一直以来,如何增加产仔数都是养猪业亟待解决的问题。母猪有较高的排卵率以及较高的受精率,所以影响产仔数的关键因素在于早期胚胎的丢失[1]。早期胚胎发育阻滞是一种因细胞无法继续分裂而导致囊胚数量下降的现象[2],通过大量研究证实,它与母源调控向胚胎调控的过渡时期一致。母源基因的降解、胚胎基因的激活在母源调控向胚胎调控的过渡时期会伴随发生,由于此时许多调控机制较差,所以任何细微的变化及干扰都可能造成早期胚胎发育阻滞[3]。卵母细胞生长成熟过程中积累的mRNA和蛋白质是母体调控的重要基础。随着母体物质的消耗,胚胎自身需要开始合成生长所需的物质,因此需要激活合子基因组,这个过程称为合子基因组激活(zygotic genome activation, ZGA)[4-5]。早期胚胎发育所需的能量(ATP)来源主要有2条途径,分别是葡萄糖参与的糖酵解途径和中间产物为丙酮酸盐的氧化磷酸化途径[6]。在合子基因组激活之前,合子不能在缺乏乳酸和丙酮酸的培养基中存活,因为此时合子不能通过糖酵解途径对葡糖糖进行利用[7]。然而当培养基中只留下丙酮酸盐时,胚胎可以存活,但不能发育到2细胞阶段之后[8], 所以丙酮酸是此时重要的能量物质。胚胎发育阻滞的时期虽然会因物种的不同而有所差异,但都发生在胚胎从输卵管向子宫转移的过程中,而丙酮酸和乳酸正是此时输卵管液中所含的重要物质[3]。综上所述,推测丙酮酸可能是影响早期胚胎发育的一种关键物质。本文总结了丙酮酸在早期胚胎发育中的应用,为提高早期胚胎发育质量、增加母猪产仔数以及母猪妊娠前后的精准饲喂提供新的可能。
1 丙酮酸代谢丙酮酸产生于细胞质中,有以下几个来源(图 1)。一个主要来源是葡萄糖通过厌氧糖酵解分解成2个丙酮酸分子,最终由丙酮酸激酶产生。绝大部分丙酮酸是通过乳酸脱氢酶氧化乳酸产生的,也可以通过胞质苹果酸酶由苹果酸重新合成。丙酮酸对其三羧酸(tricarboxylic acid cycle, TCA)循环代谢物(如柠檬酸盐、苹果酸盐和草酰乙酸盐)穿梭胞质和线粒体之间起重要作用。丙酮酸的最后一个重要来源是三碳氨基酸的分解代谢。首先以丙氨酸和2-氧戊二酸为底物,然后在丙氨酸转氨酶(ALT)催化下转化为谷氨酸和丙酮酸。线粒体基质中也有丙氨酸转氨酶活性成分,丙氨酸可以转运到线粒体中,然后转化为丙酮酸[9-10]。此外,丝氨酸、苏氨酸、甘氨酸和半胱氨酸也能够转化为丙酮酸。在大多数组织中,由这些途径产生的大量胞质丙酮酸转运至线粒体。同时,丙酮酸也可以通过双向细胞质酶——乳酸脱氢酶(LDH)还原成乳酸,包括2种不同的形式:LDH-A有利于从丙酮酸转化成乳酸,LDH-B有利于从乳酸转化为丙酮酸[11]。
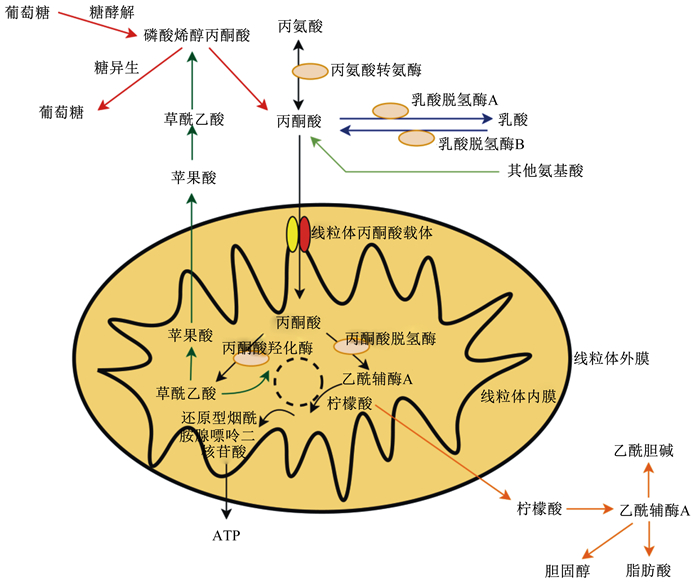
|
图 1 丙酮酸代谢途径 Fig. 1 Pyruvate metabolic pathways |
有研究证明,丙酮酸是卵母细胞和合子能够直接利用的主要能量底物[12]。牛的卵丘卵母细胞复合体(cumulus oocyte complexes, COCs)成熟过程中添加丙酮酸钠后,成熟率和受精率均显著提高[13]。当培养液中不含葡萄糖时,牛的肺动脉内皮细胞无法生长,但向其中添加10 mmol/L的丙酮酸后则可以继续生长,表明丙酮酸在某些情况下可以替代葡萄糖成为很好的能量来源[14]。此外,有研究表明卵丘卵母细胞在不含葡萄糖的培养基中,消耗的丙酮酸是裸卵的5~6倍[15]。从以上结果可以看出,丙酮酸在培养基中可以作为能量底物被代谢。
2.2 丙酮酸作为抗氧化剂丙酮酸在氧化还原中扮演着重要角色, 参与胚胎的抗氧化应激[16]。丙酮酸可以抵抗由过氧化氢造成的氧化损伤[17],过氧化氢通过bcl-2和bax基因的线粒体通路诱导内皮细胞凋亡,而丙酮酸可以阻断此通路[18]。与此结果一致,Lamirande等[19]试验结果表明,在同时含有丙酮酸与乳酸的培养基中,精子避免了由活性氧(ROS)造成的氧化损伤。而且,当过氧化氢使细胞外信号调节激酶1/2(ERK1/2)下调、p38丝裂原活化蛋白激酶(MAPK)磷酸化时,丙酮酸可以逆转这些过程[20]。
此外,抗氧化应激的关键机制还原型烟酰胺腺嘌呤二核苷酸/烟酰胺腺嘌呤二核苷酸(NADH/NAD)和还原型谷胱甘肽/氧化型谷胱甘肽(GSH/GSSG)也会受到丙酮酸的影响。首先,丙酮酸可以转化为乳酸改变NADH/NAD,然后通过增强柠檬酸的代谢改变GSH/GSSG[21]。综上所述,丙酮酸在抗氧化应激中发挥着重要作用。
2.3 其他作用神经递质的释放、细胞生长和增殖等一系列反应都离不开一个关键激活信号——胞质内钙离子浓度的增加[22],而调节细胞内钙离子信号正是丙酮酸一个潜在作用。丙酮酸可以影响钙离子通道(CRAC),主要是通过激活CRAC释放钙离子,提高钙离子进入细胞的量[23], 从而影响通过钙离子调控的细胞进程[24]。
3 早期胚胎发育 3.1 胚胎发育阻滞当早期胚胎在体外培养到一定发育阶段时,出现细胞无法分裂、囊胚数减少等问题,此时便发生了胚胎发育阻滞。胚胎发育阻滞在牛、猴、人等大型哺乳动物中非常普遍,但胚胎发育阻滞出现时期由于物种不同而存在差异,如小鼠、大鼠胚胎发育阻滞发生在2细胞期,人一般在4~8细胞期[25-26],牛、山羊、绵羊在8~16细胞期,兔在桑葚胚期[1],猪主要在4细胞期[27]。有研究报道P66Shc蛋白作为一种氧化应激调节物质能够在发育阻滞的胚胎中高表达,这表明胚胎发育阻滞受胚胎内ROS含量的影响[28]。
3.2 合子基因组激活植入前,哺乳动物胚胎发育从受精后开始,并在输卵管中由单细胞发育到囊胚。猪胚胎发育到囊胚需要7 d,且猪胚胎的脂质水平要高于小鼠和人类胚胎[29]。植入前的哺乳动物胚胎面临着一个关键时期——ZGA,如果合子基因组转录失败,它们的细胞周期就会停止[30]。因为成熟的MⅡ卵母细胞和胚胎在前几个卵裂期被认为转录沉默[31],所以在受精前,卵母细胞合成并积累大量的RNA转录本,母源物质在ZGA之前对胚胎发育进行编程。第1波基因转录在母源物质降解和合子基因开始表达中起着重要作用[32]。在小鼠和其他物种中观察到了次要转录波(次要ZGA)和主要转录波(主要ZGA)[25, 30, 33]。然而,ZGA的机制,特别是在哺乳动物中的机制,并不完全清楚。
与ZGA相关的过程依赖于染色质的各种三维结构变化和基因组的表观遗传变化[34-35],且表观遗传重编程对于早期胚胎发育过程中的特定基因调控至关重要[36]。组蛋白修饰的全基因组重编程发生在哺乳动物胚胎发育过程中,包括组蛋白去甲基化、去乙酰化,以及特定染色质区域的其他修饰[37-39]。组蛋白N末端的赖氨酸乙酰化在调节整体染色质结构和基因转录中起关键作用[40-42],通过中和与双链DNA相互作用的N末端的电荷或者提供转录因子与溴域的结合位点来促进转录激活[43]。因此,在植入前阶段,组蛋白乙酰化的增强有助于ZGA在胚胎中的表达。
3.3 ROS对早期胚胎发育的影响ROS在早期胚胎发育中的确切作用尚未完全确定。相比之下,在培养体系中使用含抗氧化剂的商业培养基可以维持胚胎中促氧化剂-抗氧化剂的平衡,从而改善胚胎营养环境[44]。在体外培养中,ROS对体外受精(in vitro fertilization, IVF)有重要影响[44],培养基中的ROS主要来源于胚胎的新陈代谢及其周围环境[45]。氧化应激被定义为各种ROS稳态水平增加导致超过机体抗氧化防御能力的现象[46]。氧化应激对许多类型的细胞都会产生影响,如对早期胚胎发育的阻滞和延缓现象[47]。研究表明,培养过细胞的废弃培养液中ROS的浓度与胚胎卵裂或囊胚形成的程度相关[48-51]。氧化应激会对细胞环境产生有害影响,同时对受精造成负面影响,损害胚胎细胞生长、诱导细胞凋亡,从而导致胚胎破碎[52]。
细胞为了维持正常的氧化还原稳态,需要借助包括抗氧化酶和非酶抗氧化剂的抗氧化系统来实现,例如维生素A、维生素C、维生素E、GSH和矿物质锌、硒[53-54]。非酶类抗氧化剂有不同的作用机制:硒是重要的谷胱甘肽过氧化物酶(GPX)和硫氧还原蛋白等抗氧化酶的组成部分[55];维生素E(生育酚)防止脂质过氧化[56];N-乙酰半胱氨酸(NAC)是GSH前体[54];NAC和丙酮酸可作为ROS的清除剂[57-59]。有研究表明,丙酮酸在氧化应激模型中的保护作用,不仅可以通过中和过氧化氢(H2O2)和ROS实现,还可以维持线粒体氧化应激下的膜电位并减少ROS的生成[20, 60-62]。丙酮酸是几种代谢途径的关键中间化合物,通过糖酵解途径从葡萄糖中产生,也可以通过糖异生转化为碳水化合物。丙酮酸是线粒体ATP产生的关键底物[18],是细胞的重要能量供应者,其主要途径有2种: 第1种为通过厌氧发酵,在缺氧时产生乳酸;第2种为在有氧时促进TCA循环。
4 丙酮酸对早期胚胎发育的影响哺乳动物的成功繁殖在很大程度上取决于卵母细胞的质量,这对受精后的胚胎发育至关重要。除了遗传因素外,卵母细胞的质量在很大程度上受卵泡环境及其在排卵后独立生存能力的影响[63]。排卵后,卵母细胞几乎完全依靠其约100 000个线粒体中的ATP存活,直到致密的桑葚胚期后开始进行糖酵解,然后将囊胚植入子宫[64]。虽然不同哺乳动物的卵母细胞代谢底物基本相同,但是它们对这些底物的依赖性各不相同。卵母细胞在卵泡发育过程中通过卵丘细胞获得丙酮酸,丙酮酸是大多数哺乳动物卵母细胞重要的代谢底物[64]。
影响受精后发育的另一重要因素是精子质量。哺乳动物的精子依赖于三磷酸腺苷的有效生成,为获能和受精提供能量[65]。前进运动对于精子穿过子宫颈到达输卵管是必要的,剧烈且不对称的游动模式有助于精子从输卵管上皮中释放,随后穿过卵母细胞膜[66-67]。极度活跃的运动和酪氨酸磷酸化是获能的2个标志,是1个精子为受精做准备的过程。葡萄糖、丙酮酸和乳酸以高浓度存在于输卵管液中[68],它们通常用作哺乳动物精子的能量底物[69-71]。根据其环境中的氧供应量和代谢底物的组成,哺乳动物的精子在不同的代谢途径之间切换,以此来解决能量需求问题[72]。
4.1 合子基因组激活之前葡萄糖代谢的测量表明,着床前,胚胎卵裂期的葡萄糖消耗量通常比囊胚期低10倍以上[73]。代谢过程(如TCA循环)与细胞的整体能量学一致,因此也会减弱[74]。令人惊讶的是,当培养基中不添加蛋白质和氨基酸时,小鼠胚胎可以正常发育,尽管1细胞和2细胞胚胎氨基酸积累程度、线粒体耗氧量和葡萄糖氧化量均处于非常低的水平,但它们仍完成了整个稳态过程[75]。早期胚胎的低代谢活性可使ROS保持较低的水平,从而避免了细胞全能性阶段的DNA损伤[76]。
胚胎消耗各代谢产物的程度不尽相同。例如,只有一部分丙酮酸在线粒体中被完全氧化或被乳酸脱氢酶还原为乳酸[73]。乳酸和丙酮酸都存在于输卵管液中,并以相似的比例包含在体外培养体系中。合子不能在缺乏乳酸和丙酮酸的培养基中存活。当仅保留丙酮酸时,胚胎则会有一定活力,但不能越过合子基因组激活[8]。在这些条件下,胚胎细胞中NAD+/NADH较低,因此无法有效利用乳酸。葡萄糖在桑葚期之前基本不被氧化,添加的葡萄糖不能转化为丙酮酸[73],所以额外添加丙酮酸对胚胎发育是必须的。
4.2 合子基因组激活之时在小鼠中,受精卵经历3~4轮细胞分裂,在8细胞阶段紧密结合,然后产生桑葚胚。第1步分化产生1个含有内细胞团的囊胚细胞,胚胎固有的祖细胞和周围的外胚层细胞形成胚外组织。小鼠植入前发育大约需要4 d,人类大约需要6 d,猪大约需要7 d,然后囊胚在子宫着床[77-78]。为了能够完成植入前的发育,胚胎必须激活合子基因组。此过程中,母本和父本基因组的许多结构和表观遗传发生了变化,这些变化使胚胎发生了重编程。如此规模的基因组重编程需要诸多代谢物的支持,例如蛋白质合成和DNA甲基化必不可少的α-酮戊二酸(α-KG)、蛋白质乙酰化所需的乙酰辅酶A、底物磷酸化所需的ATP、糖基化所需的UDP-乙酰基葡萄糖胺(UDP-GlcNAc)[79-80],而这些过程都涉及对丙酮酸的利用。
丙酮酸首先在丙酮酸脱氢酶复合体(pyruvate dehydrogenase complex, PDHC)作用下转化为乙酰辅酶A,进而调节TCA循环。丙酮酸脱氢酶复合体包含一个限速亚单位——丙酮酸脱氢酶复合体α亚单位(PDH E1α,PDHA1),复合体的主要成分为丙酮酸脱氢酶(PDH)。丙酮酸脱氢酶磷酸酶(PDP)会使PDHA1去磷酸化(激活),而丙酮酸脱氢酶激酶(PDK)会使PDHA1磷酸化(失活)[81-82]。在一项关于小鼠和人类胚胎的研究中发现,线粒体PDHA1和TCA循环相关酶在ZGA过程中瞬时移动到细胞核,并在表观遗传重编程中发挥重要作用[83]。然而,迄今为止,只有少数关于ZGA期间PDHA1核积累的时间和功能的研究被报道。PDH通常在线粒体中将丙酮酸转化为乙酰辅酶A,然后TCA循环代谢产物可以被其他核酶利用。但Zhou等[84]研究结果表明,在ZGA期间,PDH转移到猪胚胎的细胞核并高度表达,通过产生足够量的乙酰辅酶A来维持组蛋白乙酰化水平,核PDH是猪合子基因转录和胚胎发育所必需的。
5 小结丙酮酸通过降低卵母细胞内ROS含量、提高GSH含量,影响卵母细胞母体基因的表达,从而促进卵母细胞成熟。可能是因为在不同的营养条件下,PDK通过磷酸化PDHA1调节能量平衡。丙酮酸可以加快精子获能,可能是通过提高精子内源性ATP水平来实现,从而进一步引发过度活化和酪氨酸磷酸化。作为能量底物和抗氧化剂,丙酮酸具有双重功能作用,保护细胞免受体外环境的压力。在受精卵合子基因组激活之前,丙酮酸是主要的供能物质,合子基因组激活时,大量生命过程被启动,线粒体却表现出较低的活性,可能是由于此时PDHA1和TCA循环相关酶瞬时转移到核中,并在核中进行对丙酮酸的利用以及进行表观重编程。
通过丙酮酸目前在猪生产上的应用发现,丙酮酸是低蛋白质饲粮中谷氨酸的有效替代品,可以减少猪的氮排泄和氮消耗[85], 但在繁殖性能中的功能及表现还没有报道。细胞内许多独立功能的发挥离不开丙酮酸的参与,缺乏外源提供丙酮酸的细胞会面临氧化应激和能量供给不足的双重压力,乳酸可以在没有丙酮酸的情况下使用,但是由于NAD+/NADH低,乳酸转化为丙酮酸的速率不足以维持一类代谢物的水平[84]。PDH是一种必需酶,在哺乳动物胚胎的代谢和表观遗传调控中具有双重作用,并且其他与丙酮酸代谢有关的一些线粒体代谢酶可能在哺乳动物ZGA中发挥重要的保守作用,其在线粒体和核之间的再定位机制还有待研究[84]。在猪早期胚胎发育中,关于丙酮酸的研究还未延伸到合子发育,但其作用却不容忽视。
| [1] |
GEISERT R D, SCHMITT R A M. Early embryonic survival in the pig: can it be improved?[J]. Journal of Animal Science, 2002, 80(E-Suppl_1): E54-E65. |
| [2] |
NASR-ESFAHANI M H, AITKEN J R, JOHNSON M H. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo[J]. Development, 1990, 109(2): 501-507. |
| [3] |
QUINN P, HARLOW G M. The effect of oxygen on the development of preimplantation mouse embryos in vitro[J]. Journal of Experimental Zoology, 1978, 206(1): 73-80. DOI:10.1002/jez.1402060108 |
| [4] |
TADROS W, LIPSHITZ H D. The maternal-to-zygotic transition: a play in two acts[J]. Development, 2009, 136(18): 3033-3042. DOI:10.1242/dev.033183 |
| [5] |
KAGEYAMA S, GUNJI W, NAKASATO M, et al. Analysis of transcription factor expression during oogenesis and preimplantation development in mice[J]. Zygote, 2007, 15(2): 117-128. DOI:10.1017/S096719940700411X |
| [6] |
MANES C, LAI N C. Nonmitochondrial oxygen utilization by rabbit blastocysts and surface production of superoxide radicals[J]. Journal of Reproducfion and Fertility, 1995, 104(1): 69-75. DOI:10.1530/jrf.0.1040069 |
| [7] |
BRINSTER R T. Studies on the development of mouse embyros in vitro. Ⅱ.The effect of energy source[J]. Journal of Experimental Zoology, 2004, 158(1): 59-68. |
| [8] |
BROWN J J G, WHITTINGHAM D G. The roles of pyruvate, lactate and glucose during preimplantation development of embryos from F1 hybrid mice in vitro[J]. Development, 1991, 112(1): 99-105. |
| [9] |
HOPPER S, SEGAL H L. Comparative properties of glutamic-alanine transaminase from several sources[J]. Archives of Biochemistry and Biophysics, 1964, 105(3): 501-505. DOI:10.1016/0003-9861(64)90042-6 |
| [10] |
MENDES-MOURÃO J, HALESTRAP A, CRISP D M, et al. The involvement of mitochondrial pyruvate transport in the pathways of gluconeogenesis from serine and alanine in isolated rat and mouse liver cells[J]. FEBS Letters, 1975, 53(1): 29-32. DOI:10.1016/0014-5793(75)80674-0 |
| [11] |
DAWSON D M, GOODFRIEND T L, KAPLAN N O, et al. Lactic dehydrogenases: functions of the two types rates of synthesis of the two major forms can be correlated with metabolic differentiation[J]. Science, 1964, 143(3609): 929-933. DOI:10.1126/science.143.3609.929 |
| [12] |
BIGGERS J D, WHITTINGHAM D G, DONAHUE R P. The pattern of energy metabolism in the mouse oöcyte and zygote[J]. Proceedings of the National Academy of Sciences of the United States of America, 1967, 58(2): 560-567. DOI:10.1073/pnas.58.2.560 |
| [13] |
GESHI M, TAKENOUCHI N, YAMAUCHI N, et al. Effects of sodium pyruvate in nonserum maturation medium on maturation, fertilization, and subsequent development of bovine oocytes with or without cumulus cells[J]. Biology of Reproduction, 2000, 63(6): 1730-1734. DOI:10.1095/biolreprod63.6.1730 |
| [14] |
CHUNG S J, LEE S H, LEE Y J, et al. Pyruvate protection against endothelial cytotoxicity induced by blockade of glucose uptake[J]. Journal of Biochemistry and Molecular Biology, 2004, 37(2): 239-245. |
| [15] |
DOWNS S M, HUMPHERSON P G, LEESE H J. Pyruvate utilization by mouse oocytes is influenced by meiotic status and the cumulus oophorus[J]. Molecular Reproduction and Development, 2000, 62(1): 113-123. |
| [16] |
O'FALLON J V, WRIGHT R W, J r. Pyruvate revisited: a non-metabolic role for pyruvate in preimplantation embryo development[J]. Theriogenology, 1995, 43(1): 288. DOI:10.1016/0093-691X(95)92442-C |
| [17] |
MORALES H, TILQUIN P J, REES J F, et al. Pyruvate prevents peroxide-induced injury of in vitro preimplantation bovine embryos[J]. Molecular Reproduction and Development, 1999, 52(2): 149-157. DOI:10.1002/(SICI)1098-2795(199902)52:2<149::AID-MRD5>3.0.CO;2-4 |
| [18] |
KANG Y H, CHUNG S J, KANG I J, et al. Intramitochondrial pyruvate attenuates hydrogen peroxide-induced apoptosis in bovine pulmonary artery endothelium[J]. Molecular and Cellular Biochemistry, 2001, 216(1): 37-46. |
| [19] |
DE LAMIRANDE E, GAGNON C. Reactive oxygen species and human spermatozoa.Ⅱ.Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility[J]. Journal of Andrology, 1992, 13(5): 379-386. |
| [20] |
LEE Y J, KANG I J, BÜNGER R, et al. Mechanisms of pyruvate inhibition of oxidant-induced apoptosis in human endothelial cells[J]. Microvascular Research, 2003, 66(2): 91-101. DOI:10.1016/S0026-2862(03)00052-9 |
| [21] |
KASHIWAGI A, NISHIO Y, ASAHINA T, et al. Pyruvate improves deleterious effects of high glucose on activation of pentose phosphate pathway and glutathione redox cycle in endothelial cells[J]. Diabetes, 1997, 46(12): 2088-2095. DOI:10.2337/diab.46.12.2088 |
| [22] |
CARAFOLI E. Calcium signaling: a tale for all seasons[J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(3): 1115-1122. DOI:10.1073/pnas.032427999 |
| [23] |
BAKOWSKI D, PAREKH A B. Regulation of store-operated calcium channels by the intermediary metabolite pyruvic acid[J]. Current Biology, 2007, 17(12): 1076-1081. DOI:10.1016/j.cub.2007.05.041 |
| [24] |
MUALLEM S. Calcium signaling: pyruvate and CRAC meet at the crossroads[J]. Current Biology, 2007, 17(14): R549-R551. DOI:10.1016/j.cub.2007.05.037 |
| [25] |
YAN L, YANG M, GUO H, et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells[J]. Nature Structural & Molecular Biology, 2013, 20: 1131-1139. |
| [26] |
DE SOUSA P A, WATSON A J, SCHULTZ R M. Transient expression of a translation initiation factor is conservatively associated with embryonic gene activation in murine and bovine embryos[J]. Biology of Reproduction, 1998, 59(4): 969-977. DOI:10.1095/biolreprod59.4.969 |
| [27] |
MAGNANI L, JOHNSON C M, CABOT R A. Expression of eukaryotic elongation initiation factor 1A differentially marks zygotic genome activation in biparental and parthenogenetic porcine embryos and correlates with in vitro developmental potential[J]. Reproduction, Fertility and Development, 2008, 20(7): 818-825. DOI:10.1071/RD08072 |
| [28] |
FAVETTA L A, JOHN E J, S t., KING W A, et al. High levels of p66shc and intracellular ROS in permanently arrested early embryos[J]. Free Radical Biology and Medicine, 2007, 42(8): 1201-1210. DOI:10.1016/j.freeradbiomed.2007.01.018 |
| [29] |
ROMEK M, GAJDA B, KRZYSZTOFOWICZ E, et al. Lipid content of non-cultured and cultured pig embryo[J]. Reproduction in Domestic Animals, 2009, 44(1): 24-32. DOI:10.1111/j.1439-0531.2007.00984.x |
| [30] |
XUE L, CAI J Y, MA J, et al. Global expression profiling reveals genetic programs underlying the developmental divergence between mouse and human embryogenesis[J]. BMC Genomics, 2013, 14: 568. DOI:10.1186/1471-2164-14-568 |
| [31] |
DAHL J A, JUNG I, AANES H, et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition[J]. Nature, 2016, 537(7621): 548-552. DOI:10.1038/nature19360 |
| [32] |
LEE M T, BONNEAU A R, TAKACS C M, et al. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition[J]. Nature, 2013, 503(7476): 360-364. DOI:10.1038/nature12632 |
| [33] |
AOSHIMA K, INOUE E, SAWA H, et al. Paternal H3K4 methylation is required for minor zygotic gene activation and early mouse embryonic development[J]. EMBO Reports, 2015, 16(7): 803-812. DOI:10.15252/embr.201439700 |
| [34] |
DEKKER J, MIRNY L. The 3D genome as moderator of chromosomal communication[J]. Cell, 2016, 164(6): 1110-1121. DOI:10.1016/j.cell.2016.02.007 |
| [35] |
FRASER R, LIN C J. Epigenetic reprogramming of the zygote in mice and men: on your marks, get set, go![J]. Reproduction, 2016, 152(6): R211-R222. DOI:10.1530/REP-16-0376 |
| [36] |
HACKETT J A, SURANI M A. DNA methylation dynamics during the mammalian life cycle[J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2013, 368(1609): 20110328. DOI:10.1098/rstb.2011.0328 |
| [37] |
ZHANG B J, ZHENG H, HUANG B, et al. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development[J]. Nature, 2016, 537(7621): 553-557. DOI:10.1038/nature19361 |
| [38] |
AKIYAMA T, NAGATA M, AOKI F. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(19): 7339-7344. DOI:10.1073/pnas.0510946103 |
| [39] |
MA P P, SCHULTZ R M. Histone deacetylase 1(HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos[J]. Developmental Biology, 2008, 319(1): 110-120. DOI:10.1016/j.ydbio.2008.04.011 |
| [40] |
EBERHARTER A, BECKER P B. Histone acetylation: a switch between repressive and permissive chromatin: second in review series on chromatin dynamics[J]. EMBO Reports, 2002, 3(3): 224-229. DOI:10.1093/embo-reports/kvf053 |
| [41] |
GRUNSTEIN M. Histone acetylation in chromatin structure and transcription[J]. Nature, 1997, 389(6649): 349-352. DOI:10.1038/38664 |
| [42] |
SHOGREN-KNAAK M, ISHⅡ H, SUN J M, et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions[J]. Science, 2006, 311(5762): 844-847. DOI:10.1126/science.1124000 |
| [43] |
BANNISTER A J, KOUZARIDES T. Regulation of chromatin by histone modifications[J]. Cell Research, 2011, 21(3): 381-395. DOI:10.1038/cr.2011.22 |
| [44] |
AGARWAL A, SAID T M, BEDAIWY M A, et al. Oxidative stress in an assisted reproductive techniques setting[J]. Fertility and Sterility, 2006, 86(3): 503-512. DOI:10.1016/j.fertnstert.2006.02.088 |
| [45] |
LAN K C, LIN Y C, CHANG Y C, et al. Limited relationships between reactive oxygen species levels in culture media and zygote and embryo development[J]. Journal of Assisted Reproduction and Genetics, 2018, 36(2): 325-334. |
| [46] |
AGARWAL A, SALEH R A, BEDAIWY M A. Role of reactive oxygen species in the pathophysiology of human reproduction[J]. Fertility and Sterility, 2003, 79(4): 829-843. DOI:10.1016/S0015-0282(02)04948-8 |
| [47] |
GUERIN P, EL MOUATASSIM S, MÉNÉZO Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings[J]. Human Reproduction Update, 2001, 7(2): 175-189. DOI:10.1093/humupd/7.2.175 |
| [48] |
LEE T H, LEE M S, LIU C H, et al. The association between microenvironmental reactive oxygen species and embryo development in assisted reproduction technology cycles[J]. Reproductive Sciences, 2012, 19(7): 725-732. DOI:10.1177/1933719111432858 |
| [49] |
BEDAIWY M A, MAHFOUZ R Z, GOLDBERG J M, et al. Relationship of reactive oxygen species levels in day 3 culture media to the outcome of in vitro fertilization/intracytoplasmic sperm injection cycles[J]. Fertility and Sterility, 2010, 94(6): 2037-2042. DOI:10.1016/j.fertnstert.2009.12.020 |
| [50] |
MARTÍN-ROMERO F J, MIGUEL-LASOBRAS E M, DOMÍNGUEZ-ARROYO J A, et al. Contribution of culture media to oxidative stress and its effect on human oocytes[J]. Reproductive BioMedicine Online, 2008, 17(5): 652-661. DOI:10.1016/S1472-6483(10)60312-4 |
| [51] |
BEDAIWY M A, FALCONE T, MOHAMED M S, et al. Differential growth of human embryos in vitro: role of reactive oxygen species[J]. Fertility and Sterility, 2004, 82(3): 593-600. DOI:10.1016/j.fertnstert.2004.02.121 |
| [52] |
PASZKOWSKI T, CLARKE R N. Fertilization and early embryology: antioxidative capacity of preimplantation embryo culture medium declines following the incubation of poor quality embryos[J]. Human Reproduction, 1996, 11(11): 2493-2495. DOI:10.1093/oxfordjournals.humrep.a019146 |
| [53] |
BAUMEISTER P, HUEBNER T, REITER M, et al. Reduction of oxidative DNA fragmentation by ascorbic acid, zinc and N-acetylcysteine in nasal mucosa tissue cultures[J]. Anticancer Research, 2009, 29(11): 4571-4574. |
| [54] |
NEGRE-SALVAYRE A, SALVAYRE R, AUGÉ N, et al. Hyperglycemia and glycation in diabetic complications[J]. Antioxidants & Redox Signaling, 2009, 11(12): 3071-3109. |
| [55] |
EBERT R, ULMER M, ZECK S, et al. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro[J]. Stem Cells, 2006, 24(5): 1226-1235. DOI:10.1634/stemcells.2005-0117 |
| [56] |
TRABER M G, ATKINSON J. Vitamin E, antioxidant and nothing more[J]. Free Radical Biology and Medicine, 2007, 43(1): 4-15. DOI:10.1016/j.freeradbiomed.2007.03.024 |
| [57] |
RUSHWORTH G F, MEGSON I L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits[J]. Pharmacology & Therapeutics, 2014, 141(2): 150-159. |
| [58] |
MANDL J, SZARKA A, BÁNHEGYI G. Vitamin C: update on physiology and pharmacology[J]. British Journal of Pharmacology, 2009, 157(7): 1097-1110. DOI:10.1111/j.1476-5381.2009.00282.x |
| [59] |
BABICH H, LIEBLING E J, BURGER R F, et al. Choice of DMEM, formulated with or without pyruvate, plays an important role in assessing the in vitro cytotoxicity of oxidants and prooxidant nutraceuticals[J]. In Vitro Cellular & Developmental Biology-Animal, 2009, 45(5/6): 226-233. |
| [60] |
LONG L H, HALLIWELL B. Artefacts in cell culture: pyruvate as a scavenger of hydrogen peroxide generated by ascorbate or epigallocatechin gallate in cell culture media[J]. Biochemical and Biophysical Research Communications, 2009, 388(4): 700-704. DOI:10.1016/j.bbrc.2009.08.069 |
| [61] |
WANG X F, PEREZ E, LIU R, et al. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells[J]. Brain Research, 2007, 1132: 1-9. DOI:10.1016/j.brainres.2006.11.032 |
| [62] |
TEJERO-TALDO M I, CAFFREY J L, SUN J, et al. Antioxidant properties of pyruvate mediate its potentiation of β-adrenergic inotropism in stunned myocardium[J]. Journal of Molecular and Cellular Cardiology, 1999, 31(10): 1863-1872. DOI:10.1006/jmcc.1999.1020 |
| [63] |
BRADLEY J, SWANN K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos[J]. The International Journal of Developmental Biology, 2019, 63(3/4/5): 93-103. |
| [64] |
DUMOLLARD R, CAMPBELL K, HALET G, et al. Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes[J]. Developmental Biology, 2008, 316(2): 431-440. DOI:10.1016/j.ydbio.2008.02.004 |
| [65] |
HO H C, GRANISH K A, SUAREZ S S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and Not CAMP[J]. Developmental Biology, 2002, 250(1): 208-217. DOI:10.1006/dbio.2002.0797 |
| [66] |
DEMOTT R P, SUAREZ S S. Hyperactivated sperm progress in the mouse oviduct[J]. Biology of Reproduction, 1992, 46(5): 779-785. DOI:10.1095/biolreprod46.5.779 |
| [67] |
STAUSS C R, VOTTA T J, SUAREZ S S. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida[J]. Biology of Reproduction, 1995, 53(6): 1280-1285. DOI:10.1095/biolreprod53.6.1280 |
| [68] |
DE AGOSTINI LOSANO J D, DE SOUZA RAMOS ANGRIMANI D, LEITE R F, et al. Spermatic mitochondria: role in oxidative homeostasis, sperm function and possible tools for their assessment[J]. Zygote, 2018, 26(4): 251-260. DOI:10.1017/S0967199418000242 |
| [69] |
HOSHI K, TSUKIKAWA S, SATO A. Importance of Ca2+, K+ and glucose in the medium for sperm penetration through the human zona pellucida[J]. The Tohoku Journal of Experimental Medicine, 1991, 165(2): 99-104. DOI:10.1620/tjem.165.99 |
| [70] |
WILLIAMS A C, FORD W C L. The role of glucose in supporting motility and capacitation in human spermatozoa[J]. Journal of Andrology, 2001, 22(4): 680-695. |
| [71] |
MUKAI C, OKUNO M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement[J]. Biology of Reproduction, 2004, 71(2): 540-547. DOI:10.1095/biolreprod.103.026054 |
| [72] |
HERENG T H, ELGSTØEN K B P, CEDERKVIST F H, et al. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa[J]. Human Reproduction, 2011, 26(12): 3249-3263. DOI:10.1093/humrep/der317 |
| [73] |
LANE M, GARDNER D K. Lactate regulates pyruvate uptake and metabolism in the preimplantation mouse embryo[J]. Biology of Reproduction, 2000, 62(1): 16-22. DOI:10.1095/biolreprod62.1.16 |
| [74] |
BARBEHENN E K, WALES R G, LOWRY O H. Measurement of metabolites in single preimplantation embryos; a new means to study metabolic control in early embryos[J]. Journal of Embryology & Experimental Morphology, 1978, 43: 29-46. |
| [75] |
BRINSTER R L. Incorporation of carbon from glucose and pyruvate into the preimplantation mouse embryo[J]. Experimental Cell Research, 1969, 58(1): 153-158. DOI:10.1016/0014-4827(69)90125-6 |
| [76] |
BAUMANN C G, MORRIS D G, SREENAN J M, et al. The quiet embryo hypothesis: molecular characteristics favoring viability[J]. Molecular Reproduction & Development, 2010, 74(10): 1345-1353. |
| [77] |
COCKBURN K, ROSSANT J. Making the blastocyst: lessons from the mouse[J]. Journal of Clinical Investigation, 2010, 120(4): 995-1003. DOI:10.1172/JCI41229 |
| [78] |
LI L, ZHENG P, DEAN J. Maternal control of early mouse development[J]. Development, 2010, 137(6): 859-870. DOI:10.1242/dev.039487 |
| [79] |
HARDIVILLÉ S, HART G W. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation[J]. Cell Metabolism, 2014, 20(2): 208-213. DOI:10.1016/j.cmet.2014.07.014 |
| [80] |
MARTINEZ-PASTOR B, COSENTINO C, MOSTOSLAVSKY R. A tale of metabolites: the cross-talk between chromatin and energy metabolism[J]. Cancer Discovery, 2013, 3(5): 497-501. DOI:10.1158/2159-8290.CD-13-0059 |
| [81] |
PATEL M S, KOROTCHKINA L G. Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases[J]. Experimental & Molecular Medicine, 2001, 33(4): 191-197. |
| [82] |
PATEL M S, NEMERIA N S, FUREY W, et al. The pyruvate dehydrogenase complexes: structure-based function and regulation[J]. Journal of Biological Chemistry, 2014, 289(24): 16615-16623. DOI:10.1074/jbc.R114.563148 |
| [83] |
NAGARAJ R, SHARPLEY M S, CHI F, et al. Nuclear localization of mitochondrial TCA cycle enzymes as a critical step in mammalian zygotic genome activation[J]. Cell, 2017, 168(1/2): 210-223. |
| [84] |
ZHOU W J, NIU Y J, NIE Z W, et al. Nuclear accumulation of pyruvate dehydrogenase alpha 1 promotes histone acetylation and is essential for zygotic genome activation in porcine embryos[J]. Biochimica et Biophysica Acta: Molecular Cell Research, 2020, 1867(4): 118648. DOI:10.1016/j.bbamcr.2020.118648 |
| [85] |
LI Y X, TANG Z R, LI T J, et al. Pyruvate is an effective substitute for glutamate in regulating porcine nitrogen excretion[J]. Journal of Animal Science, 2018, 96(9): 3804-3814. DOI:10.1093/jas/sky237 |




