乳酸菌(lactic acid bacteria,LAB)是一类能利用可发酵碳水化合物产生大量乳酸的细菌总称[1],主要包括乳酸杆菌、双歧杆菌、乳酸乳球菌和链球菌等菌属。在动物机体内,LAB除了可以调节肠道菌群、促进肠道蠕动、保护肠道生态平衡以及改善肠道功能外,还能提高机体免疫能力,抑制腐败菌滋生,提高饲料转化率[2],在畜牧业等领域中有重要的应用价值,有望成为抗生素的替代品之一[3]。大量研究显示,LAB的上述功能可能与其细胞壁的不同成分——肽聚糖、磷壁酸、多糖、蛋白质及其次级代谢产物胞外多糖(expolysaccharides,EPS)有关(图 1)[4-5]。其中,EPS是一种分子量较高的长链聚合物,主要由蓝藻、真菌和细菌等微生物代谢产生[6],存在形式包括构成细菌细胞壁成分的荚膜多糖,以及分泌到周围环境中的黏多糖[7]。虽然有研究表明植物乳杆菌磷壁酸能够抑制细胞外调节蛋白激酶(extracellular regulated protein kinases, ERK)和p38激酶的磷酸化以及抑制核因子-κB(nuclear factor-kappa B,NF-κB)的活化,从而降低细胞因子白细胞介素(interleukin,IL)中IL-8的产生[3],但是国内外相关研究主要集中在EPS上,因为EPS能够为机体提供能量,参与机体多种生命活动,是生命有机体的重要组成成分,并具有多种生物学功能。所以,有关EPS的研究逐渐成为相关学科的前沿研究领域。EPS作为LAB的重要次级代谢产物[8],随着研究的不断深入,其抗肿瘤、抗氧化、抗病毒、免疫调节和肠道菌群调节等生物学功能(图 2)不断被发现,并得到广泛关注和认可[9-12]。本文将就乳酸菌胞外多糖(LAB-EPS)的分类、结构、生物学功能及其在生产中的应用进行综述,为LAB-EPS能早日在生产中得到广泛应用提供理论依据。
|
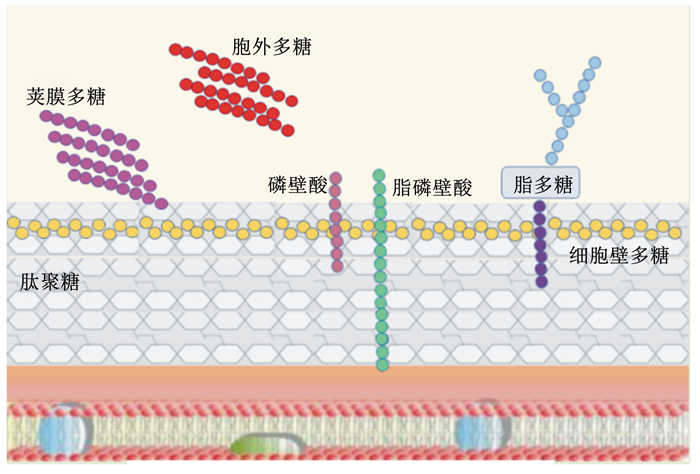
图 1 LAB细胞表面相关多糖 Fig. 1 Cell surface-associated polysaccharides of LAB[4] |
|
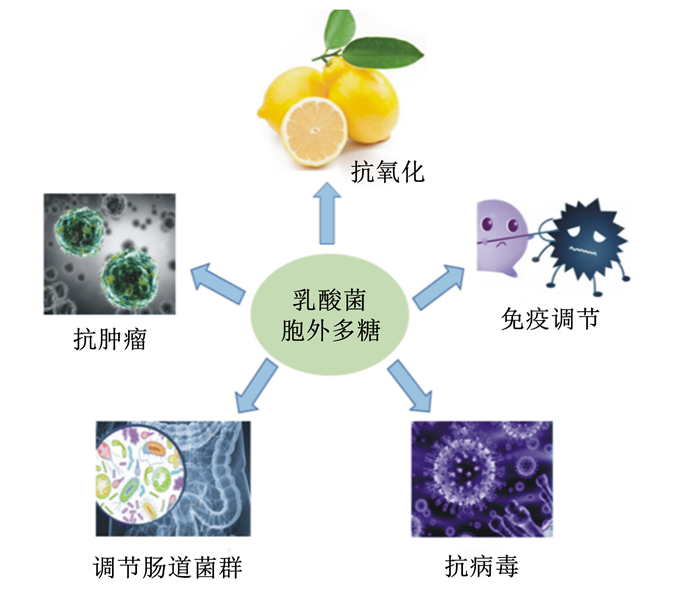
图 2 LAB-EPS主要生物学功能 Fig. 2 Main biological function of LAB-EPS |
与大多数其他细菌一样,LAB能够产生多种类型的多糖或聚糖,具有丰富的结构多样性。EPS作为LAB的重要次级代谢产物,根据位置不同,可被分为结合在细胞表面的“荚膜多糖”和释放到周围环境中的“黏多糖”,由于2种多糖经常混合在一起难以分开,所以统称为EPS。根据其化学成分的差异,EPS可分为同型多糖(homopolysaccharides,HoPS)和异型多糖(heteropolysaccharides,HePS)。HoPS是指由1种单糖脱水缩合而成的多糖;HePS是指2种及2种以上单糖通过脱水缩合形成3~8个重复单元所构成的多糖[13]。与HoPS相比,HePS包含不同单糖的重复单元(表 1),主要包括不同比例的葡萄糖、半乳糖和鼠李糖,有的还包含N-乙酰基-D-氨基葡萄糖、N-乙酰基-半乳糖胺、糖醛酸或一些非碳水化合物取代基,例如丙酮酸、乙酸盐、磷酸盐和琥珀酸盐等,它们在EPS发挥生物学功能时起关键作用[14-24],因此,HePS的生物合成及其结构更为复杂。
|
|
表 1 LAB分泌的中性或酸性异型多糖的结构 Table 1 Structures of neutral or acidic heteropolysaccharides produced by LAB |
抗肿瘤是LAB-EPS的主要生物学功能之一,1982年Shiomi等[25]初次提出LAB-EPS具有抗肿瘤作用之后,EPS的抗肿瘤功能便成为科研工作者的研究热点。研究表明,LAB-EPS发挥其抗肿瘤的活性主要是通过2种方式:一是直接抑制肿瘤细胞生长;二是调动机体的免疫系统,激发免疫细胞通过先天性免疫或适应性免疫对肿瘤细胞做出反应。用嗜酸乳杆菌EPS刺激人结肠癌细胞Caco-2和小鼠,激活了关键转录蛋白NF-κB和p38丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)启动,此外,还显著上调了IL-1α、单核细胞趋化蛋白-1、肿瘤坏死因子-α(tumor necrosis factor α,TNF-α)和重组人穿透素-3免疫相关基因的表达,且呈现剂量依赖性变化[26]。El-Deeb等[27]发现嗜酸乳杆菌20079的EPS通过对凋亡和NF-κB通路的调节实现对Caco-2的抑制作用;嗜酸乳杆菌20079的EPS处理Caco-2后,G0/G1期凋亡细胞比例增加,细胞凋亡率可高达80.65%,同时还具有上调NF-κB抑制蛋白α、p53和转化生长因子基因表达的潜力。Sun等[28]通过对流式细胞仪结果的分析也得到了相似的结论,嗜热链球菌CH9的EPS能够将人肝癌细胞HepG-2周期阻滞于G0/G1期。Di等[6]研究发现,干酪乳杆菌SB27的EPS可直接抑制结肠癌细胞HT-29的增殖,并上调B淋巴细胞瘤-2相关死亡启动子(Bcl-2-associated death promoter,Bad)、B淋巴细胞瘤-2相关X蛋白(Bax)、半胱氨酸天冬氨酸蛋白酶-3(caspase-3)和半胱氨酸天冬氨酸蛋白酶-8(caspase-8)基因的表达,诱导HT-29细胞凋亡引起形态学改变。此外,副干酪乳杆菌M5的EPS也可以激活氧化应激和内质网应激通路,进而诱导HT-29细胞凋亡[29]。
2.2 抗氧化在正常情况下,体内活性氧物质(reactive oxygen species,ROS)是有氧代谢的副产物,其中包括羟基自由基、超氧阴离子自由基和过氧化氢等[30],ROS的产生和消除维持着氧化-抗氧化平衡,这在调节信号通路的传导和细胞增殖中起着重要作用[31]。当平衡被破坏时,ROS水平升高,导致自由基的产生,这些自由基可能对蛋白质、脂质和DNA造成有害影响[32],导致机体氧化应激,引起细胞氧化损伤并发展为多系统疾病[33]。虽然人工合成的抗氧化剂能有效减缓氧化过程,但其安全性却受到质疑[34]。
因此,寻找具有高抗氧化活性和低细胞毒性的抗氧化剂成为研究热点,LAB-EPS也因此备受科研人员关注[35]。目前研究显示,大多数LAB-EPS无论在体内还是体外均具有抗氧化功能,能够参与自由基清除,从而作为天然的安全抗氧化剂发挥作用,但是抗氧化的效果普遍低于抗坏血酸。Tang等[36]在德氏乳杆菌SRFM-1中分离出3种EPS,具有较强的超氧阴离子自由基、羟基自由基、1, 1-二苯基-2-三硝基苯肼(DPPH)自由基清除活性和亚铁离子螯合活性。植物乳杆菌KX041的EPS可以充当电子供体直接与自由基反应[37],植物乳杆菌JLAU103的EPS还能够通过与过渡金属离子催化剂螯合来发挥抗氧化功能[35]。刘煜珺等[38]还发现植物乳杆菌Y42的EPS发挥抗氧化功能是通过上调HT-29细胞中过氧化氢酶(CAT)、超氧化物歧化酶(SOD)和谷胱甘肽过氧化物酶(GSH-Px)等抗氧化酶系的表达量和活性实现的。此外,通过衰老模型小鼠体内试验发现,瑞士乳杆菌的EPS在上调小鼠血清、脑组织和肝脏中多种抗氧化酶的活性和总抗氧化能力(T-AOC)的同时,还能够下调丙二醛的水平,证实了瑞士乳杆菌的EPS在体内同样具有抗氧化功能[39]。由此可见,LAB-EPS主要是通过清除氧自由基和提高机体内相关抗氧化酶活性实现其抗氧化功能,使其可以作为功能性饲料添加剂进行开发利用。
但是大量研究表明,菌株、培养基和培养条件等对LAB-EPS抗氧化功能均有影响[40](表 2)。因此,筛选具有能够分泌高抗氧化活性EPS的LAB菌株,通过优化培养条件等提高EPS抗氧化功能的研究也逐渐受到科研工作者的关注。
|
|
表 2 部分LAB氧自由基清除能力 Table 2 Oxygen free radical scavenging ability of some LAB |
病毒的有效消除依赖于被感染细胞产生促炎性免疫反应的能力,并发展为能够限制病毒复制的Th1型免疫。这种反应的特征是促炎细胞因子和趋化因子的产生,包括干扰素(interferon,IFN)、TNF-α和各种IL(如IL-12、IL-18及IL-23等)以及单核/巨噬细胞、自然杀伤(NK)细胞、T淋巴细胞的激活。研究表明,益生菌及其代谢物可以通过改善先天性免疫和适应性免疫来保护机体免受病毒感染,从而缩短病程,减少发病次数,减少病毒脱落,使肠道通透性正常化并增加病毒特异性抗体的产生[49]。Sirichokchatchawan等[10]研究证实,植物乳杆菌培养液上清液和活菌均可以在非洲绿猴肾细胞(Vero细胞)上抑制猪流行性腹泻病毒感染和复制,并且上清液抗病毒活性具有明显剂量和细菌数量依赖性。Kim等[50]证明了植物乳杆菌LRCC5310的EPS通过减少腹泻持续时间,抑制肠上皮病变,减少轮状病毒在肠道的复制,缩短哺乳小鼠的恢复时间,表现出明显的抗轮状病毒活性。Kanmani等[49]研究发现,德氏乳杆菌的EPS具有改善肠道抗病毒反应和防止肠道病毒(如轮状病毒)感染的潜力。此外,德氏乳杆菌TUA4408L的EPS还可以通过减少病毒复制来提高猪肠上皮细胞对轮状病毒感染的抵抗力,并通过调节Toll样受体3(Toll-like receptor 3, TLR3)激活抗病毒先天免疫应答[51]。王莹莹[52]发现,嗜酸乳杆菌的EPS可以促进猪睾丸细胞的免疫活性,抑制猪传染性胃肠炎病毒感染,在作用早期EPS可以协同猪传染性胃肠炎病毒诱导猪睾丸细胞的TLR3表达和NF-κB的活化,上调IL-6、IL-8和IFN-γ的表达,并调节细胞的先天性免疫反应,提高细胞的抗病毒能力。由此可见,LAB-EPS不仅能够抑制病毒的感染和复制,还可以通过激活动物机体先天性免疫应答和适应性免疫应答发挥抗病毒功能,在畜牧生产中具有重要的研究价值。
2.4 免疫调节LAB-EPS是一种天然的大分子活性物质,安全性高且来源的可追溯性强,在调节机体免疫过程中具有良好的免疫原性,是理想的天然免疫佐剂。研究发现,大多数LAB及其EPS可通过提高单核细胞吞噬能力,促进T、B淋巴细胞的增殖,提高NK细胞杀肿瘤活性和促有丝分裂活性,诱导细胞因子来调节机体免疫系统,从而提高机体的免疫防御能力来抵抗病原体。Zhu等[53]通过体外试验证明,植物乳杆菌RS20D分泌的EPS可以刺激巨噬细胞释放一氧化氮(NO),并在mRNA水平上调促炎细胞因子的基因表达。此外,EPS除了可以通过提高胞内酶活性及释放活性氧激活巨噬细胞RAW264.7,发挥免疫增强作用[54],还能显著提高巨噬细胞RAW264.7的增殖和吞噬活性,并诱导NO、TNF-α、IL-1β和IL-6的产生[5]。由副干酪乳杆菌DG产生的EPS还能够增强人单核细胞系THP-1中TNF-α、IL-6、IL-8和巨噬细胞炎症蛋白3α的表达[55]。综上所述,大多数LAB-EPS主要是通过调节免疫相关基因,激活巨噬细胞,刺激淋巴细胞增殖并释放NO、IL和TNF-α等来表现免疫调节活性[56]。因此,无论是体外试验还是体内试验均显示出LAB-EPS具有良好的免疫调节作用,很多LAB-EPS发挥免疫调节作用的同时也具有一定的抗肿瘤和抗病毒作用。由此推测,LAB-EPS很可能是通过增强机体免疫力,促进淋巴细胞、巨噬细胞等增殖,合成分泌相应细胞因子,从而抑制肿瘤细胞生长,提高动物机体抵抗病毒感染能力,发挥抗肿瘤、抗病毒作用。
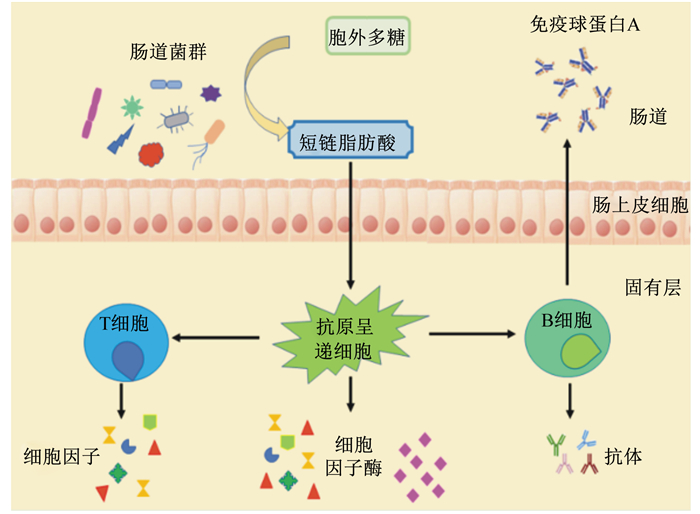
2.5 调节肠道菌群动物肠道中聚居着1013~1014个微生物,是一个复杂的微生态体系。肠道微生物的组成与机体健康密切相关,在维持生理平衡中起着至关重要的作用。同时,肠道微生物的构建与肠道免疫系统的发育也有密不可分的联系,大多数LAB-EPS能促进肠道有益微生物的生长,改善其多样性[57]。EPS可以作为肠道微生物的碳源,因为肠道微生物的基因组编码碳水化合物活性酶(carbohydrate active enzymes,CAZymes),而人和其他哺乳动物的基因组则没有编码足够的CAZymes,因此,肠道微生物能够将EPS降解为短链脂肪酸,如乙酸、丙酸和丁酸等[58]。这些短链脂肪酸对于维持肠上皮功能、控制肠上皮生长、刺激免疫系统、预防结直肠癌并减少各种炎症性疾病有重要的作用[59](图 3)。有研究显示,LAB-EPS可能通过抑制细菌细胞表面的特异性黏附因子来减少有害菌对肠上皮细胞的黏附[48],选择性促进有益菌的增殖,抑制有害菌的增殖和有害物质的产生[60],进而改善肠道微生物的多样性和平衡[57]。大量体外研究结果显示,LAB-EPS可以有效抑制阪崎肠杆菌、大肠杆菌、单核细胞增生李斯特菌、金黄色葡萄球菌、白色念珠菌、鼠疫杆菌和鼠伤寒沙门氏菌等有害菌的生长[61-64],其中有些EPS可以同时抑制多种有害菌的增殖,具有一定的广谱抗菌性。邵丽[65]研究表明,鼠李糖乳杆菌KF5的EPS可以部分被人的粪便菌群利用,促进双歧杆菌的增殖,改变粪便菌群的组成,提高菌群的丰度,促进短链脂肪酸产生,具有潜在的益生特性。李胜杰[66]用双歧杆菌WBIN03的EPS对小鼠进行灌胃,发现EPS可以促进乳杆菌和厌氧总菌的生长,并抑制肠道内肠杆菌、肠球菌及拟杆菌的生长,进一步证明EPS是一种潜在的益生物质。
|
图 3 LAB-EPS与肠道微生物的相互作用 Fig. 3 Interaction between LAB-EPS and intestinal microbiota[58] |
通过上述研究发现,每种LAB-EPS生物学功能并不是单一的,同一种EPS可能具有不同的功能,不同的EPS可能具有相同的作用机制。除了上述生物学功能以外,研究发现,EPS对细菌生物膜形成有明显抑制作用,并通过减弱细胞表面修饰或减少细胞间相互作用以抑制细菌细胞的初始自动聚集和细胞附着,从而可以用于治疗和预防由产生生物膜的致病细菌引起的传染病[67]。植物乳杆菌BGCG11产生的EPS通过下调IL-1β和诱导型一氧化氮合酶(iNOS)mRNA以及增加具有抗炎活性的IL-6和IL-10细胞因子的水平,在大鼠中表现出较高抗炎活性[68]。EPS还能与病原菌细胞壁相互作用,降解细胞壁和质膜引起蛋白质溶解和必需物质的外露,从而导致病原菌死亡[69]。Ai等[70]发现干酪乳杆菌LC2W的EPS能显著降低自发性高血压大鼠的收缩压,马乳酒样乳杆菌WT-2B产生的EPS可以通过抑制血管紧张素Ⅰ转换酶的活性来降低血压[71]。在体外试验中,植物乳杆菌BR2的EPS可以降低45%的胆固醇水平[72]。
3 LAB-EPS在畜牧生产中的应用EPS作为LAB的重要次级代谢产物之一,随着研究不断深入,其各种生物学功能逐渐被人们所熟知,所以,目前已经广泛应用于农业、食品、医药和化妆品等研究领域。在食品工业中,EPS可以改善食品品质[73],提高酸奶的黏度[74]、奶酪产量[75]和冰淇淋的黏性及假塑性[76]。此外,EPS还能阻碍淀粉的凝沉并降低陈化速率,从而延长面包的保质期,提高面包质量[77]。因此,EPS具有非常可靠的安全性。
在过去几十年中,为了追求较高的经济效益,抗生素被大量广泛地应用于畜牧生产中,正是由于抗生素的滥用,导致环境污染、畜禽产品药物残留及耐药菌株出现[78-79]等问题越来越严重。2020年我国已全面禁止抗生素在饲料添加剂中使用,随之可能会出现动物生产性能降低、病死率增加等问题,所以,开发抗生素替代品作为饲料添加剂以保证畜禽健康生长成为研究热点[80]。EPS作为LAB的重要次级代谢产物,具有多种生物学功能,是非常有潜力的抗生素替代物之一[81]。研究发现,在饲粮中添加LAB能够下调IL-6、IL-8及TNF-α的表达和分泌,显著增加仔猪防御肽pBD2、PG1-5和pBD2的分泌表达,还能增强肠上皮细胞防御功能并通过调节肠道菌群来增强肠道屏障作用[81]。而LAB可以通过EPS与肠上皮细胞的黏附定殖在肠道中,EPS还可以促进LAB生长,提高其抗逆性及益生潜力[82],并形成保护层帮助LAB逃避免疫监测,促进肠道内LAB的定殖,同时还能降低肠道致病菌柠檬酸杆菌的定植水平[83]。此外,EPS还能够通过竞争性抑制机制干扰细菌表面上特定黏附因子与肠道黏膜表面受体结合,从而降低细菌致病性[26]。据报道,饲喂能够产生EPS的罗氏乳杆菌发酵的饲粮,可降低断奶仔猪回肠、盲肠和结肠中产肠毒素的大肠杆菌的定殖水平[84],有效缓解因肠道菌群平衡破坏导致的疾病。此外,Ashfaq等[85]将EPS添加到雏鸡饲粮中,发现EPS能够显著提高肠道内LAB的丰度,并抑制常见肠道病原体如大肠杆菌、沙门氏菌和肠球菌的生长,具有替代抗生素的潜力。Rajoka等[86]对从母鸡盲肠内分离的LAB所产生的EPS进行体外试验研究,EPS表现出较强的抗大肠杆菌和鼠伤寒沙门氏菌活性,对羟基自由基、DPPH自由基和超氧阴离子自由基有较强的清除能力,同时还能抑制Caco-2的生长,表现出一定的抗肿瘤活性。由此推断,LAB-EPS通过调节肠道菌群结构、抑制有害菌定殖及调节免疫反应等作用,提高机体抗病能力,从而减少抗生素在生产中的应用。
4 小结与动物和植物来源的EPS相比,LAB产生的EPS具有许多优点,例如具有较强的可操作性、较高的繁殖能力和高级性能等,但到目前为止,其结构和活性关系尚未完全清楚。因此,我们需要对EPS的结构和活性关系进行深入研究,并构建其潜在生物学功能和工业应用之间的桥梁。目前,EPS应用研究主要集中于食品生产等领域,虽然也有研究显示,在畜牧业中LAB-EPS可以通过其抑菌活性、免疫增强作用、促进营养物质消化吸收和调节肠道菌群等方面的作用进而维持动物肠道的微生态平衡,提高动物的免疫力及生产性能,但是相关研究多数集中在体外试验及小鼠体内试验。有关EPS应用于畜禽的研究并不多,其中主要原因集中在LAB-EPS产率较低,无法满足畜禽体内试验所需剂量。另外,不同LAB分泌的EPS生物学功能也存在较大差异,其作用机理等也均处于初步研究阶段尚不明确。所以,关于如何提高LAB-EPS的产量和生物学功能,及其在生产实践中的应用和作用机理等研究均有广阔的前景,同时LAB-EPS也有望成为抗生素替代物之一。
| [1] |
ZHU H, YANG T, XING Y X, et al. Advances in nutritional function of extracellular polysaccharides from lactic acid bacteria[J]. Revista Cientifica-Facultad De Ciencias Veterinarias, 2020, 30(1): 345-353. |
| [2] |
LEBLANC J G, LEVIT R, SAVOY DE GIORI G, et al. Application of vitamin-producing lactic acid bacteria to treat intestinal inflammatory diseases[J]. Applied Microbiology and Biotechnology, 2020, 104(8): 3331-3337. DOI:10.1007/s00253-020-10487-1 |
| [3] |
KIM K W, KANG S S, WOO S J, et al. Lipoteichoic acid of probiotic Lactobacillus plantarum attenuates poly Ⅰ: C-induced IL-8 production in porcine intestinal epithelial cells[J]. Frontiers in Microbiology, 2017, 8: 1827. DOI:10.3389/fmicb.2017.01827 |
| [4] |
ZEIDAN A A, POULSEN V K, JANZEN T, et al. Polysaccharide production by lactic acid bacteria: from genes to industrial applications[J]. FEMS Microbiology Reviews, 2017, 41(Suppl.1): S168-S200. |
| [5] |
XIU L, SHENG S X, HU Z P, et al. Exopolysaccharides from Lactobacillus kiferi as adjuvant enhanced the immuno-protective against Staphylococcus aureus infection[J]. International Journal of Biological Macromolecules, 2020, 161: 10-23. DOI:10.1016/j.ijbiomac.2020.06.005 |
| [6] |
DI W, ZHANG L W, WANG S M, et al. Physicochemical characterization and antitumour activity of exopolysaccharides produced by Lactobacillus casei SB27 from yak milk[J]. Carbohydrate Polymers, 2017, 171: 307-315. DOI:10.1016/j.carbpol.2017.03.018 |
| [7] |
SANALIBABA P, CAKMAK G A. Exopolysaccharides production by lactic acid bacteria[J]. Applied Microbiology: Open Access, 2016, 2(2): 1000115. |
| [8] |
LYNCH K M, ZANNINI E, COFFEY A, et al. Lactic acid bacteria exopolysaccharides in foods and beverages: isolation, properties, characterization, and health benefits[J]. Annual Review of Food Science and Technology, 2018, 9: 155-176. DOI:10.1146/annurev-food-030117-012537 |
| [9] |
WANG K, LI W, RUI X, et al. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810[J]. International Journal of Biological Macromolecules, 2014, 63: 133-139. DOI:10.1016/j.ijbiomac.2013.10.036 |
| [10] |
SIRICHOKCHATCHAWAN W, TEMEEYASEN G, NILUBOL D, et al. Protective effects of cell-free supernatant and live lactic acid bacteria isolated from thai pigs against a pandemic strain of porcine epidemic diarrhea virus[J]. Probiotics and Antimicrobial Proteins, 2018, 10(2): 383-390. DOI:10.1007/s12602-017-9281-y |
| [11] |
LIU Z Q, ZHANG Z H, QIU L, et al. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04[J]. Journal of Dairy Science, 2017, 100(9): 6895-6905. DOI:10.3168/jds.2016-11944 |
| [12] |
AYYASH M, ABU-JDAYIL B, ITSARANUWAT P, et al. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47:structural characteristics, rheological properties, bioactivities and impact on fermented camel milk[J]. Food Chemistry, 2020, 333: 127418. DOI:10.1016/j.foodchem.2020.127418 |
| [13] |
ANGELIN J, KAVITHA M. Exopolysaccharides from probiotic bacteria and their health potential[J]. International Journal of Biological Macromolecules, 2020, 162: 853-865. DOI:10.1016/j.ijbiomac.2020.06.190 |
| [14] |
XU Y M, CUI Y L, YUE F F, et al. Exopolysaccharides produced by lactic acid bacteria and Bifidobacteria : structures, physiochemical functions and applications in the food industry[J]. Food Hydrocolloids, 2019, 94: 475-499. DOI:10.1016/j.foodhyd.2019.03.032 |
| [15] |
LI W, TANG W Z, JI J, et al. Characterization of a novel polysaccharide with anti-colon cancer activity from Lactobacillus helveticus MB2-1[J]. Carbohydrate Research, 2015, 411: 6-14. DOI:10.1016/j.carres.2014.12.014 |
| [16] |
LI W, JI J, CHEN X H, et al. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1[J]. Carbohydrate Polymers, 2014, 102: 351-359. DOI:10.1016/j.carbpol.2013.11.053 |
| [17] |
MAKINO S, SATO A, GOTO A, et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1[J]. Journal of Dairy Science, 2016, 99(2): 915-923. DOI:10.3168/jds.2015-10376 |
| [18] |
ZHOU K, ZENG Y T, YANG M L, et al. Production, purification and structural study of an exopolysaccharide from Lactobacillus plantarum BC-25[J]. Carbohydrate Polymers, 2016, 144: 205-214. DOI:10.1016/j.carbpol.2016.02.067 |
| [19] |
AI L Z, GUO Q B, DING H H, et al. Structure characterization of exopolysaccharides from Lactobacillus casei LC2W from skim milk[J]. Food Hydrocolloids, 2016, 56: 134-143. DOI:10.1016/j.foodhyd.2015.10.023 |
| [20] |
GÓRSKA-FRACZEK S, SANDSTRÖM C, KENNE L, et al. The structure and immunoreactivity of exopolysaccharide isolated from Lactobacillus johnsonii strain 151[J]. Carbohydrate Research, 2013, 378: 148-153. DOI:10.1016/j.carres.2013.05.012 |
| [21] |
FONTANA C, LI S Y, YANG Z N, et al. Structural studies of the exopolysaccharide from Lactobacillus plantarum C88 using NMR spectroscopy and the program CASPER[J]. Carbohydrate Research, 2015, 402: 87-94. DOI:10.1016/j.carres.2014.09.003 |
| [22] |
GÓRSKA-FRACZEK S, SANDSTRÖM C, KENNE L, et al. Structural studies of the exopolysaccharide consisting of a nonasaccharide repeating unit isolated from Lactobacillus rhamnosus KL37B[J]. Carbohydrate Research, 2011, 346(18): 2926-2932. DOI:10.1016/j.carres.2011.10.024 |
| [23] |
SÄWÉN E, HUTTUNEN E, ZHANG X, et al. Structural analysis of the exopolysaccharide produced by Streptococcus thermophilus ST1 solely by NMR spectroscopy[J]. Journal of Biomolecular NMR, 2010, 47(2): 125-134. DOI:10.1007/s10858-010-9413-0 |
| [24] |
MARSHALL V M, DUNN H, ELVIN M, et al. Structural characterisation of the exopolysaccharide produced by Streptococcus thermophilus EU20[J]. Carbohydrate Research, 2001, 331(4): 413-422. DOI:10.1016/S0008-6215(01)00052-0 |
| [25] |
SHIOMI M, SASAKI K, MUROFUSHI M, et al. Antitumor activity in mice of orally administered polysaccharide from kefir grain[J]. Japanese Journal of Medical Science and Biology, 1982, 35(2): 75-80. DOI:10.7883/yoken1952.35.75 |
| [26] |
LI L, JIANG Y J, YANG X Y, et al. Immunoregulatory effects on Caco-2 cells and mice of exopolysaccharides isolated from Lactobacillus acidophilus NCFM[J]. Food & Function, 2014, 5(12): 3261-3268. |
| [27] |
EL-DEEB N M, YASSIN A M, AL-MADBOLY L A, et al. A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-κB inflammatory pathways in human colon cancer[J]. Microbial Cell Factories, 2018, 17(1): 29. DOI:10.1186/s12934-018-0877-z |
| [28] |
SUN N X, LIU H P, LIU S J, et al. Purification, preliminary structure and antitumor activity of exopolysaccharide produced by Streptococcus thermophilus CH9[J]. Molecules, 2018, 23(11): 2898. DOI:10.3390/molecules23112898 |
| [29] |
胡盼盼. 乳酸菌胞外多糖诱导结肠癌HT-29细胞凋亡的机制研究[D]. 硕士学位论文. 哈尔滨: 哈尔滨工业大学, 2015. HU P P. The mechanism of exopolysaccharide-induced apoptosis in HT-29 cancer cell[D]. Master's Thesis. Harbin: Harbin Institute of Technology, 2015. (in Chinese) |
| [30] |
SCHIEBER M, CHANDEL N S. ROS function in redox signaling and oxidative stress[J]. Current Biology, 2014, 24(10): R453-R462. DOI:10.1016/j.cub.2014.03.034 |
| [31] |
CAO P, ZHANG Y, HUANG Z, et al. The preventative effects of procyanidin on binge ethanol-induced lipid accumulation and ROS overproduction via the promotion of hepatic autophagy[J]. Molecular Nutrition & Food Research, 2019, 63(18): 1801255. |
| [32] |
RAGURAMAN V, L S A, J J, et al. Sulfated polysaccharide from Sargassum tenerrimum attenuates oxidative stress induced reactive oxygen species production in in vitro and in zebrafish model[J]. Carbohydrate Polymers, 2019, 203: 441-449. DOI:10.1016/j.carbpol.2018.09.056 |
| [33] |
KRUK J, ABOUL-ENEIN H, KŁADNA A, et al. Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis[J]. Free Radical Research, 2019, 53(5): 497-521. DOI:10.1080/10715762.2019.1612059 |
| [34] |
LI S Y, ZHAO Y J, ZHANG L, et al. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods[J]. Food Chemistry, 2012, 135(3): 1914-1919. DOI:10.1016/j.foodchem.2012.06.048 |
| [35] |
MIN W H, FANG X B, WU T, et al. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103[J]. Journal of Bioscience and Bioengineering, 2019, 127(6): 758-766. DOI:10.1016/j.jbiosc.2018.12.004 |
| [36] |
TANG W Z, DONG M S, WANG W L, et al. Structural characterization and antioxidant property of released exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1[J]. Carbohydrate Polymers, 2017, 173: 654-664. DOI:10.1016/j.carbpol.2017.06.039 |
| [37] |
WANG X, SHAO C G, LIU L, et al. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041[J]. International Journal of Biological Macromolecules, 2017, 103: 1173-1184. DOI:10.1016/j.ijbiomac.2017.05.118 |
| [38] |
刘煜珺, 张雨晴, 高原, 等. 乳杆菌胞外多糖抗氧化活性研究[J]. 中国食品学报, 2019, 19(6): 21-35. LIU Y J, ZHANG Y Q, GAO Y, et al. Studies on the antioxidative activity of exopolysaccharide from Lactobacillus strains[J]. Journal of Chinese Institute of Food Science and Technology, 2019, 19(06): 21-35 (in Chinese). |
| [39] |
白丽娟. 马奶酒中产胞外多糖瑞士乳杆菌的筛选及多糖的结构和抗氧化活性研究[D]. 博士学位论文. 沈阳: 沈阳农业大学, 2017. BAI L J. Screening exopolysaccharide-producing L. helveticus and structure, antioxidant activity of exopolysaccharides[D]. Ph. D. Thesis. Shenyang: Shenyang Agricultural University, 2017. (in Chinese) |
| [40] |
OLEKSY-SOBCZAK M, KLEWICKA E. Optimization of media composition to maximize the yield of exopolysaccharides production by Lactobacillus rhamnosus strains[J]. Probiotics and Antimicrobial Proteins, 2020, 12(2): 774-783. DOI:10.1007/s12602-019-09581-2 |
| [41] |
XIAO L Y, LI Y Y, TIAN J J, et al. Influences of drying methods on the structural, physicochemical and antioxidant properties of exopolysaccharide from Lactobacillus helveticus MB2-1[J]. International Journal of Biological Macromolecules, 2020, 157: 220-231. DOI:10.1016/j.ijbiomac.2020.04.196 |
| [42] |
RIAZ RAJOKA M S, JIN M L, ZHAO H B, et al. Functional characterization and biotechnological potential of exopolysaccharide produced by Lactobacillus rhamnosus strains isolated from human breast milk[J]. LWT, 2018, 89: 638-647. DOI:10.1016/j.lwt.2017.11.034 |
| [43] |
ZHANG J, ZHAO X, JIANG Y Y, et al. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir[J]. Journal of Dairy Science, 2017, 100(8): 6025-6041. DOI:10.3168/jds.2016-12480 |
| [44] |
TRABELSI I, KTARI N, BEN SLIMA S, et al. Evaluation of dermal wound healing activity and in vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus sp. Ca6[J]. International Journal of Biological Macromolecules, 2017, 103: 194-201. DOI:10.1016/j.ijbiomac.2017.05.017 |
| [45] |
ABEDFAR A, ABBASZADEH S, HOSSEININEZHAD M, et al. Physicochemical and biological characterization of the EPS produced by L. acidophilus isolated from rice bran sourdough[J]. LWT, 2020, 127: 109373. DOI:10.1016/j.lwt.2020.109373 |
| [46] |
WANG K, LI W, RUI X, et al. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810[J]. International Journal of Biological Macromolecules, 2014, 67: 71-78. DOI:10.1016/j.ijbiomac.2014.02.056 |
| [47] |
LI S Q, SHAH N P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275[J]. Food Chemistry, 2014, 165: 262-270. DOI:10.1016/j.foodchem.2014.05.110 |
| [48] |
MAO J W, YIN J, GE Q, et al. In vitro antioxidant activities of polysaccharides extracted from Moso Bamboo-Leaf[J]. International Journal of Biological Macromolecules, 2013, 55: 1-5. DOI:10.1016/j.ijbiomac.2012.12.027 |
| [49] |
KANMANI P, ALBARRACIN L, KOBAYASHI H, et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells[J]. Molecular Immunology, 2018, 93: 253-265. DOI:10.1016/j.molimm.2017.07.009 |
| [50] |
KIM K, LEE G, THANH H D, et al. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response[J]. Journal of Dairy Science, 2018, 101(7): 5702-5712. DOI:10.3168/jds.2017-14151 |
| [51] |
KANMANI P, ALBARRACIN L, KOBAYASHI H, et al. Genomic characterization of Lactobacillus delbrueckii TUA4408L and evaluation of the antiviral activities of its extracellular polysaccharides in porcine intestinal epithelial cells[J]. Frontiers in Immunology, 2018, 9: 2178. DOI:10.3389/fimmu.2018.02178 |
| [52] |
王莹莹. 嗜酸乳杆菌胞外多糖对TGEV感染ST细胞TLR3及部分细胞因子表达的影响[D]. 硕士学位论文. 哈尔滨: 东北农业大学, 2015. WANG Y Y. The expression of TLR3 and some cytokines in ST cells induced by TGEV infection and Lactobacillus acidophilus exopolysaccharides pretreatment[D]. Master's Thesis. Harbin: Northeast Agricultural University, 2015. (in Chinese) |
| [53] |
ZHU Y T, WANG X J, PAN W S, et al. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity[J]. International Journal of Biological Macromolecules, 2019, 121: 342-349. DOI:10.1016/j.ijbiomac.2018.09.201 |
| [54] |
李琪, 杜佳峰, 高洁, 等. 副干酪乳杆菌VL8胞外多糖对巨噬细胞RAW264.7免疫激活作用研究[J]. 食品研究与开发, 2020, 41(3): 208-213. LI Q, DU J F, GAI J, et al. Immune activation of macrophage RAW264.7 by Lactobacillus paracasei VL8 exopolysaccharides[J]. Food Research and Development, 2020, 41(3): 208-213 (in Chinese). |
| [55] |
BALZARETTI S, TAVERNITI V, GUGLIELMETTI S, et al. A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells[J]. Applied and Environmental Microbiology, 2017, 83(3): e02702-16. |
| [56] |
ZHOU Y, CUI Y H, QU X J. Exopolysaccharides of lactic acid bacteria: structure, bioactivity and associations: a review[J]. Carbohydrate Polymers, 2019, 207: 317-332. DOI:10.1016/j.carbpol.2018.11.093 |
| [57] |
KORCZ E, KERÉNYI Z, VARGA L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: potential health benefits with special regard to cholesterol-lowering effects[J]. Food & Function, 2018, 9(6): 3057-3068. |
| [58] |
CHAISUWAN W, JANTANASAKULWONG K, WANGTUEAI S, et al. Microbial exopolysaccharides for immune enhancement: fermentation, modifications and bioactivities[J]. Food Bioscience, 2020, 35: 100564. DOI:10.1016/j.fbio.2020.100564 |
| [59] |
TANG C, DING R X, SUN J, et al. The impacts of natural polysaccharides on intestinal microbiota and immune responses—a review[J]. Food & Function, 2019, 10(5): 2290-2312. |
| [60] |
童良琴, 曲亚军, 陈敏. 乳酸菌胞外多糖的研究进展[J]. 中国生物工程杂志, 2015, 35(11): 85-91. TONG L Q, QU Y J, CHEN M. Research advance on exopolysaccharides synthesized by lactic acid bacteria[J]. China Biotechnology, 2015, 35(11): 85-91 (in Chinese). |
| [61] |
LI S J, HUANG R H, SHAH N P, et al. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315[J]. Journal of Dairy Science, 2014, 97(12): 7334-7343. DOI:10.3168/jds.2014-7912 |
| [62] |
JEONG D, KIM D H, KANG I B, et al. Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir[J]. Food Control, 2017, 78: 436-442. DOI:10.1016/j.foodcont.2017.02.033 |
| [63] |
ALLONSIUS C N, VAN DEN BROEK M F L, DE BOECK I, et al. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides[J]. Microbial Biotechnology, 2017, 10(6): 1753-1763. DOI:10.1111/1751-7915.12799 |
| [64] |
WANG J, ZHAO X, YANG Y W, et al. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32[J]. International Journal of Biological Macromolecules, 2015, 74: 119-126. DOI:10.1016/j.ijbiomac.2014.12.006 |
| [65] |
邵丽. 产胞外多糖乳杆菌的筛选及其多糖的分离、结构和生物活性研究[D]. 博士学位论文. 无锡: 江南大学, 2015. SHAO L. Screening of exopolysaccharide-producing lactobacilli and separation, structure and bioactivities of exopolysaccharide[D]. Ph. D. Thesis. Wuxi: Jiangnan University, 2015. (in Chinese) |
| [66] |
李胜杰. 两歧双歧杆菌WBIN03胞外多糖的抗菌/抗氧化活性及其对小鼠免疫和肠道菌群影响的研究[D]. 硕士学位论文. 南昌: 南昌大学, 2014. LI S J. Research on the antioxidant and antibacterial active of exopolysaccharides from Bifidobacterium bifidum WBIN03 and its benefical effects on the immunity and inteatinal microbiota in mice[D]. Master's Thesis. Nanchang: Nanchang University, 2014. (in Chinese) |
| [67] |
KANMANI P, SUGANYA K, KUMAR R S, et al. Synthesis and functional characterization of antibiofilm exopolysaccharide produced by Enterococcus faecium MC13 isolated from the gut of fish[J]. Applied Biochemistry and Biotechnology, 2013, 169(3): 1001-1015. DOI:10.1007/s12010-012-0074-1 |
| [68] |
DINIĆ M, PECIKOZA U, DJOKIĆ J, et al. Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats[J]. Frontiers in Pharmacology, 2018, 9: 1. DOI:10.3389/fphar.2018.00001 |
| [69] |
FOOLADI T, SOUDI M R, ALIMADADI N, et al. Bioactive exopolysaccharide from Neopestalotiopsis sp. strain SKE15:production, characterization and optimization[J]. International Journal of Biological Macromolecules, 2019, 129: 127-139. DOI:10.1016/j.ijbiomac.2019.01.203 |
| [70] |
AI L Z, ZHANG H, GUO B H, et al. Preparation, partial characterization and bioactivity of exopolysaccharides from Lactobacillus casei LC2W[J]. Carbohydrate Polymers, 2008, 74(3): 353-357. DOI:10.1016/j.carbpol.2008.03.004 |
| [71] |
MAEDA H, ZHU X, SUZUKI S, et al. Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens WT-2B[J]. Journal of Agricultural and Food Chemistry, 2004, 52(17): 5533-5538. DOI:10.1021/jf049617g |
| [72] |
SASIKUMAR K, KOZHUMMAL VAIKKATH D, DEVENDRA L, et al. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods[J]. Bioresource Technology, 2017, 241: 1152-1156. DOI:10.1016/j.biortech.2017.05.075 |
| [73] |
ZHANG H Y, PENG J X, LI X, et al. A nano-bio interfacial protein corona on silica nanoparticle[J]. Colloids and Surfaces B: Biointerfaces, 2018, 167: 220-228. DOI:10.1016/j.colsurfb.2018.04.021 |
| [74] |
HAN X, YANG Z, JING X P, et al. Improvement of the texture of yogurt by use of exopolysaccharide producing lactic acid bacteria[J]. Biomed Research International, 2016, 2016: 7945675. |
| [75] |
LLUIS-ARROYO D, FLORES-NÁJERA A, CRUZ-GUERRERO A, et al. Effect of an exopolysaccharide-producing strain of Streptococcus thermophilus on the yield and texture of mexican manchego-type cheese[J]. International Journal of Food Properties, 2014, 17(8): 1680-1693. DOI:10.1080/10942912.2011.599091 |
| [76] |
DERTLI E, MAYER M J, COLQUHOUN I J, et al. EpsA is an essential gene in exopolysaccharide production in Lactobacillus johnsonii FI9785[J]. Microbial Biotechnology, 2016, 9(4): 496-501. DOI:10.1111/1751-7915.12314 |
| [77] |
LYNCH K M, COFFEY A, ARENDT E K. Exopolysaccharide producing lactic acid bacteria: their techno-functional role and potential application in gluten-free bread products[J]. Food Research International, 2018, 110: 52-61. DOI:10.1016/j.foodres.2017.03.012 |
| [78] |
TOUTAIN P L, FERRAN A A, BOUSQUET-MELOU A, et al. Veterinary medicine needs new green antimicrobial drugs[J]. Frontiers in Microbiology, 2016, 7: 1196. |
| [79] |
NEUMAN H, FORSYTHE P, UZAN A, et al. Antibiotics in early life: dysbiosis and the damage done[J]. FEMS Microbiology Reviews, 2018, 42(4): 489-499. |
| [80] |
WANG J, JI H F, WANG S X, et al. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota[J]. Frontiers in Microbiology, 2018, 9: 1953. DOI:10.3389/fmicb.2018.01953 |
| [81] |
AZAD M A K, SARKER M, LI T J, et al. Probiotic species in the modulation of gut microbiota: an overview[J]. Biomed Research International, 2018, 2018: 9478630. |
| [82] |
CAGGIANIELLO G, KLEEREBEZEM M, SPANO G. Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms[J]. Applied Microbiology and Biotechnology, 2016, 100(9): 3877-3886. DOI:10.1007/s00253-016-7471-2 |
| [83] |
FANNING S, HALL L J, CRONIN M, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(6): 2108-2113. DOI:10.1073/pnas.1115621109 |
| [84] |
YANG Y, GALLE S, LE M H, et al. Feed fermentation with reuteran- and levan-producing Lactobacillus reuteri reduces colonization of weanling pigs by enterotoxigenic Escherichia coli[J]. Applied and Environmental Microbiology, 2015, 81(17): 5743-5752. DOI:10.1128/AEM.01525-15 |
| [85] |
ASHFAQ I, AMJAD H, AHMAD W, et al. Growth inhibition of common enteric pathogens in the intestine of broilers by microbially produced dextran and levan exopolysaccharides[J]. Current Microbiology, 2020, 77(9): 2128-2136. DOI:10.1007/s00284-020-02091-3 |
| [86] |
RAJOKA M S R, MEHWISH H M, HAYAT H F, et al. Characterization, the antioxidant and antimicrobial activity of exopolysaccharide isolated from poultry origin Lactobacilli[J]. Probiotics and Antimicrobial Proteins, 2019, 11(4): 1132-1142. DOI:10.1007/s12602-018-9494-8 |




