2. 扬州大学农业科技发展研究院, 扬州 225009
2. Institutes of Agricultural Science and Technology Development, Yangzhou University, Yangzhou 225009, China
现代肉鹅高度集约化生产模式下,产量快速增长的同时也伴随着免疫应激的增多,如病原微生物、疫苗接种、运输及环境污染等诸多因素都会引起免疫应激[1]。免疫应激严重影响肉鹅生长性能并导致炎症反应的发生[2]。因此,采取一定措施缓解免疫应激,对提高生产效益和保障肉鹅健康具有重要意义。目前,通过营养调控来缓解免疫应激已成为动物营养学的重要研究方向[3]。
甜菜碱(betaine,Bet)又称为三甲基甘氨酸,最早从甜菜副产物中提取而来,广泛分布于动物、植物和微生物中,具有甲基供体和渗透调节功能[4]。Wang等[5]研究显示,饲粮中添加0.05%甜菜碱提高了肉鸭前期生长性能。Chen等[6]研究表明,饲粮中添加0.05%和0.10%甜菜碱有利于改善肉鸡生长性能。杨雨等[7]研究同样表明,饲粮中添加0.06%~0.18%甜菜碱有利于提高仔鹅前期生长性能。甜菜碱在酒精性或非酒精性脂肪肝、癌症、糖尿病等疾病中发挥抗炎作用[8-11],主要与甜菜碱能抑制核因子-κB(nuclear factor-κB,NF-κB)和核苷酸结合寡聚化结构域样受体家族热蛋白结构域3(nucleotide-binding oligomerization domain-like receptor protein 3,NLRP3)炎症小体活性、调节能量代谢以及减轻细胞凋亡有关[12]。结合甜菜碱的特性与生产中的研究,人们发现饲粮中添加甜菜碱有利于畜禽缓解多种应激[13-14]。因此,本试验通过研究饲粮中添加甜菜碱对脂多糖(lipopolysaccharide,LPS)刺激仔鹅生长性能、器官指数、血清生化指标及脾脏炎性因子表达的影响,以阐明甜菜碱对仔鹅免疫应激反应和生长性能的调节作用。
1 材料与方法 1.1 试验材料甜菜碱为昕洋碱750,有效成分含量为75%,购自北京某科技发展有限公司。LPS来源于大肠杆菌(Escherichia coli)O55:B5,有效成分含量为97%,购自美国Sigma-Aldrich公司。试验动物为168只15日龄体重相近且健康的江南白鹅公鹅,其试验前的饲养管理一致。
1.2 试验设计与饲养管理试验采用2×2双因子随机设计,主因子分别为LPS(腹腔注射LPS或生理盐水)和甜菜碱(饲粮中不添加或添加0.06%甜菜碱,添加量参照杨雨等[7])。试验选取168只15日龄的江南白鹅公鹅,随机分为4组,每组6个重复,每个重复7只鹅。试验设计见表 1。
|
|
表 1 试验设计 Table 1 Experiment design |
分别于16、18、20日龄早晨对试验鹅进行LPS刺激,注射剂量与时间参照Yang等[15],21、28日龄称重、屠宰采样。试验分为应激期(16~21日龄)和恢复期(22~28日龄),试验期13 d。试验鹅网上平养,饲喂干粉料,自由采食、饮水,自然光照。其他饲养管理按常规进行。
饲粮配方主要参考扬州大学家禽生产与营养实验室历年的研究成果[16-17],以玉米、豆粕为基础原料配制基础饲粮,基础饲粮组成及营养水平见表 2。
|
|
表 2 基础饲粮组成及营养水平(风干基础) Table 2 Composition and nutrient levels of the basal diet (air-dry basis) |
分别于21和28日龄,按重复对试验鹅进行称重(空腹6 h)并记录,统计各组试验鹅的采食量和增重情况,并计算应激期和恢复期的平均日采食量(ADFI)、平均日增重(ADG)和料重比(F/G)。
1.3.2 免疫器官指数分别于21和28日龄,从各重复选取接近平均体重的试验鹅1只,屠宰后迅速完整剥离并摘取胸腺、脾脏和法氏囊,剔除脂肪和其他黏附组织,称重并计算免疫器官指数。
1.3.3 血液指标分别于21和28日龄,翅静脉采血并分离血清,用UniCel DXC 800 Synchron全自动生化分析仪检测血清中总蛋白(TP)、白蛋白(ALB)、球蛋白(GLO)含量,并计算白蛋白/球蛋白(白球比,A/G)。
1.3.4 脾脏炎症因子mRNA相对表达量分别于21和28日龄,从各重复选取接近平均体重的试验鹅1只,屠宰后迅速无菌采集脾脏组织,液氮速冻后立即置于-70 ℃冰箱,用于测定脾脏炎症因子mRNA相对表达量。用总RNA提取试剂盒[天根生化科技(北京)有限公司]提取脾脏中总RNA,用Nanodrop 2000超微量分光光度计检测RNA浓度和纯度,用反转录试剂盒[天根生化科技(北京)有限公司]进行反转录,得到的cDNA稀释后通过CFX ConnectTM实时荧光定量PCR仪对脾脏炎症因子白细胞介素-1β(IL-1β)、白细胞介素-6(IL-6)、肿瘤坏死因子-α(TNF-α)及转化生长因子-β(TGF-β)mRNA相对表达量进行相对定量分析,以β-肌动蛋白(β-actin)作为内参基因,基因引物信息见表 3。反应体系为20 μL:SYBR Green PCR Master Mix (2×) 10 μL,上、下游引物各1.4 μL,cDNA模板1 μL、无酶水6.2 μL。反应条件:95 ℃预变性2 min,95 ℃变性5 s、60 ℃退火10 s,共39个循环。采用2-ΔΔCt法计算mRNA相对表达量。
|
|
表 3 基因引物信息 Table 3 Primer sequences of genes |
试验数据采用Excel 2017进行初步整理,利用SPSS 20.0统计软件的一般线性模型(GLM)程序进行双因素方差分析,模型主效应包括LPS、甜菜碱及两者的互作效应。当有互作效应时,采用Duncan氏法进行多重比较。结果用平均值和均值标准误(SEM)表示。P≤0.05表示差异显著,0.05<P<0.10表示差异有显著趋势。采用GraphPad Prism 7.0软件作图。
2 结果与分析 2.1 甜菜碱对LPS刺激仔鹅生长性能的影响如表 4所示,在应激期,LPS刺激显著降低仔鹅21日龄体重、ADFI和ADG(P≤0.05),显著提高F/G(P≤0.05),而添加甜菜碱具有提高仔鹅21日龄体重(P=0.090)和ADFI(P=0.080)的趋势,LPS刺激和添加甜菜碱对仔鹅21日龄体重有互作效应趋势(P=0.075),LPS刺激和添加甜菜碱对仔鹅ADFI有显著互作效应(P≤0.05)。这表明饲粮中添加甜菜碱可以缓解由LPS刺激引起ADFI的降低。
|
|
表 4 甜菜碱对LPS刺激仔鹅生长性能的影响 Table 4 Effects of Bet on growth performance of geese challenged by LPS |
在恢复期,LPS刺激显著降低仔鹅28日龄体重、ADFI和F/G(P≤0.05),对ADG无显著影响(P>0.05);添加甜菜碱对仔鹅恢复期生长性能无显著影响(P>0.05)。
2.2 甜菜碱对LPS刺激仔鹅器官指数的影响如表 5所示,21日龄时,LPS刺激显著降低仔鹅胸腺指数(P≤0.05),而添加甜菜碱显著提高了仔鹅胸腺指数(P≤0.05)。LPS刺激和添加甜菜碱对各器官指数均无显著互作效应(P>0.05)。
|
|
表 5 甜菜碱对LPS刺激仔鹅器官指数的影响 Table 5 Effects of Bet on organ indices of geese challenged by LPS |
如表 6所示,21日龄时,LPS刺激显著降低仔鹅血清白蛋白含量和白球比(P≤0.05),显著提高血清球蛋白含量(P≤0.05);LPS刺激和添加甜菜碱对仔鹅血清白蛋白含量有显著互作效应(P≤0.05)。这表明饲粮中添加甜菜碱能够一定程度上阻止LPS刺激对仔鹅血清中白蛋白含量的降低作用。
|
|
表 6 甜菜碱对LPS刺激仔鹅血清生化指标的影响 Table 6 Effects of Bet on serum biochemical parameters of geese challenged by LPS |
如图 1所示,21日龄时,LPS刺激显著提高仔鹅脾脏IL-1β mRNA相对表达量(P≤0.05),而添加甜菜碱显著降低脾脏IL-1β mRNA相对表达量(P≤0.05)。28日龄时,LPS刺激和添加甜菜碱对仔鹅脾脏炎症因子mRNA相对表达量均无显著影响(P>0.05)。
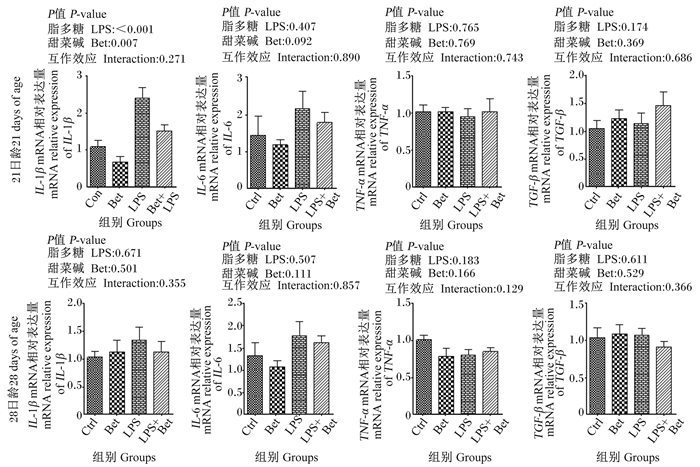
|
图 1 甜菜碱对LPS刺激仔鹅脾脏炎性因子mRNA相对表达量的影响 Fig. 1 Effects of Bet on mRNA relative expression of inflammatory factors in spleen of geese challenged by LPS |
LPS是革兰氏阴性细菌的主要致病性因子,由脂质A、核心多糖及O抗原3部分组成[18]。腹腔或肌肉注射LPS是目前构建动物免疫应激模型最常用的方式。免疫应激使机体产生全身炎性反应,引发食欲减退、采食量下降、嗜睡等生理和行为的变化[19],进而导致生长性能下降。Liu等[20]在肉仔鸡12、14、33和35日龄分别腹腔注射0.5 mg/kg BW的LPS,发现LPS刺激对肉仔鸡1~21日龄、22~42日龄及1~42日龄ADG和ADFI均有不良影响。本试验中,LPS刺激显著降低了仔鹅21日龄体重和16~21日龄ADFI、ADG,提高了16~21日龄F/G,这与前人在肉鸡方面的研究结果[21-22]类似,同时提示本次免疫应激模型构建成功。甜菜碱作为有效饲料添加剂,具有促生长、改善胴体组成及提高肉品质的作用[23]。Liu等[24]试验表明,饲粮中添加0.05%、0.10%、0.20%甜菜碱可以减轻热应激对黄羽肉鸡采食量和体重的不利影响,且存在剂量效应。Hamidi等[25]报道,饲粮中添加0.12%甜菜碱能显著提高混合球虫感染肉鸡的ADG,可能与甜菜碱对肠道上皮细胞和肠道黏膜的保护作用有关。Wang等[26]研究表明,饲粮中添加甜菜碱通过增强肠组织功能、降低腹泻率、促进营养物质消化来改善断奶仔猪的生长性能。本试验研究发现,在应激期,饲粮中添加甜菜碱具有提高21日龄体重和ADFI的趋势,LPS刺激和添加甜菜碱对仔鹅21日龄体重有互作效应趋势,对ADFI有显著互作效应,表明甜菜碱能缓解LPS刺激对仔鹅生长性能的影响,其原因可能与甜菜碱具有诱食性进而提高了采食量有关。
3.2 甜菜碱对LPS刺激仔鹅器官指数的影响胸腺、脾脏和法氏囊是家禽的主要免疫器官,这3个器官的指数是评价家禽免疫状况的重要指标[27]。胸腺是细胞免疫的重要器官,脾脏是产生抗体、补体等免疫物质的最大免疫器官,法氏囊是家禽特有的体液免疫器官。本试验中,LPS刺激导致21日龄仔鹅胸腺指数显著下降,这与孙骁等[28]报道仔猪LPS刺激的结果一致。研究表明,胸腺萎缩与胸腺细胞凋亡有关,胸腺组织和胸腺组织中的淋巴细胞同时随着年龄增长而减少[29]。而饲粮中添加甜菜碱缓解了LPS刺激对仔鹅胸腺的不利影响,表明甜菜碱可以增强仔鹅的免疫功能,可能与甜菜碱提供甲基、促进蛋白质合成,进而促进细胞生长有关[30]。安立龙等[31]报道,饲粮中添加0.1%甜菜碱可以减弱热应激对肉鸡胸腺、脾脏组织的损伤作用,保护组织结构的完整性。酒精性肝损伤模型试验中,甜菜碱主要通过降低炎性因子表达、减少脂质过氧化及预防细胞凋亡来发挥护肝功能[32-33]。
3.3 甜菜碱对LPS刺激仔鹅血清生化指标的影响血清中某些成分变化在一定程度上可以反映动物机体的生理状态。血清总蛋白来自肝脏合成和肠道吸收,主要包括白蛋白和球蛋白。白蛋白是血清总蛋白的主要蛋白质成分,可维持血浆胶体渗透压,在体内代谢物质的运输、营养和组织修复等方面都起着重要的作用[34],球蛋白由机体免疫器官产生,反映机体免疫力,当机体受到病原体攻击时,免疫系统就会增加球蛋白的产生。本试验中,LPS刺激导致21日龄仔鹅血清白蛋白含量显著降低,球蛋白含量显著升高,白球比显著降低,表明机体受到应激,产生炎症反应。而LPS刺激与添加甜菜碱对血清白蛋白含量存在显著互作效应,说明饲粮中添加甜菜碱能够一定程度上阻止LPS刺激对血清白蛋白含量的降低作用,添加甜菜碱有利于白蛋白合成,提高对组织损伤的修复作用。Remus等[35]提出饲粮中添加甜菜碱对血清总蛋白、白蛋白和球蛋白含量具有改善作用,主要与甜菜碱能提供甲基、促进蛋白质合成有关。Yang[36]等研究同样表明,饲粮中添加0.06%甜菜碱可以提高仔鹅血清总蛋白含量。然而Ghasemi等[37]研究发现,饲粮中添加1%甜菜碱对饲喂不同蛋白质水平的热应激肉鸡血清总蛋白含量没有显著影响,但可以提高抗新城疫和传染性支气管炎病毒的抗体滴度。
3.4 甜菜碱对LPS刺激仔鹅炎性细胞因子表达的影响天然免疫是机体抵抗病原微生物侵袭的第1道防线,研究表明,LPS是活化机体免疫系统中巨噬细胞(先天免疫细胞)的有效诱导物,促使炎性因子IL-1β、IL-6和TNF-α等的合成与分泌,引发炎症反应[38]。Li等[39]通过体外试验证明,LPS攻毒小鼠巨噬细胞后炎性因子(IL-1β、IL-6、TNF-α)和趋化因子等mRNA相对表达量显著上升。本试验中,LPS刺激显著提高21日龄仔鹅脾脏IL-1β mRNA相对表达量,对IL-6 mRNA相对表达量有增加趋势。Li等[40]和Shen等[41]在LPS刺激肉鸡试验中也观察到类似结果。本研究发现,饲粮中添加甜菜碱显著降低21日龄仔鹅脾脏IL-1β mRNA相对表达量。IL-1β在机体应对感染和炎症损伤中具有重要作用,IL-1β是NF-κB下游基因之一。Xia等[42]指出,甜菜碱可抑制NF-κB的活化从而降低IL-1β的表达。Zhang等[43]研究发现,甜菜碱降低炎性因子IL-6 mRNA相对表达量与抑制其启动子区域的甲基化有关。TGF-β是重要的抗炎因子,具有促进细胞生长、分化和免疫调节作用,LPS刺激和添加甜菜碱对TGF-β mRNA相对表达量都没有显著影响。
4 结论LPS刺激降低仔鹅生长性能和免疫性能,而饲粮中添加0.06%甜菜碱可以改善仔鹅生长性能,提高胸腺指数,降低脾脏促炎因子IL-1β mRNA相对表达量,从而在一定程度上缓解LPS刺激对仔鹅的负面影响。甜菜碱对LPS刺激应激期的缓解作用比恢复期更为明显。
| [1] |
LI R, SONG Z H, ZHAO J F, et al. Dietary L-theanine alleviated lipopolysaccharide-induced immunological stress in yellow-feathered broilers[J]. Animal Nutrition, 2018, 4(3): 265-272. DOI:10.1016/j.aninu.2018.05.002 |
| [2] |
郑灶波. 不同环境应激对肉鹅免疫力、肠道微生物及生长性能的影响[D]. 硕士学位论文. 广州: 仲恺农业工程学院, 2017. ZHENG Z B. The effects of environmental stress on immunity, intestinal microbes and growth performance in goose[D]. Master's Thesis. Guangzhou: Zhongkai University of Agriculture and Engineering, 2017. (in Chinese) |
| [3] |
刘玉兰. 仔猪免疫应激及其营养调控研究进展[J]. 中国畜牧杂志, 2017, 53(10): 1-3, 11. LIU Y L. Research progress on piglet immune stress and nutritional regulation[J]. Chinese Journal of Animal Science, 2017, 53(10): 1-3, 11 (in Chinese). |
| [4] |
EKLUND M, BAUER E, WAMATU J, et al. Potential nutritional and physiological functions of betaine in livestock[J]. Nutrition Research Reviews, 2005, 18(1): 31-48. DOI:10.1079/NRR200493 |
| [5] |
WANG Y Z, XU Z R, FENG J. The effect of betaine and DL-methionine on growth performance and carcass characteristics in meat ducks[J]. Animal Feed Science and Technology, 2004, 116(1/2): 151-159. |
| [6] |
CHEN R, ZHUANG S, CHEN Y P, et al. Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway[J]. Poultry Science, 2018, 97(12): 4297-4305. DOI:10.3382/ps/pey303 |
| [7] |
杨雨, 阳金金, 万雨, 等. 饲粮中添加甜菜碱对仔鹅生长性能及其调控因子的影响[J]. 动物营养学报, 2020, 32(9): 4140-4147. YANG Y, YANG J J, WANG Y, et al. Effects of dietary betaine on growth performance, serum contents of growth hormone and insulin-like growth factor-Ⅰ, slaughter performance and viscera indices of geese[J]. Chinese Journal of Animal Nutrition, 2020, 32(9): 4140-4147 (in Chinese). |
| [8] |
JI C, SHINOHARA M, VANCE D, et al. Effect of transgenic extrahepatic expression of betaine-homocysteine methyltransferase on alcohol or homocysteine-induced fatty liver[J]. Alcoholism: Clinical & Experimental Research, 2008, 32(6): 1049-1058. |
| [9] |
KATHIRVEL E, MORGAN K, NANDGIRI G, et al. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2010, 299(5): G1068-G1077. DOI:10.1152/ajpgi.00249.2010 |
| [10] |
KAR F, HACIOGLU C, KACAR S, et al. Betaine suppresses cell proliferation by increasing oxidative stress-mediated apoptosis and inflammation in DU-145 human prostate cancer cell line[J]. Cell Stress and Chaperones, 2019, 24(5): 871-881. DOI:10.1007/s12192-019-01022-x |
| [11] |
HUANG B Q, HU X L, HU J, et al. Betaine alleviates cognitive deficits in diabetic rats via PI3K/Akt signaling pathway regulation[J]. Dementia and Geriatric Cognitive Disorders, 2020. DOI:10.1159/000508624 |
| [12] |
ZHAO G F, HE F, WU C L, et al. Betaine in inflammation: mechanistic aspects and applications[J]. Frontiers in Immunology, 2018, 9: 1070. DOI:10.3389/fimmu.2018.01070 |
| [13] |
RATRIYANTO A, MOSENTHIN R. Osmoregulatory function of betaine in alleviating heat stress in poultry[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(6): 1634-1650. DOI:10.1111/jpn.12990 |
| [14] |
SHEDID S M, ABDEL-MAGIED N, SAADA H N. Role of betaine in liver injury induced by the exposure to ionizing radiation[J]. Environmental Toxicology, 2019, 34(2): 123-130. DOI:10.1002/tox.22664 |
| [15] |
YANG L, LIU G, ZHU X Q, et al. The anti-inflammatory and antioxidant effects of leonurine hydrochloride after lipopolysaccharide challenge in broiler chicks[J]. Poultry Science, 2019, 98(4): 1648-1657. DOI:10.3382/ps/pey532 |
| [16] |
CHEN Y J, YANG H M, WAN X L, et al. The effect of different dietary levels of sodium and chloride on performance and blood parameters in goslings (1 to 28 days of age)[J]. Journal of Animal Physiology and Animal Nutrition, 2020, 104(2): 507-516. DOI:10.1111/jpn.13273 |
| [17] |
张得才. 蚕沙的营养价值评定及其在仔鹅饲粮中的应用研究[D]. 硕士学位论文. 扬州: 扬州大学, 2015. ZHANG D C. Study on nutrition value and application in diets of silkworm excrement for geese[D]. Master's Thesis. Yangzhou: Yangzhou University, 2015. (in Chinese) |
| [18] |
WANG X F, LI Y L, SHEN J, et al. Effect of Astragalus polysaccharide and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers[J]. International Journal of Biological Macromolecules, 2015, 76: 188-194. DOI:10.1016/j.ijbiomac.2015.02.040 |
| [19] |
KIM K, EHRLICH A, PERNG V, et al. Algae-derived β-glucan enhanced gut health and immune responses of weaned pigs experimentally infected with a pathogenic E. coli[J]. Animal Feed Science and Technology, 2019, 248: 114-125. DOI:10.1016/j.anifeedsci.2018.12.004 |
| [20] |
LIU L, SHEN J, ZHAO C, et al. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide[J]. International Journal of Biological Macromolecules, 2015, 72: 624-632. DOI:10.1016/j.ijbiomac.2014.08.057 |
| [21] |
WANG W W, LI Z, HAN Q Q, et al. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers[J]. British Journal of Nutrition, 2016, 116(11): 1878-1888. DOI:10.1017/S0007114516004116 |
| [22] |
CHEN Y P, ZHANG H, CHENG Y F, et al. Dietary L-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age[J]. British Journal of Nutrition, 2018, 119(11): 1254-1262. DOI:10.1017/S0007114518000740 |
| [23] |
武志敏. 甜菜碱和苜蓿皂甙对断奶仔猪生长性能及免疫功能的影响[D]. 硕士学位论文. 大庆: 黑龙江八一农垦大学, 2010. WU Z M. Effect of betaine alfalfa saponin on growth performance and immune function of weaned piglets[D]. Master's Thesis. Daqing: Heilongjiang Bayi Agricultural University, 2010. |
| [24] |
LIU W C, YUAN Y L, SUN C Y, et al. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress[J]. Animals, 2019, 9(8): 506. DOI:10.3390/ani9080506 |
| [25] |
HAMIDI H, JAHANIAN R, POURREZA J. Effect of dietary betaine on performance, immunocompetence and gut contents osmolarity of broilers challenged with a mixed coccidial infection[J]. Asian Journal of Animal and Veterinary Advances, 2010, 5(3): 193-201. DOI:10.3923/ajava.2010.193.201 |
| [26] |
WANG H C, LI S S, XU S Y, et al. Betaine improves growth performance by increasing digestive enzymes activities, and enhancing intestinal structure of weaned piglets[J]. Animal Feed Science and Technology, 2020, 267: 114545. DOI:10.1016/j.anifeedsci.2020.114545 |
| [27] |
ZHENG X C, WU Q J, SONG Z H, et al. Effects of oridonin on growth performance and oxidative stress in broilers challenged with lipopolysaccharide[J]. Poultry Science, 2016, 95(10): 2281-2289. DOI:10.3382/ps/pew161 |
| [28] |
孙骁, 李梓丹, 刘华, 等. 饲粮纤维对脂多糖刺激仔猪生长性能、器官相对重量及炎性细胞因子mRNA表达量的影响[J]. 动物营养学报, 2019, 31(11): 5018-5028. SUN X, LI Z D, LIU H, et al. Effects of dietary on growth performance, relative weight of organs and mRNA expression of inflammatory cytokines in lipopolysaccharide-challenged piglets[J]. Chinese Journal of Animal, 2019, 31(11): 5018-5028 (in Chinese). |
| [29] |
MARUŠIĆ M, TURKALJ-KLJAJIĆ M, PETROVEČKI M, et al. Indirect demonstration of the lifetime function of human thymus[J]. Clinical and Experimental Immunology, 1998, 111(2): 450-456. DOI:10.1046/j.1365-2249.1998.00470.x |
| [30] |
效梅, 安立龙, 冯业, 等. 中药-甜菜碱复方对高温环境中三黄鸡组织器官生长发育的影响[J]. 家畜生态学报, 2008, 29(6): 56-62. XIAO M, AN L L, FENG Y, et al. Effects of Chinese medicine and betaine compound prescription on growth and development in three yellow broilers under high temperature[J]. Acta Ecologae Abimalis Domastici, 2008, 29(6): 56-62 (in Chinese). |
| [31] |
安立龙, 效梅, 黄志毅, 等. 不同剂量甜菜碱对热应激肉鸡组织器官发育的影响[J]. 家畜生态学报, 2005, 26(3): 40-46. AN L L, XI AO, HUANG Z Y, et al. The influence of betaine on growth of tissue and organ for broiler under heat stress[J]. Acta Ecologae Abimalis Domastici, 2005, 26(3): 40-46 (in Chinese). |
| [32] |
SHI Q Z, WANG L W, ZHANG W, et al. Betaine inhibits Toll-like receptor 4 expression in rats with ethanol-induced liver injury[J]. World Journal of Gastroenterology, 2010, 16(7): 897-903. |
| [33] |
JI C, KAPLOWITZ N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice[J]. Gastroenterology, 2003, 124(5): 1488-1499. DOI:10.1016/S0016-5085(03)00276-2 |
| [34] |
ATTIA Y A, HASSAN R A, QOTA E M A. Recovery from adverse effects of heat stress on slow-growing chicks in the tropics 1:effect of ascorbic acid and different levels of betaine[J]. Tropical Animal Health and Production, 2009, 41(5): 807-818. DOI:10.1007/s11250-008-9256-9 |
| [35] |
REMUS J, VIRTANEN E, ROSI L, et al. Effect of betaine on nutrient utilization of 21-day-old broilers during coccidiosis[C]//Proceedings of the 10th European symposium on poultry nutrition. Turkish Branch: World's Poultry Science Association, 1995: 371-372.
|
| [36] |
YANG Z, WANG Z Y, YANG H M, et al. Effects of dietary methionine and betaine on slaughter performance, biochemical and enzymatic parameters in goose liver and hepatic composition[J]. Animal Feed Science and Technology, 2017, 228: 48-58. DOI:10.1016/j.anifeedsci.2017.04.003 |
| [37] |
GHASEMI H A, NARI N. Effect of supplementary betaine on growth performance, blood biochemical profile, and immune response in heat-stressed broilers fed different dietary protein levels[J]. Journal of Applied Poultry Research, 2020, 29(2): 301-313. DOI:10.1016/j.japr.2019.11.004 |
| [38] |
MCNELIS J C, OLEFSKY J M. Macrophages, immunity, and metabolic disease[J]. Immunity, 2014, 41(1): 36-48. DOI:10.1016/j.immuni.2014.05.010 |
| [39] |
LI H, YOON J H, WON H J, et al. Isotrifoliol inhibits pro-inflammatory mediators by suppression of TLR/NF-κB and TLR/MAPK signaling in LPS-induced RAW264.7 cells[J]. International Immunopharmacology, 2017, 45: 110-119. DOI:10.1016/j.intimp.2017.01.033 |
| [40] |
LI K, ZHANG P F, SHI B L, et al. Dietary Artemisia ordosica extract alleviating immune stress in broilers exposed to lipopolysaccharide[J]. Italian Journal of Animal Science, 2017, 16(2): 301-307. DOI:10.1080/1828051X.2016.1274242 |
| [41] |
SHEN J, WU S R, GUO W, et al. Epigenetic regulation of pro-inflammatory cytokine genes in lipopolysaccharide-stimulated peripheral blood mononuclear cells from broilers[J]. Immunobiology, 2017, 222(2): 308-315. DOI:10.1016/j.imbio.2016.09.009 |
| [42] |
XIA Y Y, CHEN S, ZHU G Q, et al. Betaine inhibits interleukin-1β production and release: potential mechanisms[J]. Frontiers in Immunology, 2018, 9: 2670. DOI:10.3389/fimmu.2018.02670 |
| [43] |
ZHANG Y H, YU Y, OU C B, et al. Alleviation of infectious-bursal-disease-virus-induced bursal injury by betaine is associated with DNA methylation in IL-6 and interferon regulatory factor 7 promoter[J]. Poultry Science, 2019, 98(10): 4457-4464. DOI:10.3382/ps/pez280 |




