条件下体内代谢、转化以及生物利用率是否存在差异,对姜黄素相关作用机制的研究及应用具有指导意义。
|
Curcumin:姜黄素;Curcumin sulphate:姜黄素硫化物;Dihydrocurcumin:二氢姜黄素;Bicyclopentadione:环戊二烯;Tetrahydrocurcumin:四氢姜黄素;Curcumin glucuronide:姜黄素葡萄糖醛酸;Hexahydrocurcumin:六氢姜黄素;Hexahydrocurcuminol:六氢姜黄醇;Dihydroferulic acid:二氢阿魏酸;Ferulic acid:阿魏酸。 图 2 姜黄素的代谢 Fig. 2 Metabolism of curcumin[9] |
Nrf2是转录因子家族成员,是细胞氧化还原反应中最强的转录调控因子,通过与ARE相互作用,调节细胞内的氧化还原稳态[20]。动物胃肠道是活性氧(reactive oxygen species,ROS)的主要来源,ROS增多时,机体自身激活Nrf2-ARE信号通路,诱导下游相关抗氧化蛋白/酶和Ⅱ相代谢酶表达,缓解机体氧化应激[21]。肠道屏障功能对维持动物胃肠道的健康稳定状态至关重要,研究显示Nrf2在维持肠道黏膜屏障完整性中起重要作用[22]。Wang等[23]研究表明,姜黄素可通过诱导血红素氧合酶1转录激活改善过氧化氢(H2O2)介导的人肠上皮细胞紧密连接和肠黏膜屏障功能受损。饲粮添加200 mg/kg的姜黄素可通过Keap1-Nrf2途径改善宫内生长受限仔猪空肠抗氧化功能,从而进一步改善空肠紧密连接和免疫功能,缓解宫内生长受限仔猪空肠氧化应激[24]。此外,饲粮添加400或800 mg/kg的姜黄素提高了1~21日龄北京鸭空肠黏膜抗氧化酶活性,并降低DNA损伤的标志物8-羟基脱氧鸟苷和脂质过氧化物丙二醛含量,血红素氧合酶1和核因子Nrf2表达上调[25]。研究报道,从姜黄中分离出的184种致毒性的化合物中,发现姜黄素及其衍生物可能引起剂量依赖性肝毒性[26]。Qiu等[27]评估了姜黄素对小鼠的亚慢性毒性效果,结果表明,高剂量的姜黄素诱导小鼠产生ROS并使超氧化物歧化酶和谷胱甘肽硫转移酶活性降低。过量或长期摄入姜黄素可能通过氧化应激、炎症和代谢紊乱引发机体代谢失衡。迄今为止,姜黄素对动物毒性作用特别是对胃肠道副作用的报道非常少,因此确定姜黄素是否安全及其在畜禽饲料中适宜添加量至关重要。近年来研究发现,肠道微生物代谢物可通过激活芳香基芳烃受体(aryl hydrocarbon receptor,AhR)-Nrf2依赖性途径上调紧密连接蛋白表达,增强肠道黏膜屏障功能[28]。越来越多的研究表明,姜黄素对肠道菌群具有有益作用,有利于益生菌菌群的生长,并减少有害菌菌株含量[6, 29-30]。目前关于姜黄素对畜禽具体的抗氧化作用机制仍不十分明确,姜黄素是否通过调节肠道微生物代谢,激活AhR-Nrf2途径改善肠道屏障功能未见报道。
2.2 姜黄素通过抗细胞凋亡途径改善肠道黏膜屏障细胞凋亡或程序性细胞死亡对大多数细胞生物的正常功能和存活必不可少。目前认为,细胞凋亡可分为死亡受体途径、线粒体途径和内质网途径。近年来研究表明,姜黄素可通过抗凋亡机制降低细胞色素C和半胱天冬酶3表达以及提高线粒体B细胞淋巴瘤2(B-cell lymphoma 2, Bcl2)家族中抗凋亡蛋白表达抑制氧化应激诱导的肠上皮细胞凋亡,保护肠黏膜屏障功能[31]。在X射线诱导的小鼠肠黏膜损伤模型中,姜黄素可上调抗凋亡基因Bcl2的转录及蛋白表达,抑制凋亡相关半胱天冬酶3的活化,减轻肠上皮细胞凋亡[32]。Kim等[33]研究报道,姜黄素可以降低脱氧核苷酸末端转移酶介导的dUTP缺口末端标记(terminal dexynucleotidyl transferase-mediated dUTP nick end labeling,TUNEL)样性DNA片段和凋亡相关蛋白水平,阻断革兰氏阴性菌创伤弧菌(Vibrio vulnificus)引发宿主胃肠细胞凋亡。线粒体能量代谢是细胞最基本、最重要的活动之一。有研究发现,姜黄素作为脂溶性物质可以特异性的富集于线粒体中,维持细胞能量代谢、细胞保护和生物合成,缓解应激源导致的肠上皮细胞氧化损伤。Ruan等[34]报道,姜黄素可抑制赭曲霉毒素A诱导的北京鸭空肠黏膜凋亡相关基因的表达,并下调线粒体转录因子A、B1和B2的mRNA表达水平,提高空肠黏膜紧密连接蛋白的表达。以上研究揭示,姜黄素可通过线粒体途径调控细胞凋亡,缓解肠黏膜细胞凋亡造成的肠道通透性增加及其屏障功能的降低。目前,有关姜黄素介导的线粒体凋亡途径的具体机制尚不明确。
2.3 姜黄素通过调控细胞自噬改善肠道黏膜屏障自噬是一种重要的细胞机制,在正常生理过程中起“管家”作用,对于肠道稳态维持,肠道生态调节,适当的肠道免疫反应和抗菌保护至关重要。细胞自噬依赖于自噬相关蛋白(autophagy-related protein,ATG)和Ras相关蛋白以及与自噬蛋白相结合的关键蛋白,如雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)、自噬标志蛋白1(Beclin1)和LC3[35-37]。姜黄素是一种天然的自噬调节剂,体内和体外试验研究表明,姜黄素诱导的自噬由许多信号通路介导,其中包括磷脂酰肌醇3-激酶(phosphatidylinositol-3-kinases,PI3K)/蛋白质丝氨酸苏氨酸激酶(protein-serine-threonine kinase,Akt)/mTOR、腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)、丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)/细胞外调节激酶(extracellular regulated protein kinases 1/2,ERK1/2)、Bcl2信号级联和Rab GTPase激活蛋白网络[38]。在人结肠癌细胞中,姜黄素通过抑制Akt/mTOR信号通路直接靶向激活转录因子EB(transcription factor EB,TFEB),增加下游靶分子溶酶体膜蛋白1和自噬相关基因ATG9B的表达,进而激活自噬和增加溶酶体功能[39]。线粒体自噬在清除功能障碍线粒体,保证机体能量供应,以及肠黏膜损伤修复中发挥重要作用。Cao等[40]研究发现,姜黄素通过激活AMPK信号途径诱导TFEB的核移位启动PTEN诱导假定激酶1(PTEN induced putative kinase 1,PINK1)/帕金依赖性线粒体自噬缓解氧化应激,减轻猪肠上皮细胞线粒体损伤,保护肠道屏障功能。
沉默信息调节因子2相关酶1(silent information regulator factor 2 homolog 1,SIRT1)广泛表达于小肠和结肠等肠上皮细胞,是烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD+)依赖的组蛋白去乙酰化酶,在细胞氧化、细胞凋亡、炎症和能量代谢、肠道菌群结构等方面发挥重要作用[41-42]。在氧化应激条件下,SIRT1可通过PI3K/Beclin1、过氧化物酶体增殖物激活受体γ共活化因子1α(peroxisome proliferator-activated receptor gamma coactivator 1-alpha,PGC-1α)和mTOR途径介导调节胚胎干细胞自噬和线粒体功能,同时刺激肠道干细胞生长及促进紧密连接完整性,从而增强肠道屏障功能[43-45]。Zhang等[46]研究姜黄素对小鼠葡聚糖硫酸钠(DSS)引起的溃疡性结肠炎保护作用,发现姜黄素可下调结肠Beclin1表达,上调磷酸化的mTOR和SIRT1的表达,抑制小鼠肠炎级联反应,明显改善结肠组织结构。叉头框转录因子O亚族(forkhead box transcription factor O,FoxO)是一类关键的自噬调控因子,以FoxO1和FoxO3的作用最广泛。可通过激活细胞自噬活性,在心脏、肝脏、肠道及骨骼肌等多种器官组织中,参与细胞增殖、代谢和存活等过程。姜黄素增加人脐静脉内皮细胞自噬过程中乙酰化FoxO1水平,加强乙酰化FoxO1与ATG7间的相互作用,促进自噬,提供抗氧化应激保护作用[47]。近年来研究发现,SIRT1可能是宿主与微生物组相互作用的关键因子,SIRT1可以调节肠道菌群结构来预防肠道炎症[48]。而自噬缺陷可破坏肠道内稳态,影响肠道菌群组成,损害细胞内细菌清除率和扩大肠内炎症反应[49-50]。但尚不清楚姜黄素是否可通过SIRT1调控细胞自噬改善肠黏膜微生物屏障。
2.4 姜黄素通过Toll样受体(Toll-like receptor,TLR)4/NF-κB/激活蛋白1(activator protein 1,AP-1)信号改善肠道黏膜屏障TLR作为模式识别受体,可识别病原微生物双链DNA、单链RNA、脂多糖(lipopolysaccharide,LPS)、脂蛋白及鞭毛蛋白,从而激活细胞内信号通路,伴随促炎症细胞因子、趋化因子和干扰素的产生,调节适应性免疫反应[51]。部分TLR(TLR1、TLR2、TLR4和TLR5)可通过含Toll-IL-1受体结构域接头蛋白/髓样分化因子88(myeloid differentiation factor 88,MyD88)/NF-κB信号通路、Janus活化激酶/信号转导与转录激活子信号通路、MAPK、Notch通路和PI3K/Akt等信号通路,参与肠黏膜炎症基因的转录调节[52-53]。已有研究表明,姜黄素可抑制MyD88依赖性和非依赖性的信号传导机制[54]。此外,姜黄素可与TLR结构域外髓样分化蛋白2结合,抑制对LPS的先天免疫应答。姜黄素还可以在下游步骤(包括肿瘤坏死因子受体相关因子6和IL-1受体相关激酶)以及免疫调节(如单核细胞趋化蛋白1和巨噬细胞炎症蛋白)和信号传导相关细胞因子阻断作用而抑制TLR信号传导[54-55]。在结肠癌上皮细胞Caco-2模型中,姜黄素可减弱LPS诱导的促炎症细胞因子释放和紧密连接蛋白破坏,这很可能是通过TLR依赖性信号传导降低导致[56]。在动物模型中,Gan等[57]研究证实,姜黄素可下调断奶仔猪肠道TLR4信号通路,抑制IL-1β和TNF-α等关键炎症因子的释放,减轻TLR介导的肠道炎症,最终提高肠道免疫屏障功能。
在TLR下游,NF-κB和AP-1作为转录因子与DNA结合调节炎症、细胞分化、增殖和凋亡基因的表达,对肠道黏膜屏障的调控起重要作用。NF-κB信号途径可通过细胞因子受体配体、模式识别受体、ROS、肿瘤坏死因子受体蛋白、T细胞受体和B细胞受体激活[58]。姜黄素可通过抑制NF-κB抑制蛋白激酶(inhibitor of NF-κB kinase,IKK)的活性来防止NF-κB抑制蛋白(IκB)磷酸化,并抑制NF-κB的活化。Tian等[59]研究报道,姜黄素可能通过抑制NF-κB的活化而降低TNF-α的分泌,促进肠道通透性的恢复以及增强紧密连接蛋白-1(zonula occludens,ZO-1)表达保护大鼠肠黏膜组织免受缺血再灌注的损伤。Wang等[56]研究发现,姜黄素预处理减弱LPS对肠上皮细胞和巨噬细胞中主要促炎症细胞因子IL-1β的分泌,降低IL-1β诱导的肠上皮细胞中p38-MAPK的激活,并抑制肌动蛋白轻链激酶诱导的紧密连接相关蛋白磷酸化,免受LPS诱导的肠道通透性增加。Eckert等[60]用姜黄素类似物治疗T84肠上皮单层细胞发现,姜黄素类似物降低了IL-6诱导炎症相关的细胞旁通透性,这可能是由于TLR4/NF-κB依赖性信号传导降低所致。AP-1家族成员是磷酸蛋白,他们的活性受激酶和磷酸激酶相互作用影响。MAPK的磷酸化、蛋白激酶A和C以及糖原合成激酶3都影响AP-1的活性和功能。在AP-1信号传导中,姜黄素可以直接或间接的抑制MAPK、ERK1/2、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)和p38信号途径从而限制炎症靶基因的转录释放[61]。姜黄素预处理小鼠巨噬细胞可降低ERK、p38和JNK的磷酸化,有效抑制LPS诱导的促炎症细胞因子TNF-α释放和免疫调节剂microRNA-155的表达[62]。此外,姜黄素可能通过AP-1依赖途径减少树突状细胞中细胞黏附和T细胞激活相关的细胞间黏附分子1和CD11c的表达[63]。
2.5 姜黄素通过辅助性T细胞(T-helper, Th)17/调节性T细胞(T-regulatory cell, Treg)调节肠道黏膜屏障CD4+ Th主要分为Th1、Th2、Th17和Treg,在机体抵御病原体方面起核心作用。Th17和Treg主要分布在肠道屏障表面,特别是在肠黏膜中,分别在保护宿主免受病原微生物侵袭和抑制过度的效应T细胞反应中起作用。Th17与Treg在功能上相互制约,两者之间的平衡对肠黏膜稳态具有重要意义[64]。姜黄素能够潜在抑制树突状细胞的成熟、诱导耐受性选择,调节树突状细胞的活化来增强Treg细胞的抑制功能,促进炎症性肠炎中受损的肠黏膜修复[65]。Zhao等[66]发现姜黄素可以增加被LPS激活的小鼠树突状细胞中代谢型谷氨酸受体表达,降低小鼠树突状细胞产生促炎症因子IL-6和IL-23,同时与Th17细胞相关的细胞因子IL-17A和维甲酸相关孤核受体γt表达也降低,进而诱导产生Treg和抑制Th17的分化,在炎症性肠病和自身免疫性疾病的调节起至关重要作用。此外,肠道菌群可通过分泌炎症细胞因子、小分子物质或短链脂肪酸等物质介导或影响Th17/Treg免疫平衡,调节肠道屏障功能。姜黄素可增加产丁酸盐细菌丰度,并伴随着结肠黏膜中CD4+Foxp3+Treg和CD103+CD8α调节性树突状细胞的扩增,从而抑制DSS诱导的小鼠结肠炎发展[67]。因此,姜黄素通过免疫细胞间相互作用来调节肠道黏膜免疫系统,并可能在各种炎症和自身免疫疾病中具有治疗作用。同时,姜黄素对微生物组有有益作用,但姜黄素是否可以通过修饰肠道微生物群来调节Th17/Treg平衡报道很少。
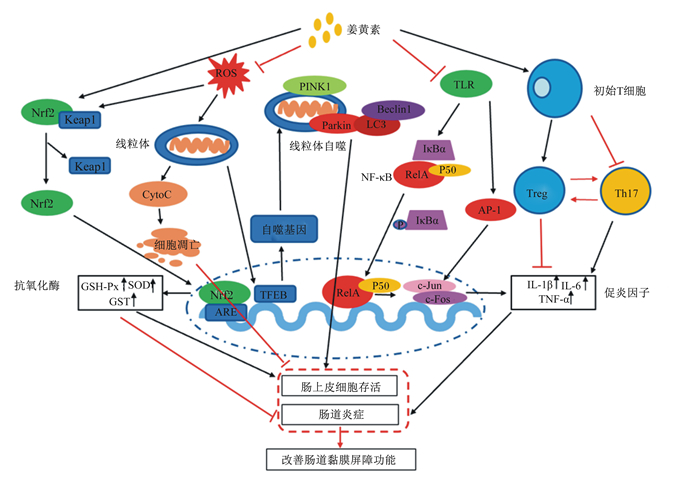
综上所述,姜黄素主要通过模式识别受体、核受体、细胞凋亡和细胞自噬等信号传导通路发挥抗氧化、抗细胞凋亡、抗炎、免疫调节和代谢调控等作用,从而改善肠道黏膜屏障功能(图 3)。
|
Nrf2:核因子E2相关因子2 nuclear factor erythroid 2-related factor 2;Keap1:Kelch样环氧氯丙烷相关蛋白1 Kelch-like ECH-associated protein 1; ARE:抗氧化反应元件antioxidant response element;GSH-Px:谷胱甘肽过氧化物酶glutathione peroxidase;SOD:超氧化物歧化酶superoxide dismutase;GST:谷胱甘肽硫转移酶glutathione S-transferase;ROS:活性氧reactive oxygen species;CytoC: 细胞色素C cytochrome C; TFEB:核转录因子EB transcription factor EB;PINK1:PTEN诱导假定激酶1 PTEN induced putative kinase 1;Beclin1:自噬标志蛋白1;TLR: Toll样受体Toll-like receptor;NF-κB:核因子-κB nuclear factor-κB;IκBα:核因子-κB抑制蛋白α nuclear factor-κB inhibitor α;AP-1:激活蛋白1 activator protein 1;Treg:调节性T细胞T-regulatory cell;Th17:辅助性T细胞17 T-helper 17;IL-1β:白细胞介素-1β interleukin-1β;IL-6:白细胞介素-6 interleukin-6;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α。 图 3 姜黄素调节肠道黏膜屏障功能的分子机制 Fig. 3 Molecular mechanism of curcumin regulating intestinal mucosal barrier function |
畜禽营养不良、应激、病毒和病原体侵袭等引起的肠道性疾病会破坏肠道内稳态,诱发大量炎症因子的释放,引起肠道上皮细胞凋亡、肠黏膜细胞间紧密连接被破坏,肠道微生物区系发生改变,导致肠道黏膜屏障功能受损。目前,姜黄素在畜禽生产应用方面的研究仍然有限。本文通过动物疾病、肠上皮细胞、小鼠类动物模型阐述姜黄素调控肠道屏障功能的作用机制,为姜黄素对畜禽肠道黏膜屏障功能的保护作用提供科学依据。然而,还需要进一步深入研究姜黄素在不同动物、不同生理状态下的吸收与代谢及改善肠道黏膜屏障功能的作用机制、作用靶点,姜黄素诱导的能量代谢对机体天然免疫细胞的分化与成熟机制,姜黄素的低生物学利用率与肠道微生物之间的互作关系,肠道微生物及其代谢物对相关的信号通路的作用机制,确定姜黄素对胃肠道副作用及其在畜禽饲料中的适宜添加量, 以期为生产中维护畜禽肠道健康提供科学指导。
| [1] |
HUSSAIN Z, EI THU H, AMJAD M W, et al. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: a review of new trends and future perspectives[J]. Materials Science and Engineering: C, 2017, 77: 1316-1326. DOI:10.1016/j.msec.2017.03.226 |
| [2] |
KAUR H, HE B, ZHANG C H, et al. Piperine potentiates curcumin-mediated repression of mTORC1 signaling in human intestinal epithelial cells: implications for the inhibition of protein synthesis and TNFα signaling[J]. Journal of Nutritional Biochemistry, 2018, 57: 276-286. DOI:10.1016/j.jnutbio.2018.04.010 |
| [3] |
KIM C Y. Inhibition of interleukin-1α-induced intestinal epithelial tight junction permeability by curcumin treatment in Caco-2 cells in Caco-2 cells[J]. Journal of Life Science, 2016, 26(9): 1082-1087. DOI:10.5352/JLS.2016.26.9.1082 |
| [4] |
GHOSH S S, HE H L, WANG J, et al. Curcumin-mediated regulation of intestinal barrier function: the mechanism underlying its beneficial effects[J]. Tissue Barriers, 2018, 6(1): e1425085. DOI:10.1080/21688370.2018.1425085 |
| [5] |
LOPRESTI A L. The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects?[J]. Advances in Nutrition, 2018, 9(1): 41-50. DOI:10.1093/advances/nmx011 |
| [6] |
SCAZZOCCHIO B, MINGHETTI L, D'ARCHIVIO M. Interaction between gut microbiota and curcumin: a new key of understanding for the health effects of curcumin[J]. Nutrients, 2020, 12(9): 2499. DOI:10.3390/nu12092499 |
| [7] |
TSUDA T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives[J]. Food & Function, 2018, 9(2): 705-714. |
| [8] |
TIAN M P, SONG R X, WANG T, et al. Inducing sustained release and improving oral bioavailability of curcumin via chitosan derivatives-coated liposomes[J]. International Journal of Biological Macromolecules, 2018, 120: 702-710. DOI:10.1016/j.ijbiomac.2018.08.146 |
| [9] |
KIMURA S, KIRIYAMA A, ARAKI K, et al. Novel strategy for improving the bioavailability of curcumin based on a new membrane transport mechanism that directly involves solid particles[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2018, 122: 1-5. DOI:10.1016/j.ejpb.2017.09.017 |
| [10] |
BAN C, JO M, PARK Y H, et al. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles[J]. Food Chemistry, 2020, 302: 125328. DOI:10.1016/j.foodchem.2019.125328 |
| [11] |
STOHS S J, CHEN O, RAY S D, et al. Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: a review[J]. Molecules, 2020, 25(6): 1397. DOI:10.3390/molecules25061397 |
| [12] |
PRASAD S, TYAGI A K, AGGARWAL B B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice[J]. Cancer Research and Treatment, 2014, 46(1): 2-18. DOI:10.4143/crt.2014.46.1.2 |
| [13] |
ZHANG Z G, CHEN Y J, XIANG L H, et al. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats[J]. Nutrients, 2017, 9(10): 1146. DOI:10.3390/nu9101146 |
| [14] |
SHEN L, JI H F. Bidirectional interactions between dietary curcumin and gut microbiota[J]. Critical Reviews in Food Science and Nutrition, 2019, 59(18): 2896-2902. DOI:10.1080/10408398.2018.1478388 |
| [15] |
PLUTA R, JANUSZEWSKI S, ULAMEK-KOZIOL M. Mutual two-way interactions of curcumin and gut microbiota[J]. International Journal of Molecular Sciences, 2020, 21(3): 1055. DOI:10.3390/ijms21031055 |
| [16] |
LUO D D, CHEN J F, LIU J J, et al. Tetrahydrocurcumin and octahydrocurcumin, the primary and final hydrogenated metabolites of curcumin, possess superior hepatic-protective effect against acetaminophen-induced liver injury: role of CYP2E1 and Keap1-Nrf2 pathway[J]. Food and Chemical Toxicology, 2019, 123: 349-362. DOI:10.1016/j.fct.2018.11.012 |
| [17] |
YANG J Y, ZHONG X C, KIM S J, et al. Comparative effects of curcumin and tetrahydrocurcumin on dextran sulfate sodium-induced colitis and inflammatory signaling in mice[J]. Journal of Cancer Prevention, 2018, 23(1): 18-24. DOI:10.15430/JCP.2018.23.1.18 |
| [18] |
ZHANG Z B, LUO D D, XIE J H, et al. Curcumin's metabolites, tetrahydrocurcumin and octahydrocurcumin, possess superior anti-inflammatory effects in vivo through suppression of TAK1-NF-κB pathway[J]. Frontiers in Pharmacology, 2018, 9: 1181. DOI:10.3389/fphar.2018.01181 |
| [19] |
TEYMOURI M, BARATI N, PIRRO M, et al. Biological and pharmacological evaluation of dimethoxycurcumin: a metabolically stable curcumin analogue with a promising therapeutic potential[J]. Journal of Cellular Physiology, 2018, 233(1): 124-140. DOI:10.1002/jcp.25749 |
| [20] |
LU M C, JI J A, JIANG Z Y, et al. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update[J]. Medicinal Research Reviews, 2016, 36(5): 924-963. DOI:10.1002/med.21396 |
| [21] |
BAI X P, CHEN Y B, HOU X Y, et al. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters[J]. Drug Metabolism Reviews, 2016, 48(4): 541-567. DOI:10.1080/03602532.2016.1197239 |
| [22] |
WEN Z Y, LIU W H, LI X, et al. A protective role of the Nrf2-Keap1 pathway in maintaining intestinal barrier function[J]. Oxidative Medicine and Cellular Longevity, 2019, 2019: 1759149. |
| [23] |
WANG N, WANG G, HAO J X, et al. Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells[J]. Digestive Diseases and Sciences, 2012, 57(7): 1792-1801. DOI:10.1007/s10620-012-2094-7 |
| [24] |
YAN E F, ZHANG J Q, HAN H L, et al. Curcumin alleviates IUGR jejunum damage by increasing antioxidant capacity through Nrf2/Keap1 pathway in growing pigs[J]. Animals, 2020, 10(1): 41. |
| [25] |
RUAN D, ZHU Y W, FOUAD A M, et al. Dietary curcumin enhances intestinal antioxidant capacity in ducklings via altering gene expression of antioxidant and key detoxification enzymes[J]. Poultry Science, 2019, 98(9): 3705-3714. DOI:10.3382/ps/pez058 |
| [26] |
BALAJI S, CHEMPAKAM B. Toxicity prediction of compounds from turmeric (Curcuma longa L)[J]. Food and Chemical Toxicology, 2010, 48(10): 2951-2959. DOI:10.1016/j.fct.2010.07.032 |
| [27] |
QIU P Y, MAN S L, LI J, et al. Overdose intake of curcumin initiates the unbalanced state of bodies[J]. Journal of Agricultural and Food Chemsitry, 2016, 64(13): 2765-2771. DOI:10.1021/acs.jafc.6b00053 |
| [28] |
SINGH R, CHANDRASHEKHARAPPA S, BODDULURI S R, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway[J]. Nature Communications, 2019, 10: 89. DOI:10.1038/s41467-018-07859-7 |
| [29] |
SHEN L, LIU L, JI H F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications[J]. Food Nutrition Research, 2017, 61: 1361780. DOI:10.1080/16546628.2017.1361780 |
| [30] |
PETERSON C T, VAUGHN A R, SHARMA V, et al. Effects of turmeric and curcumin dietary supplementation on human gut microbiota: a double-blind, reandomized, placebo-controlled pilot study[J]. Journal of Evidence-Based Integrative Medicine, 2018, 23: 2515690X. |
| [31] |
LOGANES C, LEGA S, BRAMUZZO M, et al. Curcumin anti-apoptotic action in a model of intestinal epithelial inflammatory damage[J]. Nutrients, 2017, 9(6): 578. DOI:10.3390/nu9060578 |
| [32] |
FUKUDA K, UEHARA Y, NAKATA E, et al. A diarylpentanoid curcumin analog exhibits improved radioprotective potential in the intestinal mucosa[J]. International Journal of Radiation Biology, 2016, 92(7): 388-394. DOI:10.3109/09553002.2016.1164910 |
| [33] |
KIM J Y, LEE Y M, KIM D W, et al. Nanosphere loaded with curcumin inhibits the gastrointestinal cell death signaling pathway induced by the foodborne pathogen Vibrio vulnificus[J]. Cells, 2020, 9(3): 631. DOI:10.3390/cells9030631 |
| [34] |
RUAN D, WANG W C, LIN C X, et al. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A[J]. Animal, 2019, 13(1): 42-52. DOI:10.1017/S1751731118000678 |
| [35] |
RAVANAN P, SRIKUMAR I F, TALWAR P. Autophagy: the spotlight for cellular stress responses[J]. Life Sciences, 2017, 188: 53-57. DOI:10.1016/j.lfs.2017.08.029 |
| [36] |
COSIN-ROGER J, SIMMEN S, MELHEM H, et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation[J]. Nature Communications, 2017, 8: 98. DOI:10.1038/s41467-017-00213-3 |
| [37] |
WONG M, GANAPATHY A S, SUCHANEC E, et al. Intestinal epithelial tight junction barrier regulation by autophagy-related protein ATG6/beclin[J]. American Journal of Physiology-Cell Physiology, 2019, 316(5): 753-765. DOI:10.1152/ajpcell.00246.2018 |
| [38] |
SHAKERI A, CICERO A F G, PANAHI Y, et al. Curcumin: a naturally occurring autophagy modulator[J]. Journal of Cellular Physiology, 2019, 234(5): 5643-5654. DOI:10.1002/jcp.27404 |
| [39] |
ZHANG J B, WANG J G, XU J, et al. Curcumin targets the TFEB-lysosome pathway for induction of autophagy[J]. Oncotarget, 2016, 7(46): 75659-75671. DOI:10.18632/oncotarget.12318 |
| [40] |
CAO S T, WANG C C, YAN J T, et al. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway[J]. Free Radical Biology and Medicine, 2020, 147: 8-22. DOI:10.1016/j.freeradbiomed.2019.12.004 |
| [41] |
ZHANG W J, HUANG Q B, ZENG Z H, et al. Sirt1 inhibits oxidative stress in vascular endothelial cells[J]. Oxidative Medicine and Cellular Longgevity, 2017, 2017: 7543973. |
| [42] |
REN M T, GU M L, ZHOU X X, et al. Sirtuin 1 alleviates endoplasmic reticulum stress-mediated apoptosis of intestinal epithelial cells in ulcerative colitis[J]. World Journal Gastroenterol, 2019, 25(38): 5800-5813. DOI:10.3748/wjg.v25.i38.5800 |
| [43] |
OU X, LEE M R, HUANG X X, et al. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress[J]. Stem Cells, 2014, 32(5): 1183-1194. DOI:10.1002/stem.1641 |
| [44] |
IGARASHI M, GUARENTE L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction[J]. Cell, 2016, 166(2): 436-450. DOI:10.1016/j.cell.2016.05.044 |
| [45] |
LIANG D Y, ZHUO Y S, GUO Z H, et al. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells[J]. Biochimie, 2020, 170: 10-20. DOI:10.1016/j.biochi.2019.12.001 |
| [46] |
ZHANG L Z, XUE H, ZHAO G, et al. Curcumin and resveratrol suppress dextran sulfate sodium-induced colitis in mice[J]. Molecular Medicine Reports, 2019, 19(4): 3053-3060. |
| [47] |
HAN J, PAN X Y, XU Y, et al. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage[J]. Autophagy, 2012, 8(5): 812-825. DOI:10.4161/auto.19471 |
| [48] |
WELLMAN A S, METUKURI M R, KAZGAN N, et al. Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota[J]. Gastroenterology, 2017, 153(3): 772-786. DOI:10.1053/j.gastro.2017.05.022 |
| [49] |
MARTIN P K, MARCHIANDO A, XU R L, et al. Autophagy proteins suppress protective type Ⅰ interferon signalling in response to the murine gut microbiota[J]. Nature Microbiology, 2018, 3(10): 1131-1141. DOI:10.1038/s41564-018-0229-0 |
| [50] |
LARABI A, BARNICH N, NGUYEN H T T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD[J]. Autophagy, 2020, 16(1): 38-51. DOI:10.1080/15548627.2019.1635384 |
| [51] |
LU Y, LI X R, LIU S S, et al. Toll-like receptors and inflammatory bowel disease[J]. Frontiers in Immunology, 2018, 9: 72. DOI:10.3389/fimmu.2018.00072 |
| [52] |
VIJAY K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future[J]. International Immunopharmacology, 2018, 59: 391-412. DOI:10.1016/j.intimp.2018.03.002 |
| [53] |
FITZGERALD K A, KAGAN J C. Toll-like receptors and the control of immunity[J]. Cell, 2020, 180(6): 1044-1066. DOI:10.1016/j.cell.2020.02.041 |
| [54] |
BOOZARI M, BUTLER A E, SAHEBKAR A. Impact of curcumin on Toll-like receptors[J]. Journal of Cellular Physiology, 2019, 234(8): 12471-12482. DOI:10.1002/jcp.28103 |
| [55] |
CHEN J J, DAI L, ZHAO L X, et al. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis[J]. Scientific Reports, 2015, 5: 10278. DOI:10.1038/srep10278 |
| [56] |
WANG J, GHOSH S S, GHOSH S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions[J]. American Journal of Physiology: Cell Physiology, 2017, 312(4): C438-C445. DOI:10.1152/ajpcell.00235.2016 |
| [57] |
GAN Z D, WEI W Y, LI Y, et al. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets[J]. Molecules, 2019, 24(7): 1220. DOI:10.3390/molecules24071220 |
| [58] |
LIU T, ZHANG L Y, JOO D, et al. NF-κB signaling in inflammation[J]. Signal Transduction and Targeted Therapy, 2017, 2: 17023. DOI:10.1038/sigtrans.2017.23 |
| [59] |
TIAN S Y, GUO R X, WEI S C, et al. Curcumin protects against the intestinal ischemia-reperfusion injury: involvement of the tight junction protein ZO-1 and TNF-α related mechanism[J]. Korean Journal of Physiology and Pharmacology, 2016, 20(2): 147-152. DOI:10.4196/kjpp.2016.20.2.147 |
| [60] |
ECKERT J, SCOTT B, LAWRENCE S M, et al. FLLL32, a curcumin analog, ameliorates intestinal injury in necrotizing enterocolitis[J]. Journal of Inflammation Research, 2017, 10: 75-81. DOI:10.2147/JIR.S131051 |
| [61] |
BURGE K, GUNASEKARAN A, ECKERT J, et al. Curcumin and intestinal inflammatory diseases: molecular mechanisms of protection[J]. International Journal of Molecular Sciences, 2019, 20(8): 1912. DOI:10.3390/ijms20081912 |
| [62] |
MA F Y, LIU F, DING L, et al. Anti-inflammatory effects of curcumin are associated with down regulating microRNA-155 in LPS-treated macrophages and mice[J]. Pharmaceutical Biology, 2017, 55(1): 1263-1273. DOI:10.1080/13880209.2017.1297838 |
| [63] |
ABDOLLAHI E, MOMTAZI A A, JOHNSTON T P, et al. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades?[J]. Journal of Cellular Physiology, 2018, 233(2): 830-848. DOI:10.1002/jcp.25778 |
| [64] |
OMENETTI S, PIZARRO T T. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome[J]. Frontiers in Immunology, 2015, 6: 639. |
| [65] |
ZHAO H M, XU R, HUANG X Y, et al. Curcumin improves regulatory T cells in gut-associated lymphoid tissue of colitis mice[J]. World Journal of Gastroenterology, 2016, 22(23): 5374-5383. DOI:10.3748/wjg.v22.i23.5374 |
| [66] |
ZHAO G M, LIU Y, YI X Y, et al. Curcumin inhibiting Th17 cell differentiation by regulating the metabotropic glutamate receptor-4 expression on dendritic cells[J]. International Immunopharmacology, 2017, 46: 80-86. DOI:10.1016/j.intimp.2017.02.017 |
| [67] |
OHNO M, NISHIDA A, SUGITANI Y, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells[J]. PLoS One, 2017, 12(10): e0185999. DOI:10.1371/journal.pone.0185999 |




