2. 四川农业大学动物科技学院, 成都 611130
2. College of Animal Science and Technology, Sichuan Agricultural University, Chengdu 611130, China
硒(Se)是动物必需的微量元素,在提高畜禽生长性能、抗氧化能力和改善肉品质等方面发挥着重要作用。缺硒易导致畜禽发生肌营养不良、渗出性素质等缺乏症。传统硒添加剂以亚硒酸钠为主,但因存在生物利用率低、毒性强等诸多缺点已被部分国家减少或禁止使用。因此,生物活性强、利用率高、环境污染小的有机硒和纳米硒在现代养殖中的应用越来越广泛。已有研究表明,有机硒和纳米硒在家禽生长性能、抗氧化能力和肉品质等方面的积极作用均优于无机硒[1]。
纳米硒是利用纳米技术制备而成的单质硒,粒径一般在60 nm以下,其不仅具有比普通单质硒和无机硒更好的生物活性,还具有纳米尺寸效应和抗菌抑菌的效果[2-3]。与无机硒相比,纳米硒具有较高的生物学效价、硒沉积率、稳定性以及较低的毒性[4-5];而与有机硒相比,纳米硒具有较高的产品稳定性以及低廉的价格。目前,在家禽生产中,酵母硒、硒代甲硫氨酸、纳米硒与无机硒的比较研究较多[6-9],但不同硒源对肉鸡生长性能、组织硒沉积及肌肉品质之间的比较缺乏系统研究。在此,本试验拟以肉鸡为研究对象,考察4种不同硒源(亚硒酸钠、酵母硒、羟基-硒代蛋氨酸和纳米硒)对肉鸡生长性能、血清和肌肉硒含量、抗氧化能力及肉品质的影响,以期为各硒源在肉鸡生产中的合理应用提供参考依据。
1 材料与方法 1.1 试验材料亚硒酸钠来自成都某饲料有限公司,硒含量1%;酵母硒来自宜昌某股份有限公司,硒含量0.2%;羟基-硒代蛋氨酸来自法国某公司,硒含量2%;纳米硒来自某生物科技有限公司,硒含量0.3%。
1.2 试验设计将450羽1日龄爱拔益加(AA)雄性肉鸡(购自湖北襄大农牧有限公司)按照单因素试验设计随机分为5组,每组6个重复,每个重复15只鸡。对照组(CON组)饲喂不添加硒的基础饲粮,试验组分别在基础饲粮中添加0.3 mg/kg(以硒计)的亚硒酸钠(SS组)、酵母硒(SeY组)、羟基-硒代蛋氨酸(HMSeBA组)和纳米硒(Nano-Se组)。试验期42 d,分为1~21日龄、22~42日龄2个阶段。
1.3 试验饲粮和饲养管理试验在四川农业大学动物营养研究所教学科研基地进行。采用网上笼养,自由饮水和采食,定期清粪消毒。每日观察并记录鸡只生长状况和饲料损失,其他饲养管理按照常规程序进行。基础饲粮参照我国农业行业标准《鸡饲养标准》(NY/T 33—2004)进行配制,基础饲粮组成及营养水平见表 1。SS组、SeY组、HMSeBA组和Nano-Se组饲粮硒含量(实测值)分别为:1~21日龄1 to 21 days of age,0.42、0.42、0.43和0.43 mg/kg;22~42日龄,0.47、0.47、0.49和0.48 mg/kg。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of basal diets (air-dry basis) |
试验期间,以重复为单位,于21和42日龄早晨空腹称重,并准确记录各阶段的采食量和余料量。计算各组试验1~21日龄、22~42日龄和1~42日龄的平均日采食量(ADFI)、平均日增重(ADG)和料重比(F/G)。
1.4.2 样品的采集42日龄后,依据体重相近原则,每个重复选取1只鸡,采集颈静脉血,静置40 min后4 ℃、3 500 r/min离心10 min,取上清置于-20 ℃保存备用。屠宰后取胸肌和腿肌于冻存管,放置液氮中保存备用。
1.4.3 硒含量的测定按照GB/T 13883—2008方法处理血清、胸肌和腿肌样品,采用原子吸收光谱法(AFS-230E双道原子荧光光度计,北京海光仪器公司)测定硒含量。
1.4.4 抗氧化指标的测定测定血清、胸肌和腿肌的谷胱甘肽过氧化物酶(GSH-Px)和总超氧化物歧化酶(T-SOD)活性、总抗氧化能力(T-AOC)及丙二醛(MDA)含量。所用试剂均购自南京建成生物工程研究所,具体操作步骤按照试剂盒说明书进行。
1.4.5 肉品质的测定利用色差仪和pH计分别测定屠宰后45 min、24 h胸肌和腿肌的亮度(L*)、红度(a*)、黄度(b*)值及pH。取胸肌或腿肌5 g左右称重(m1),悬空放入自封袋,并置于0~4 ℃冷库中,24 h后取出称重(m2),计算滴水损失:
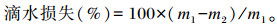
|
取胸肌或腿肌10 g左右称重(m3),放入80 ℃的恒温水浴锅中蒸煮30 min,冷却20 min后称重(m4),计算蒸煮损失:
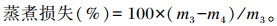
|
利用Excel 2016对数据进行初步整理,利用SPSS 22.0软件进行统计分析,对试验数据进行单因素方差分析(one-way ANOVA),差异显著者进行Duncan氏法多重比较,P < 0.05表示差异显著。所有数据均以平均值±标准差表示。
2 结果 2.1 不同硒源对AA肉鸡生长性能的影响从表 2可知,各组之间肉鸡各阶段的体重、ADG和ADFI均无显著差异(P>0.05)。与CON组、SeY组和HMSeBA组相比,Nano-Se组肉鸡1~21日龄的F/G显著降低(P<0.05);各组之间肉鸡22~42日龄、1~42日龄的F/G均无显著差异(P>0.05)。
|
|
表 2 不同硒源对AA肉鸡生长性能的影响 Table 2 Effects of different Se sources on growth performance of AA broilers |
从表 3可知,与CON组相比,SS组、SeY组、HMSeBA组和Nano-Se组肉鸡胸肌和腿肌硒含量显著升高(P<0.05)。与SS组相比,HMSeBA组和SeY组肉鸡胸肌硒含量显著升高(P<0.05)。Nano-Se组肉鸡血清、胸肌和腿肌硒含量与SS组无显著差异(P>0.05)。肉鸡胸肌和腿肌硒含量高低依次为:HMSeBA组>SeY组>Nano-Se组≈SS组>CON组。
|
|
表 3 不同硒源对AA肉鸡血清、胸肌和腿肌硒含量的影响 Table 3 Effects of different Se sources on contents of selenium in serum, breast and thigh muscle of AA broilers |
从表 4可知,与CON组相比,SS组、SeY组、HMSeBA组和Nano-Se组肉鸡血清和腿肌GSH-Px活性显著升高(P<0.05),但各硒源组之间肉鸡胸肌GSH-Px活性无显著差异(P>0.05)。各组之间肉鸡血清、胸肌和腿肌T-SOD活性、T-AOC及MDA含量无显著差异(P>0.05)。
|
|
表 4 不同硒源对AA肉鸡血清和肌肉抗氧化指标的影响 Table 4 Effects of different Se sources on indexes of antioxidant in serum and muscle of AA broilers |
从表 5可知,各组之间肉鸡胸肌和腿肌的蒸煮损失、滴水损失、pH45 min、pH24 h及肉色的L45 min*、L24 h*、b45 min*、b24 h*值均无显著差异(P>0.05)。与SeY组和HMSeBA组相比,Nano-Se组的胸肌的a45 min*值显著升高(P<0.05);但与CON组和SS组相比,Nano-Se组的胸肌a45 min*无显著差异(P>0.05)。各组之间肉鸡腿肌a45 min*和a24 h*无显著差异(P>0.05)。
|
|
表 5 不同硒源对AA肉鸡肌肉品质的影响 Table 5 Effects of different Se sources on muscle quality of AA broilers |
微量元素硒能够改善动物生长性能,但不同硒源对动物生长性能改善程度有所差异。与亚硒酸钠相比,饲粮添加纳米硒、酵母硒或硒代蛋氨酸可通过提高饲料转化效率提高肉鸡生长性能[8, 10-11]。本试验中,与CON组相比,饲粮添加0.3 mg/kg纳米硒可显著降低1~21日龄的F/G,与前人研究结果[10-11]一致。有研究表明,饲粮添加0.3 mg/kg亚硒酸钠、酵母硒、DL-硒代蛋氨酸和纳米硒对肉鸡生长性能无显著影响[9, 12-13]。本试验条件下,亚硒酸钠、酵母硒、羟基-硒代蛋氨酸和纳米硒添加对肉鸡各阶段的体重、ADG、ADFI及1~42日龄F/G无显著影响,这与Bakhshalinejad等[9]和Boostani等[12]的结论一致。本试验条件下,饲粮添加不同硒源对肉鸡各阶段的体重、ADG和ADFI无显著影响,可能是肉鸡基础饲粮中的硒含量(0.14、0.18 mg/kg)已能够满足肉鸡生长对硒的需要[14]。在1~21日龄,Nano-Se组肉鸡的F/G最低,可能是纳米硒具有一定的抗菌和抗应激能力[2-3]。本试验在夏季炎热季节进行,同时受制于试验场环境条件,可能是本试验中42日龄各组体重偏低的原因。
3.2 不同硒源对AA肉鸡血清和肌肉硒含量的影响饲粮添加硒可提高动物血清和肌肉组织硒含量,不同硒源在动物组织中的硒沉积率不尽相同。研究发现,饲粮添加0.25或0.30 mg/kg的纳米硒、亚硒酸钠、酵母硒或羟基-硒代蛋氨酸均可提高肉鸡组织硒含量,且纳米硒和羟基-硒代蛋氨酸的添加效果优于亚硒酸钠[1, 15-16]。Bakhshalinejad等[9]在肉鸡饲粮中添加不同硒源发现,纳米硒组的胸肌硒含量显著高于DL-硒代蛋氨酸组。本试验中,饲粮添加不同硒源可提高肉鸡血清、胸肌和腿肌硒含量,且Nano-Se组的血清硒含量显著高于SeY组,造成该现象的原因可能与硒在体内的代谢路径有关,无机硒主要以简单扩散的方式进行吸收代谢,有机硒主要以主动运输的方式进行吸收代谢,而纳米硒是通过被动扩散和主动转运的方式进行吸收代谢[17-18]。同时,与无机硒相比,纳米硒具有更高的表面活性和极细的粒度[3-4],因而其所含的硒更容易在组织中富集和存留。但本试验条件下,Nano-Se组的胸肌和腿肌硒含量却低于HMSeBA组和SeY组,与SS组相当,其原因还需通过试验进一步验证。
3.3 不同硒源对AA肉鸡抗氧化能力的影响抗氧化酶类是机体内极为重要的抗氧化系统之一,主要通过T-SOD、GSH-Px、T-AOC和MDA等指标来反映机体抗氧化能力。其中,GSH-Px是典型的含硒酶,其活性可能与机体组织硒含量有关[19]。饲粮添加不同硒源可通过改变组织硒含量而影响GSH-Px活性[20]。有报道认为,GSH-Px的活性不受硒源和硒浓度的影响[21-22]。无机硒、有机硒和纳米硒虽然在畜禽中都能够发挥抗氧化功能,但纳米硒的独特之处在于其清除体内过多自由基的效率高于无机硒和有机硒[23]。本试验中,与CON组相比,饲粮添加不同硒源可提高血清和腿肌GSH-Px活性;但与SS组、SeY组和HMSeBA组相比,Nano-Se组的血清和腿肌GSH-Px活性无显著差异,该结果与Meng等[21]、Cichoski等[22]和Li等[13]的研究结论一致。同时,Mikulková等[24]和Li等[25]研究发现,饲粮添加亚硒酸钠、酵母硒、硒代蛋氨酸和纳米硒对胸肌GSH-Px活性无显著影响,该结果与本试验肌肉GSH-Px活性的研究结果相似。T-AOC和T-SOD能清除体内过多的自由基,而MDA是动物机体脂质的过氧化产物,T-AOC、T-SOD活性及MDA含量均可反映机体抗氧化能力。本试验中,与CON组相比,饲粮添加不同硒源对肉鸡血清、胸肌和腿肌T-SOD、T-AOC活性和MDA含量无显著影响,可能原因是基础饲粮所含的硒已能够满足肉鸡生理需求。此外,正常生理状态下,肉鸡自身氧化与抗氧化系统处于平衡状态,额外补硒对其影响不大。
3.4 不同硒源对AA肉鸡肉品质的影响硒在体内发挥抗氧化作用的同时还可阻止多不饱和脂肪酸过氧化,而肉色又与肌肉氧化密切相关。因此,可通过防止肌肉氧化进而改善肉品质[26]。饲粮添加纳米硒可显著降低胸肌的蒸煮损失,改善肌肉a*和b*值,改善肉品质,且纳米硒改善效果优于亚硒酸钠[1, 9]。有研究发现,饲粮添加纳米硒、有机硒和亚硒酸钠对肌肉pH、滴水损失和肉色值无显著影响[27-29]。本试验中,与CON组相比,饲粮添加不同硒源对胸肌和腿肌蒸煮损失、滴水损失、pH、L*和b*值均无显著影响;且随着货架时间的延长,各组的pH和肉色也无显著差异;与HMSeBA组和SeY组相比,Nano-Se组的胸肌a45 min*显著升高,这与Bakhshalinejad等[9]和Ibrahim等[1]研究结果一致。综上所述,与羟基-硒代蛋氨酸和酵母硒相比,饲粮添加纳米硒可改善肉品质。
4 结论本试验条件下,饲粮添加不同硒源对肉鸡ADG、ADFI无显著影响,但饲粮添加纳米硒降低了肉鸡1~21日龄的F/G;饲粮添加不同硒源提高了肉鸡血清和肌肉的硒含量;饲粮添加纳米硒能够一定程度改善肉鸡肉品质。
| [1] |
IBRAHIM D, KISHAWY A T Y, KHATER S I, et al. Effect of dietary modulation of selenium form and level on performance, tissue retention, quality of frozen stored meat and gene expression of antioxidant status in Ross broiler chickens[J]. Animals, 2019, 9(6): 342. DOI:10.3390/ani9060342 |
| [2] |
SHOEIBI S, MASHREGHI M. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities[J]. Journal of Trace Elements in Medicine and Biology, 2017, 39: 135-139. DOI:10.1016/j.jtemb.2016.09.003 |
| [3] |
NABI F, ARAIN M A, HASSAN F, et al. Nutraceutical role of selenium nanoparticles in poultry nutrition: a review[J]. World's Poultry Science Journal, 2020, 33: 1-13. |
| [4] |
GOPI M, PEARLIN B, KUMAR R D, et al. Role of nanoparticles in animal and poultry nutrition: modes of action and applications in formulating feed additives and food processing[J]. International Journal of Pharmacology, 2017, 13(7): 724-731. DOI:10.3923/ijp.2017.724.731 |
| [5] |
HU C H, LI Y L, XIONG L, et al. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens[J]. Animal Feed Science and Technology, 2012, 177(3/4): 204-210. |
| [6] |
王兴华, 靳东南. 日粮中不同硒源对肉鸡生产性能和抗氧化能力的影响[J]. 山西农业科学, 2016, 44(8): 1184-1191. WANG X H, JIN D N. Effect of different selenium sources on production performance and antioxidant capacity of broilers[J]. Journal of Shanxi Agricultural Science, 2016, 44(8): 1184-1191 (in Chinese). DOI:10.3969/j.issn.1002-2481.2016.08.32 |
| [7] |
MOHAMMADI E, JANMOHAMMADI H, OLYAYEE M, et al. Nano selenium improves humoral immunity, growth performance and breast-muscle selenium concentration of broiler chickens[J]. Animal Production Science, 2020, 60(16): 1902-1910. DOI:10.1071/AN19581 |
| [8] |
邹晓庭, 郑根华, 尹兆正, 等. 不同硒源对肉鸡生长性能、胴体特性和肉质的影响[J]. 浙江大学学报(农业与生命科学版), 2005, 31(6): 773-776. ZOU X T, ZHENG G H, YIN Z Z, et al. Effects of different selenium sources on growth performance, carcass composition and meat quality in broilers[J]. Journal of Zhejiang University (Agriculture & Life Science), 2005, 31(6): 773-776 (in Chinese). DOI:10.3321/j.issn:1008-9209.2005.06.024 |
| [9] |
BAKHSHALINEJAD R, HASSANABADI A, SWICK R A. Dietary sources and levels of selenium supplements affect growth performance, carcass yield, meat quality and tissue selenium deposition in broilers[J]. Animal Nutrition, 2019, 5(3): 256-263. DOI:10.1016/j.aninu.2019.03.003 |
| [10] |
LIN X, YANG T, LI H, et al. Interactions between different selenium compounds and essential trace elements involved in the antioxidant system of laying hens[J]. Biological Trace Element Research, 2020, 193: 252-260. DOI:10.1007/s12011-019-01701-x |
| [11] |
SALEH A A, EBEID T A. Feeding sodium selenite and nano-selenium stimulates growth and oxidation resistance in broilers[J]. South African Journal of Animal Science, 2019, 49(1): 176. DOI:10.4314/sajas.v49i1.20 |
| [12] |
BOOSTANI A, SADEGHI A A, MOUSAVI S N, et al. Effects of organic, inorganic, and Nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress[J]. Livestock Science, 2015, 178: 330-336. DOI:10.1016/j.livsci.2015.05.004 |
| [13] |
LI J L, ZHANG L, YANG Z Y, et al. Effects of different selenium sources on growth performance, antioxidant capacity and meat quality of local Chinese Subei chickens[J]. Biological Trace Element Research, 2018, 181(2): 340-346. DOI:10.1007/s12011-017-1049-4 |
| [14] |
OLIVEIRA T F B, RIVERA D F R, MESQUITA F R, et al. Effect of different sources and levels of selenium on performance, meat quality, and tissue characteristics of broilers[J]. Journal of Applied Poultry Research, 2014, 23(1): 15-22. DOI:10.3382/japr.2013-00761 |
| [15] |
BRIENS M, MERCIER Y, ROUFFINEAU F, et al. 2-hydroxy-4-methylselenobutanoic acid induces additional tissue selenium enrichment in broiler chickens compared with other selenium sources[J]. Poultry Science, 2014, 93(1): 85-93. DOI:10.3382/ps.2013-03182 |
| [16] |
ZHAO L, SUN L H, HUANG J Q, et al. A novel organic selenium compound exerts unique regulation of selenium speciation, selenogenome, and selenoproteins in broiler chicks[J]. The Journal of Nutrition, 2017, 147(5): 789-797. DOI:10.3945/jn.116.247338 |
| [17] |
MEHDI Y, HORNICK J L, ISTASSE L, et al. Selenium in the environment, metabolism and involvement in body functions[J]. Molecules, 2013, 18(3): 3292-3311. DOI:10.3390/molecules18033292 |
| [18] |
孙庆艳. 不同硒源在产蛋鸡上的评价[D]. 硕士学位论文. 北京: 中国农业科学院, 2016. SUN Q Y. Evaluation of different selenium sources on laying hens[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2016. (in Chinese) |
| [19] |
ZOIDIS E, DEMIRIS N, KOMINAKIS A, et al. Meta-analysis of selenium accumulation and expression of antioxidant enzymes in chicken tissues[J]. Animal, 2014, 8(4): 542-554. DOI:10.1017/S1751731113002395 |
| [20] |
CHAOSAP C, SIVAPIRUNTHEP P, TAKEUNGWONGTRAKUL S, et al. Effects of Zn-L-selenomethionine on carcass composition, meat characteristics, fatty acid composition, glutathione peroxidase activity, and ribonucleotide content in broiler chickens[J]. Food Science of Animal Resour, 2020, 40(3): 338-349. DOI:10.5851/kosfa.2020.e9 |
| [21] |
MENG T T, LIU Y L, XIE C Y, et al. Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens[J]. Biological Trace Element Research, 2019, 189(2): 548-555. DOI:10.1007/s12011-018-1490-z |
| [22] |
CICHOSKI A J, ROTTA R B, SCHEUERMANN G, et al. Investigation of glutathione peroxidase activity in chicken meat under different experimental conditions[J]. Food Science and Technology, 2012, 32(4): 661-667. DOI:10.1590/S0101-20612012005000107 |
| [23] |
陈辉, 黄仁录, 邸科前. 纳米硒在动物营养中的应用进展[J]. 饲料工业, 2005, 26(17): 58-59. CHEN H, HUANG L N, DI K Q. Research progress on application of nano-selenium in animal nutrition[J]. Journal of Feed Industry, 2005, 26(17): 58-59 (in Chinese). DOI:10.3969/j.issn.1001-991X.2005.17.018 |
| [24] |
MIKULKOVÁ K, ILLEK J, BEZDĚKOVÁ Z, et al. Glutathione as an antioxidant marker: determination of glutathione concentration in the breast muscles and liver of broilers supplemented with different selenium sources[J]. Acta Veterinaria Brno, 2019, 88(2): 157-163. DOI:10.2754/avb201988020157 |
| [25] |
LI K X, WANG J S, YUAN D, et al. Effects of different selenium sources and levels on antioxidant status in broiler breeders[J]. Asian-Australasian Journal of Animal Sciences, 2018, 31(12): 1939-1945. DOI:10.5713/ajas.18.0226 |
| [26] |
LIU S M, SUN H X, JOSE C, et al. Phenotypic blood glutathione concentration and selenium supplementation interactions on meat colour stability and fatty acid concentrations in merino lambs[J]. Meat Science, 2011, 87(2): 130-139. DOI:10.1016/j.meatsci.2010.09.011 |
| [27] |
ZHANG K, ZHAO Q Y, ZHAN T F, et al. Effect of different selenium sources on growth performance, tissue selenium content, meat quality, and selenoprotein gene expression in finishing pigs[J]. Biological Trace Element Research, 2020, 196(2): 463-471. DOI:10.1007/s12011-019-01949-3 |
| [28] |
GÖÇMEN R, YAZGAN O, CUFADAR Y. Effect of different organic and inorganic selenium levels on performance, selenium concentrations of some tissues, glutathione peroxidase enzyme activity and meat quality in broilers[J]. The Journal of Animal & Plant Sciences, 2016, 26(4): 916-923. |
| [29] |
CAI S J, WU C X, GONG L M, et al. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers[J]. Poultry Science, 2012, 91(10): 2532-2539. DOI:10.3382/ps.2012-02160 |




